Abstract
This article describes the distribution and development of handedness for manual gestures in captive chimpanzees. Data on handedness for unimanual gestures were collected in a sample of 227 captive chimpanzees. Handedness for these gestures was compared with handedness for three other measures of hand use: tool use, reaching, and coordinated bimanual actions. Chimpanzees were significantly more right-handed for gestures than for all other measures of hand use. Hand use for simple reaching at 3 to 4 years of age predicted hand use for gestures 10 years later. Use of the right hand for gestures was significantly higher when gestures were accompanied by a vocalization than when they were not. The collective results suggest that left-hemisphere specialization for language may have evolved initially from asymmetries in manual gestures in the common ancestor of chimpanzees and humans, rather than from hand use associated with other, noncommunicative motor actions, including tool use and coordinated bimanual actions, as has been previously suggested in the literature.
One of the most pronounced manifestations of hemispheric specialization in humans is population-level right-handedness and left-hemisphere specialization for language (Hellige, 1993). Although there is cultural variation, all human populations studied to date are right-handed (Raymond & Pontier, 2004). It has been argued that right-handedness is a marker of hemispheric specialization for language functions. Among right-handed subjects, 96% show left-hemisphere dominance for speech, whereas 70% of left-handed subjects do (Knecht et al., 2000; Rasmussen & Milner, 1977).
Historically, population-level functional and neuroanatomical asymmetries have been considered a hallmark of human evolution and fundamentally linked to the emergence of language and speech, tool use, and bipedalism (see Bradshaw & Rogers, 1993, for review); however, recent studies in a host of vertebrate species have found evidence of population-level behavioral and neuroanatomical asymmetries (Rogers & Andrew, 2002; but see Crow, 1998). The evidence of population-level asymmetries in many vertebrates clearly indicates that language was not a necessary condition for the evolution of hemispheric specialization. However, the evolutionary processes that led to left-hemisphere specialization in handedness and language remain a central topic of interest in comparative neuropsychological research (Corballis, 2002; Kimura, 1993; Steklis & Raleigh, 1979).
One line of investigation that may provide a link between handedness and hemispheric specialization for language is research on manual gestural communication (Corballis, 2002; Iverson & Thelen, 1999; Kimura, 1993). It is well known that deaf humans sign primarily with the right hand and show left-hemisphere dominance in the production and perception of signs (Corina et al., 1993). It has also been shown that movement of the right hand is significantly greater when people are talking than when they are not talking, although this effect is reduced in self-reported left-handed individuals (Kimura, 1993). Ontogenetically, increased pointing with the right hand is associated with the development of speech in children (Blake, O'Rourke, & Borzellino, 1994), and the right hand is used more for signing than for nonsigned motor actions in children of deaf parents (Bonvillian, Richards, & Dooley, 1997). Collectively, these studies suggest that sign language and gestures are specialized to the left hemisphere in adults and developing children.
Recent comparative studies have shown that chimpanzees (and other great apes) intentionally and referentially communicate through manual gestures (Call & Tomasello, 1994; Hostetter, Cantero, & Hopkins, 2001; Leavens & Hopkins, 1998; Leavens, Hopkins, & Bard, 1996; Leavens, Hopkins, & Thomas, 2004; Leavens, Hostetter, Wesley, & Hopkins, 2004; Tomasello, Call, Nagell, Olguin, & Carpenter, 1994). Given the important communicative role of manual gestures in chimpanzees, evidence of lateralization in this behavior could provide important insights for evolutionary models of hemispheric specialization and language.
Recently, Corballis (2002) proposed that hemispheric specialization for language functions in humans evolved from an extant, lateralized manual communication system in the common ancestor of humans and chimpanzees. This evolutionary model differs from others in that the focus is on a direct link between lateralization in communicative behaviors of chimpanzees and left-hemisphere specialization in language for humans. In contrast, many other evolutionary models propose more indirect paths to the emergence of handedness and left-hemisphere specialization for language. For example, these models posit links with tool use, bipedalism, throwing, and coordinated bimanual hand use associated with foraging (see Bradshaw & Rogers, 1993).
The main purpose of the present study was to evaluate Corballis's (2002) hypothesis by comparing handedness for manual gestures in captive chimpanzees with handedness for three other measures of hand use: reaching, coordinated bimanual actions, and tool use. Although Corballis's hypothesis cannot be directly tested, it does predict that lateralization for communication and cognition is more developed than lateralization for other, noncommunicative motor actions (Hamilton & Vermeire, 1988). Our working hypothesis was that the precursor to left-hemisphere specialization for language in humans should be found in the hand use that shows the strongest population-level right-handedness in chimpanzees. According to Corballis, this hand use should be manual gestures, rather than noncommunicative behaviors.
A second purpose of this study was to investigate longitudinally the development of hand use for gestures in captive chimpanzees. Leavens, Hostetter, et al. (2004) previously argued that the development of gestures in captive chimpanzees is governed primarily by the physical constraints their cages impose on their ability to obtain food instrumentally. Wild chimpanzees can simply walk to the location of food and grasp it. For chimpanzees in captivity, the presence of the cage precludes the ability to grasp food outside the cage, and therefore the apes must learn to manipulate a social agent (i.e., human caretaker) to obtain the food for them.
One possible explanation for the development of gestures in chimpanzees is that reaching and grasping for objects becomes conventionalized or generalizes into gesture when objects become no longer directly attainable by grasping. That is, when chimpanzees can no longer obtain an object because of spatial constraints, they begin to develop a conventionalized gesture to request the object. If this is the case, it might be argued that handedness for gestures, in their adult form, is rooted in hand use for reaching and grasping early in development. To test this hypothesis, we correlated hand-use data for reaching and coordinated bimanual actions collected 10 years ago (see Hopkins, 1995a, 1995b) with the gesture data collected in this study. If gestures develop from the context of reaching, then hand use for simple reaching at 3 to 4 years of age should significantly predict hand use for gestures 10 years later, and there should be no association between hand use for coordinated bimanual actions at age 3 to 4 and subsequent hand use for gestures.
Method
Subjects
Subjects were 227 captive chimpanzees (Pan troglodytes) from two separate colonies of apes. The first group of subjects comprised 112 chimpanzees housed at the Department of Veterinary Sciences of The University of Texas M.D. Anderson Cancer Center (UTMDACC). The second group of subjects consisted of 115 chimpanzees housed at the Yerkes National Primate Research Center of Emory University. Subjects ranged in age from 4 to 50 years (M = 20.39, SD = 10.31). Between the two colonies, there were 127 females and 100 males. Of the 127 female subjects, 57 were mother reared, 38 were nursery reared, and 32 were wild caught. Among the 100 males, there were 38 mother-reared, 43 nursery-reared, and 19 wild-caught subjects.
Mother-reared chimpanzees were those reared by their biological mother for more than the first 30 days of life. Nursery-reared subjects were brought to the nursery before they reached 31 days of age and raised there by humans. Wild-caught chimpanzees were captured in Africa. The rearing histories and housing conditions of the chimpanzees in these colonies have been described in detail elsewhere (Hopkins, Hook, Braccini, & Schapiro, 2003).
Procedure
Gesture
The main goal of the testing procedure was to obtain discrete responses so we could measure hand use in manual gestures. At the onset of each trial, the experimenter approached the subject's home cage and offered a food item (a piece of carrot, banana, or candy). The experimenter stood approximately 1 to 1.5 m from the cage and was positioned as much as possible in the center plane of the subject. If not positioned immediately in front of the experimenter, the chimpanzee approached the front of the cage once the experimenter arrived with the food. The experimenter said the chimpanzee's name and offered the food until the subject produced a manual gesture. If by chance the chimpanzee was already gesturing toward the experimenter when he or she arrived, no response was recorded. Hand use was recorded as left or right. Two-handed gestures, which were rare, were not scored. In addition, the experimenter noted whether or not the chimpanzee vocalized while gesturing.
Subjects were tested over a 15-day period, and the goal was to obtain a minimum of 30 responses from each subject. The number of trials administered per day varied in accordance with subjects' motivation and availability for testing. Subjects were tested in both the indoor and the outdoor sections of their home cages. None of the subjects were separated from their groups or cage mates for the purposes of testing.
Other Behavioral Measures of Hand Use
As previously stated, one purpose of this study was to compare handedness for gestures with handedness for other measures of hand use: bimanual coordination (tube task), simple reaching, and tool use. We provide a brief description of each measure here. Full details of the methods and procedures are available elsewhere (Hopkins et al., 2003; Hopkins & Rabinowitz, 1997; Hopkins, Russell, Hook, Braccini, & Schapiro, 2005). A minimum of 40 responses was collected from each subject for each measure.
Tube Task
Hand preference was assessed using the tube task, a task designed to elicit coordinated bimanual actions (see Hopkins et al., 2003, for a description). For this task, peanut butter was smeared on the inside edges of polyvinyl-chloride (PVC) tubes approximately 15 cm in length and 2.5 cm in diameter. The peanut butter was smeared on both ends of the PVC pipes and placed far enough down the tubes that the chimpanzees could not lick it off completely with their mouths, but rather had to use their fingers to remove it. The PVC tubes were handed to the subjects in their home cages, and a focal sampling was used to collect individual data from each subject. The experimenter recorded whether the peanut butter was extracted by a finger of the right or left hand.
Reaching
On each trial of this task, the experimenter threw a raisin into the subject's home cage. The raisin was thrown to a location at least 3 m from the focal subject so that the chimpanzee had to locomote to the raisin, pick it up, and bring it to its mouth for consumption. The experimenter recorded whether the right or left hand was used to acquire the raisin (see Hopkins et al., 2005).
Tool Use
At both facilities, PVC pipes mounted at approximately 45° angles on rectangular plastic boards were affixed to the outside portion of the home cages. One end of each tube (the one glued to the board) was open, allowing access to honey or syrup down at the other end, which was closed by a removable PVC cap. The apparatus could be easily attached with stainless steel screws onto the cage wall of the subjects, and the subjects could individually access the open ends of the PVC tubes through the cage mesh and dip for food at the bottom. During testing, each PVC tube was first filled with honey or syrup to about one third of its whole length, which made it impossible for subjects to reach the food directly with their fingers. After the devices were attached to the cages, sticks or bamboo skewers were directly handed to the subjects or dropped into the cages from observation towers positioned around the enclosures. Each time a subject probed into a tube, the subject's hand use was recorded as left or right (see Hopkins & Rabinowitz, 1997). Any two-handed responses (including responses in which the two hands mirrored each other) were not recorded.
Juvenile Measures
Tube-task and reaching data were collected for 55 chimpanzees when they were between 3 and 4 years of age, which was approximately 10 years prior to the onset of data collection in this study (see Hopkins, 1995a, 1995b). The reaching data collected in 1994 were obtained using a different method than the procedure just described. Specifically, because the subjects were smaller and could reach through the cage mesh, discrete reaching responses were obtained by placing the food items outside the subjects' home cages. On each trial, the subject had to locomote to a specific location near where a peanut had been placed 20 to 25 cm outside the home cage and reach through the mesh to retrieve the peanut. Hand use was recorded as left or right, and each subject received 50 trials over 10 days of testing. This procedure induced strong asymmetries in the young chimpanzees. The tube data were collected in the same manner as described for the present study.
Data Analysis
For each subject and measure, a handedness index (HI) was determined by subtracting the number of left-handed responses from the number of right-handed responses and then dividing by the total number of responses. HI values ranged from −1.0 to 1.0, with the absolute value representing the strength of the lateral bias; positive values indicate right-hand bias, and negative values indicate left-hand bias. Population-level handedness was evaluated using one-sample t tests with zero as the estimated population mean, which is what would be predicted if the sample of HI scores were normally or bimodally distributed. In addition to conducting inferential analyses, we classified subjects as left-, ambiguously, or right-handed on the basis of binomial z scores calculated from the frequency of left- and right-hand responses. Subjects with z scores of −1.64 or lower and those with z scores of 1.64 or higher were classified as left- and right-handed, respectively. All others were classified as ambiguously handed. Sex, colony, and rearing effects were evaluated using analysis of variance (ANOVA) with alpha set to .05. Post hoc tests were performed using Tukey's honestly significant difference (HSD) test.
Results
Population-Level and Colony Effects for Gestures
In the initial analysis, one-sample t tests were performed on the gesture HI scores for each colony. Significant population-level right-handedness was found for both the Yerkes colony, t(114) = 6.02, p < .001, Ω = 1.12, and the UTMDACC colony, t(111) = 6.42, p < .001, Ω = 1.22 (see Fig. 1). An independent-samples t test failed to reveal significant differences between the HI scores of the two colonies.
Fig. 1.
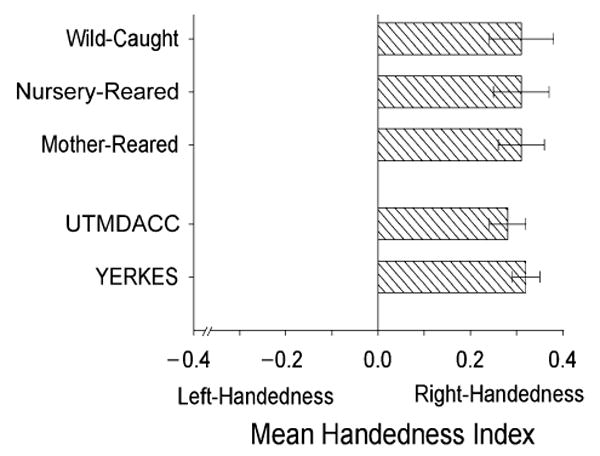
Mean handedness index scores for gesture. Results are shown for UTMDACC (University of Texas M.D. Anderson Cancer Center) and Yerkes chimpanzees separately, as well as for mother-reared, nursery-reared, and wild-caught apes in the two colonies combined. The standard error bars represent variation around the mean scores.
There were 36 left-handed, 58 ambiguously handed, and 133 right-handed subjects, a distribution that differs significantly from chance, χ2(2, N = 227) = 68.36, p < .01, Φ = 1.31. The number of right-handed subjects was significantly higher than the number of left-handed subjects, χ2(1, N = 169) = 55.68, p < .01, Φ = 1.29, and the number of ambiguously handed subjects, χ2(1, N = 191) = 29.45, p < .01, Φ = 0.85.
Sex and Rearing Effects on Manual Gestures
In the next analysis, we evaluated the effect of sex and rearing history on handedness for gesture using a factorial ANOVA. The HI scores were the dependent variable, and sex and rearing history served as between-groups variables. No significant main effects or interactions were found (see Fig. 1). Chi-square tests of independence also failed to reveal any significant associations between hand preference and either sex or rearing history of the subjects.
The Influence of Vocalization on Manual Gestures
The previous analyses focused on overall hand use, irrespective of whether or not the chimpanzees vocalized while gesturing. In this analysis, we evaluated the influence of vocal behavior on hand use for gestures. In all, 594 gestures distributed among 110 chimpanzees were accompanied by a vocalization; 137 of these gestures were made with the left hand and 457 with the right hand. A one-sample t test on HI scores derived only for the gestures accompanied by a vocalization revealed population-level right-handedness (M = .26), t(109) = 3.57, p < .01, Ω = 0.68. When this analysis was restricted to only those 40 individuals that produced 6 or more gestures accompanied by a vocalization, the significant population-level right-handedness remained present (M = .46), t(39) = 6.35, p < .001, Ω = 2.03. Last, we compared the HI scores for gestures accompanied by vocalization with HI scores for gestures not accompanied by vocalization in 40 chimpanzees that produced 6 or more responses. Gestures accompanied by a vocalization were significantly more right-handed than those not accompanied by a vocalization, t(39) = 2.30, p < .03, Ω = 0.74 (see Fig. 2).
Fig. 2.
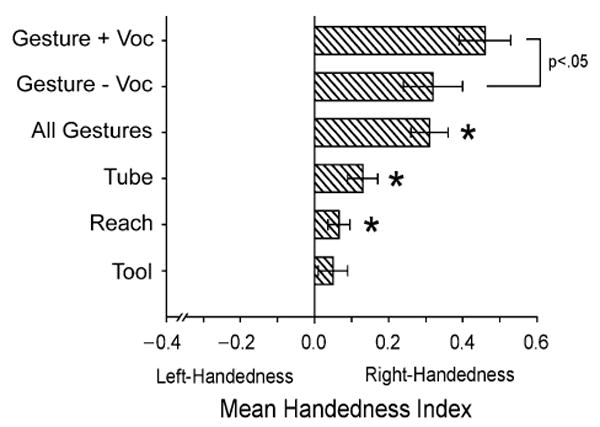
Mean handedness index scores for the four measures of hand use: tool use, simple reaching, coordinated bimanual tube task, and gesture. For gesture, results are shown separately for gestures without vocalization (“− Voc”), gestures accompanied by vocalization (“+ Voc”), and all gestures combined. Asterisks indicate the measures for which the mean score differed significantly from zero. The standard error bars represent variation around the mean scores.
Comparison in Hand Use Between Measures
This analysis was restricted to the 166 subjects for which data were available for all four handedness measures. HI scores for the four behavior measures were computed and compared using a repeated measures ANOVA. Measure (tool use, tube task, reaching, gesture) was the repeated measure; sex and rearing history served as between-groups variables. A significant main effect for measure was found, F(3, 504) = 11.62, p < .001, Ω = 0.06. Post hoc analysis indicated that the HI scores were significantly higher for gestures than for all other measures (see Fig. 2). No other significant differences between the mean HI scores were found. It is important to recognize that the difference between gestures and other measures of hand use was quantitative, not qualitative. Specifically, one-sample t tests indicated significant population-level handedness for simple reaching, t(165) = 1.91, p < .10, Ω = 0.30, and the tube task, t(165) = 3.07, p < .01, Ω = 0.48, although not for the tool-use task. Thus, the chimpanzees are right-handed for multiple measures of hand use, but significantly more right-handed for gestures than for motor actions that are not communicative in function.
Longitudinal Analysis
For the two longitudinal analyses, sex and rearing history served as covariates. In the two analyses, HI scores for reaching and the tube task at age 3 to 4 served as predictor variables, and HI score for gestures in the present study was the dependent variable. The partial correlation coefficient between simple reaching and gesture was significant (partial r = .418, df = 48, p < .01), but the partial correlation coefficient between the tube task and gesture was not (partial r = −.016, df = 42, n.s.). Chimpanzees that were right-handed for reaching at 3 to 4 years of age were right-handed for gesturing as subadults.
Discussion
The evidence of population-level right-handedness for manual gestures is consistent with previous findings for noncommunicative actions in these two colonies of chimpanzees (Hopkins et al., 2003, 2005). Moreover, the evidence of increased right-hand use for gestures accompanied by vocalizations than for those not accompanied by vocalizations is consistent with previous findings in the Yerkes colony using different testing procedures (Hopkins & Cantero, 2003). Collectively, these findings reinforce the argument that the preferences in hand use are not due to observer bias, as suggested by some researchers (Palmer, 2002). The fact that discrete responses were obtained for the gestures in this study also rules out the possibility that the observed right-handedness is due to a lack of independence of data points (McGrew & Marchant, 1997). Last, we found no evidence that rearing differences had an effect on hand use; HI scores of mother-reared, nursery-reared, and wild-caught chimpanzees were comparable. This indicates that the evidence of population-level handedness is not due to differential rearing or learning experiences by the chimpanzees (see McGrew & Marchant, 1997).
The data presented here speak directly to several evolutionary and developmental models of hemispheric specialization in human and nonhuman primates. Specifically, the results of this study clearly show that tool use was likely not the basis for the emergence of right-handedness in humans, in light of the fact that this behavior failed to show population-level handedness. The results further indicate that neither language nor bipedalism was a necessary condition for the development of population-level handedness in primates. The findings of this study support the view of Corballis (2002), who suggested that left-hemisphere asymmetries for handedness, language, and speech in humans evolved from an extant lateralized system for manual gestures in the common ancestor to humans and chimpanzees. The finding of increased use of the right hand for gestures accompanied by vocalizations is also consistent with findings in human adults and babies (see Iverson & Thelen, 1999; Kimura, 1993).
The evidence that handedness for simple reaching at 3 to 4 years of age better predicts handedness for gestures than hand use for bimanual actions supports the theory that simple reaching becomes conventionalized into gesture when objects become distal and are no longer within the field of reach of the subject. It is important to emphasize that in no way do we believe that the gestures made by the apes in this and other studies are frustrated grasping attempts. Previous studies have shown that chimpanzees and orangutans do not gesture to foods outside their home cages when no human is present (see Call & Tomasello, 1994; Leavens et al., 1996). If the chimpanzees in these studies were simply attempting to reach for and grasp the food items, then their gestures would have been independent of whether or not a human or other social agent was present.
The association between laterality in reaching and gestural communication in chimpanzees may be explained by the mirror-neuron system (Rizzolatti & Arbib, 1998). Mirror neurons are perception-action cells that fire when monkeys execute grasping actions or perceive another monkey grasping an object. Mirror neurons have been identified primarily in area F5 in monkeys, which is the homologue to Broca's area in humans. Rizzolatti and Arbib proposed that the evolution of language was initiated by the existence of this simple mirror-neuron system, which eventually incorporated more sophisticated dimensions of motor actions, including manual gestures and imitation. Combined with this theoretical framework, the results reported here suggest that grasping and gesture may have a common neural basis and that gestures may develop out of the grasping system.
For gestures, the ratio of right- to left-handed chimpanzees is a little more than 3:1, which is lower than what is reported in human populations (Raymond & Pontier, 2004). The cause of this difference is unclear. Some researchers have suggested that mutations in the human genome account for the differences in hand-preference distributions between humans and apes (Corballis, 1997). Others have suggested that the evolution of culture and social norms may have facilitated and enhanced the expression of handedness in human populations relative to apes (Hopkins, 2004). Additional genomic research on chimpanzees and humans may shed important light on the independent or interactive roles of biological and social mechanisms in the development of handedness in primates, including humans.
In conclusion, captive chimpanzees show population-level right-handedness for gestures, and lateralization is more robust in this communicative behavior than in noncommunicative motor actions. On the basis of these findings, we believe that lateralization for language and speech in humans has its neurobiological origins in asymmetries associated with manual gestures in the common ancestor of humans and chimpanzees (see Hopkins & Fernandez-Carriba, 2002). The extent to which gestures are or are not lateralized in wild chimpanzees has not yet been studied but warrants investigation. In addition, functional brain-imaging studies are needed to evaluate what the neural substrates of gestural communication are in chimpanzees in relation to known neural circuitry underlying language and speech in humans. Such work would provide important information on similarities and differences that may account for the emergence of speech and language in modern humans.
Acknowledgments
This research was supported by National Institutes of Health Grants NS-36605, NS-42867, NS-29574, U42-RR-15090, and RR-00165 to the Yerkes National Primate Research Center or The University of Texas M.D. Anderson Cancer Center (UTMDACC). The Yerkes Center and the UTMDACC Department of Veterinary Sciences are fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
References
- Blake J, O'Rourke P, Borzellino G. Form and function in the development of pointing and reaching gestures. Infant Behavior and Development. 1994;17:195–203. [Google Scholar]
- Bonvillian JD, Richards HC, Dooley TT. Early sign language acquisition and the development of hand preferences in young children. Brain and Language. 1997;58:1–22. doi: 10.1006/brln.1997.1754. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) Journal of Comparative Psychology. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton, NJ: Princeton University Press; 2002. [Google Scholar]
- Corina DP, Poizner H, Bellugi U, Feinberg T, Dowd D, O'Grady-Batch IL. Dissociation between linguistic and non-linguistic gestural system: A case of compositionality. Brain and Language. 1993;43:414–447. doi: 10.1016/0093-934x(92)90110-z. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Sexual selection, timing and the descent of man: A theory of the genetic origins of language. Current Psychology of Cognition. 1998;17:1079–1114. [Google Scholar]
- Hamilton CR, Vermeire BA. Complementary hemispheric specialization in monkeys. Science. 1988;242:1694–1696. doi: 10.1126/science.3201258. [DOI] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric asymmetry: What's right and what's left. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995a;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Developmental Psychology. 1995b;31:619–625. [Google Scholar]
- Hopkins WD. Laterality in maternal cradling and infant positional biases: Implications for the development and evolution of hand preferences in non-human primates. International Journal of Primatology. 2004;25:1243–1265. doi: 10.1023/B:IJOP.0000043961.89133.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantero M. The influence of vocalizations on preferential hand use in gestural communication by chimpanzees. Developmental Science. 2003;6:55–61. [Google Scholar]
- Hopkins WD, Fernandez-Carriba S. Laterality in communicative behaviors in nonhuman primates: A critical analysis. In: Rogers L, Andrew R, editors. Comparative vertebrate lateralization. Oxford, England: Oxford University Press; 2002. pp. 445–479. [Google Scholar]
- Hopkins WD, Hook M, Braccini S, Schapiro SJ. Population-level right handedness for a coordinated bimanual task in chimpanzees (Pan troglodytes): Replication and extension in a second colony of apes. International Journal of Primatology. 2003;24:677–689. doi: 10.1023/A:1023752816951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Rabinowitz DM. Manual specialization and tool use in captive chimpanzees (Pan troglodytes): The effect of unimanual and bimanual strategies on hand preference. Laterality. 1997;2:267–277. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter A, Cantero M, Hopkins WD. Differential use of vocal and gestural communication in chimpanzees in response to the attentional status of a human audience. Journal of Comparative Psychology. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Thelen E. Hand, mouth and brain: The dynamic emergence of speech and gesture. Journal of Consciousness Studies. 1999;6:19–40. [Google Scholar]
- Kimura D. Neuromotor mechanisms in human communication. Oxford, England: Oxford University Press; 1993. [Google Scholar]
- Knecht S, Deppe M, Draeger B, Bobe L, Lohman H, Ringelstein EB, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees (Pan troglodytes): A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Thomas R. Referential communication by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2004;118:48–57. doi: 10.1037/0735-7036.118.1.48. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Animal Behaviour. 2004;67:467–476. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence with funnel plots. American Journal of Physical Anthropology. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech function. In: Dimond SJ, Blizard DA, editors. Evolution and lateralization in the brain. Vol. 299. New York: New York Academy of Sciences; 1977. pp. 355–369. [DOI] [PubMed] [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew JR. Comparative vertebrate lateralization. Cambridge, England: Cambridge University Press; 2002. [Google Scholar]
- Steklis HD, Raleigh MJ. Behavioral and neurobiological aspects of primate vocalization and facial expression. In: Steklis HD, Raleigh MJ, editors. Social communication and behavior: An interdisciplinary series. New York: Academic Press; 1979. pp. 257–282. [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: A trans-generational study. Primates. 1994;35:137–154. [Google Scholar]


