Abstract
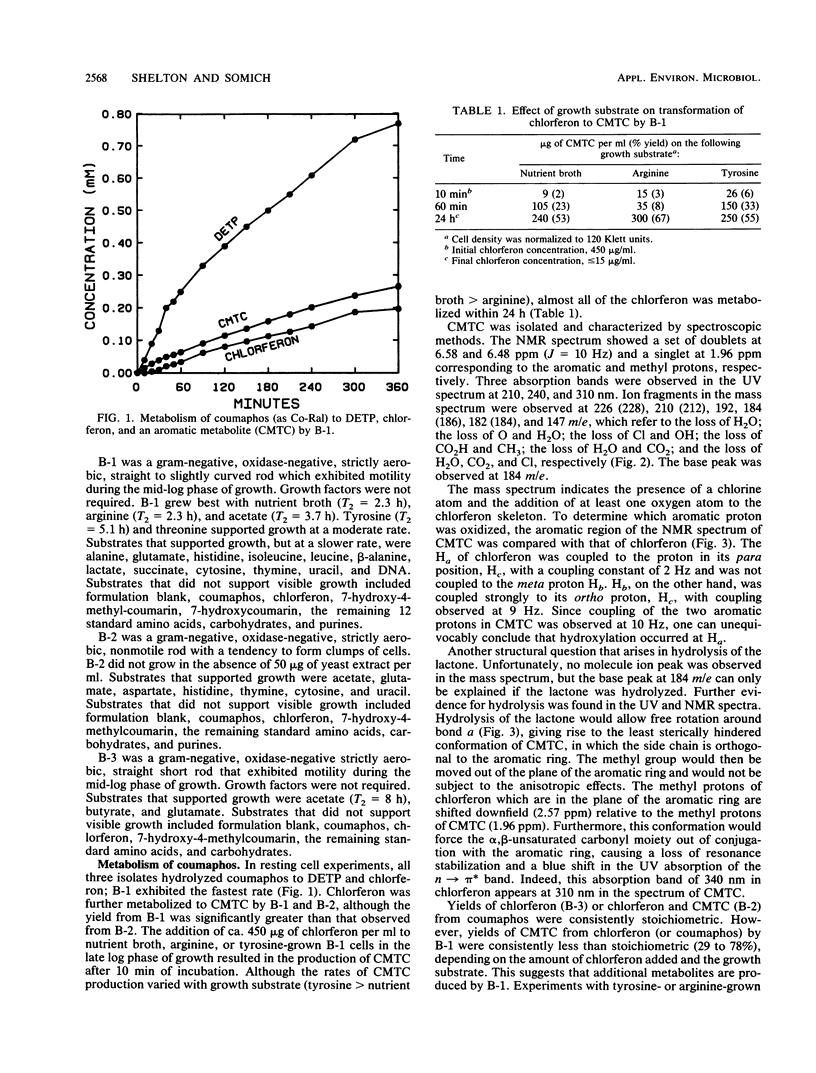
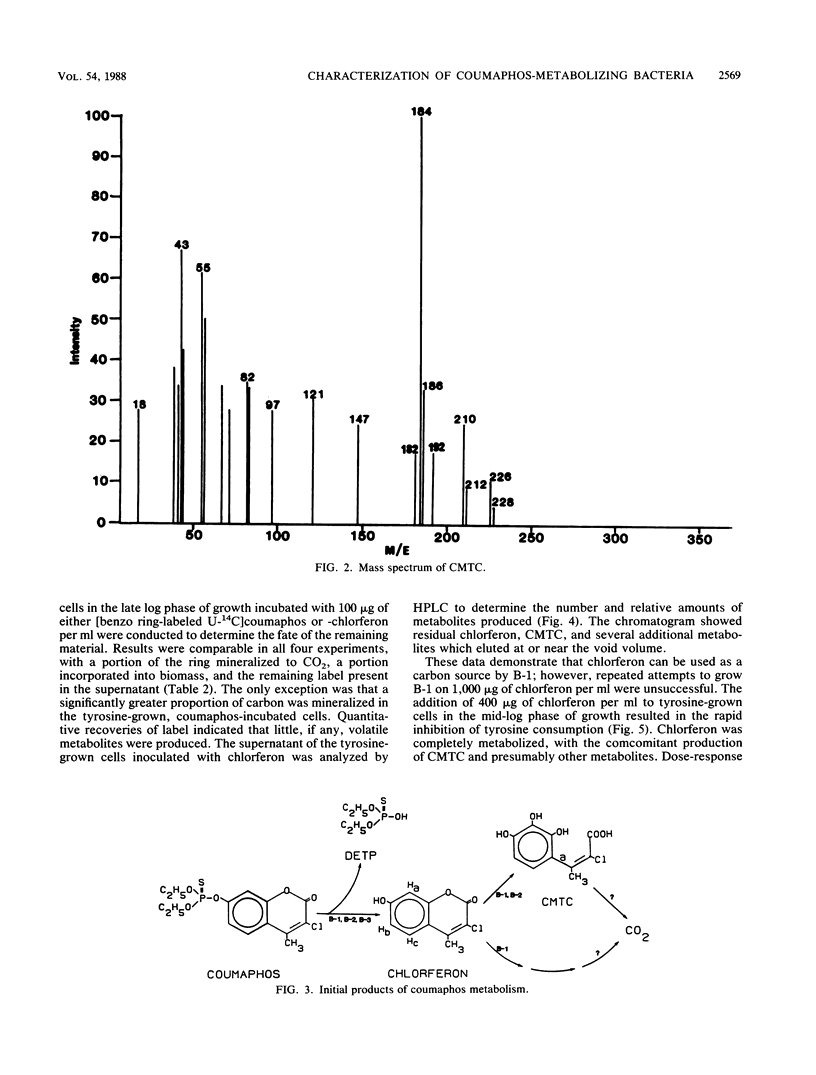
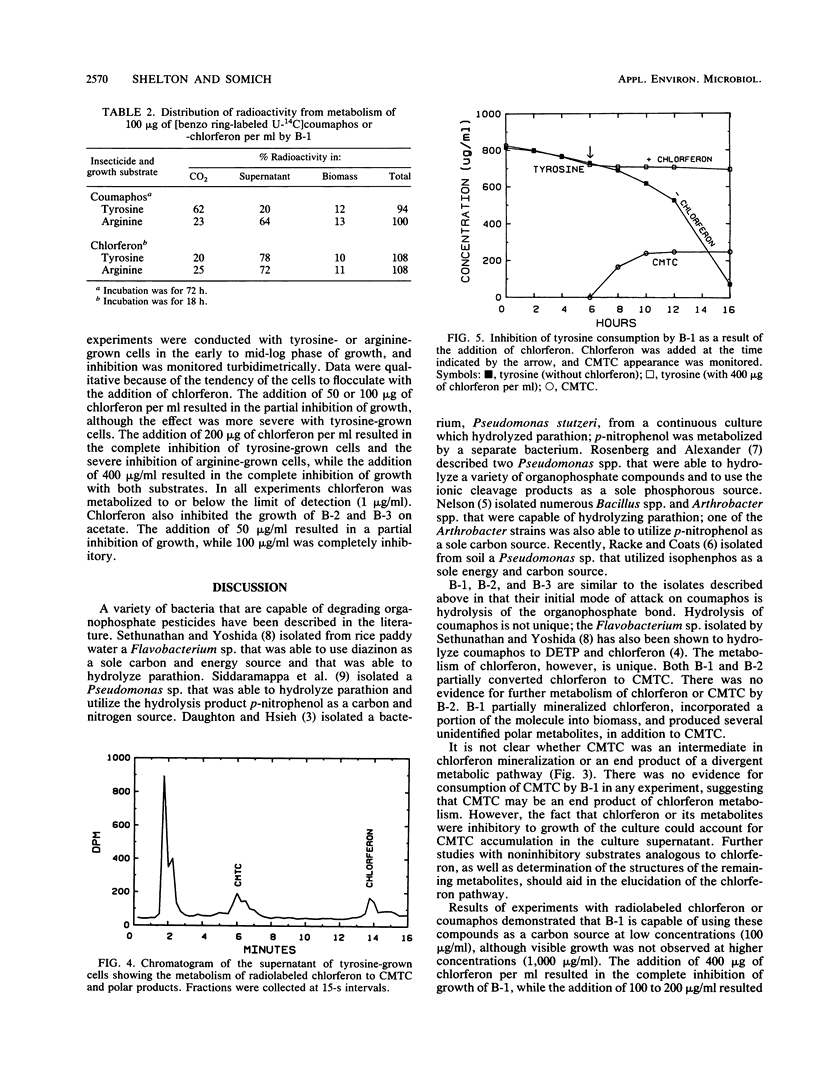
Coumaphos, an organophosphate insecticide, is used for tick control in cattle dipping vats along the U.S.-Mexican border. Recently, several vats (problem vats) have experienced a loss of efficacy because of microbial degradation. Three morphologically distinct bacteria (designated B-1, B-2, and B-3) that metabolized coumaphos were isolated from enrichment cultures that were initiated from problem vat dip material. In general, amino acids, pyrimidines, and acetate supported growth; carbohydrates were not utilized. Only B-2 required growth factors. In resting cell experiments, coumaphos was hydrolyzed to diethylthiophosphoric acid and chlorferon by all three isolates. Chlorferon was subsequently metabolized by B-1 and B-2 to α-chloro-β-methyl-2,3,4-trihydroxy-trans-cinnamic acid. Only B-1 produced additional metabolites. Experiments with [benzo ring-labeled U-14C]coumaphos or chlorferon demonstrated that B-1 was capable of both mineralizing and incorporating into biomass the aromatic portion of the molecule. The majority of label, however, was recovered in the form of soluble products, including α-chloro-β-methyl-2,3,4-trihydroxy-trans-cinnamic acid. Although B-1 had the capacity to use chlorferon as a carbon source at low concentrations (100 μg/ml), visible growth at higher concentrations (1,000 μg/ml) was not observed. The addition of 400 μg of chlorferon per ml to B-1 cells in the mid-log phase of growth resulted in complete inhibition of growth, while the addition of 100 to 200 μg of chlorferon per ml resulted in partial inhibition. The growth of B-2 and B-3 was inhibited by 100 μg of chlorferon per ml. These data suggest that, although B-1 and, to a lesser extent, B-2 and B-3 are responsible for the primary degradation of coumaphos, other organisms in the enrichment culture may play a secondary role in coumaphos degradation by removing inhibitory products of coumaphos metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner W., Staub D., Leisinger T. Bacterial degradation of dichloromethane. Appl Environ Microbiol. 1980 Nov;40(5):950–958. doi: 10.1128/aem.40.5.950-958.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. G., Hsieh D. P. Parathion utilization by bacterial symbionts in a chemostat. Appl Environ Microbiol. 1977 Aug;34(2):175–184. doi: 10.1128/aem.34.2.175-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Alexander M. Microbial cleavage of various organophosphorus insecticides. Appl Environ Microbiol. 1979 May;37(5):886–891. doi: 10.1128/aem.37.5.886-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethunathan N., Yoshida T. A Flavobacterium sp. that degrades diazinon and parathion. Can J Microbiol. 1973 Jul;19(7):873–875. doi: 10.1139/m73-138. [DOI] [PubMed] [Google Scholar]
- Siddaramappa R., Rajaram K. P., Sethunathan N. Degradation of parathion by bacteria isolated from flooded soil. Appl Microbiol. 1973 Dec;26(6):846–849. doi: 10.1128/am.26.6.846-849.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


