Abstract
Hand preferences for a coordinated bimanual task were assessed in a sample of 31 captive gorillas (Gorilla gorilla) and 19 captive orangutans (Pongo pygmaeus) and were compared with chimpanzee (Pan troglodytes) hand preferences in subjects that were matched on the basis of age, sex, and rearing history. The task required that the apes remove food from the inside edges of a symmetrical polyvinyl chloride pipe presented to them in their home cages. The results indicate significant species differences with chimpanzees showing population-level right-handedness and orangutans showing population-level left-handedness. The gorillas showed a nonsignificant trend toward right-handedness. The results are discussed in terms of possible ecological or biomechanical factors that may influence hand preferences in different ape species.
The evolution of handedness has been a topic of historical (Warren, 1980) and contemporary debate (Fagot & Vauclair, 1991; Hopkins, 1996, 1999; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Marchant & McGrew, 1991; McGrew & Marchant, 1997). The central issues surrounding evolutionary theories of handedness have focused on whether population-level right-handedness is a uniquely human adaptation and whether the mechanisms selecting for handedness are associated with the emergence of complex cognitive processes such as language or tool use (see Bradshaw & Rogers, 1993, for a review). Since the seminal review article on nonhuman primate handedness by MacNeilage et al. (1987), there have been a plethora of studies on the distribution of limb and hand preference in a variety of animal species, notably nonhuman primates. Although there continues to be considerable debate over whether population-level handedness is expressed in nonhuman primates, evidence has emerged for several measures suggesting population-level handedness in limb preferences in a variety of vertebrates (for reviews, see Bradshaw & Rogers, 1993; Hook-Costigan & Rogers, 1997; Rogers & Andrews, 2002; Ward & Hopkins, 1993).
One difficulty with the interpretation of findings on handedness between species has been the lack of common measures of hand use. The most commonly used measure of hand preference is simple reaching, and many primate species have been tested for handedness using this task (see Lehman, 1993). For simple reaching, some have reported increasing preferential use of the right hand for nonhuman primate species more closely related to humans than for species more distantly related (see Westergaard, Kuhn, & Suomi, 1998, for a review), whereas others have not reported such effects (see Hopkins & Morris, 1993; McGrew & Marchant, 1997). Direct comparative studies of simple reaching in great apes that have used the same measures and testing procedures have revealed mixed results with some authors reporting population-level right-handedness (Olson, Ellis, & Nadler, 1990) and others not (Heestand, 1986). However, simple reaching is not a particularly complex motor task and is not the most sensitive measure of hand preference in nonhuman primates. This is indicated by the fact that many subjects fail to exhibit a significant hand preference at the individual level for simple reaching (i.e., there are many subjects classified as nonpreferent or ambidextrous). The lack of consistent findings between species for simple reaching may reflect that situational factors, such as posture (Westergaard et al., 1998), food position (Welles, 1976), or grip morphology (Christel, 1994; Hopkins, Cantalupo, Wesley, Hostetter, & Pilcher, 2002; Tonooka & Matsuzawa, 1995), have mediating effects on the use of the right or left hand.
The lack of common measures of hand use for more complex tasks that are not subject to situational factors, such as coordinated bimanual tasks, has precluded direct comparison of handedness findings in nonhuman primates, and this limits the degree to which conclusions can be drawn on the evolution of handedness. For example, in a brief review of handedness findings in great apes, Hopkins and Pearson (2000) concluded that chimpanzees and gorillas exhibited population-level right-handedness whereas bonobos and orangutans did not (see also Rogers & Kaplan, 1996). One limitation of this interpretation is that orangutans and bonobos have been the least studied of the great apes, and the kinds of measures used to assess handedness in these species were very limited and quite different compared with those used with gorillas and chimpanzees. Thus, the conclusions offered by Hopkins and Pearson (2000) are constrained by these confounding variables when comparing species. What is clearly needed are comparative studies of handedness in great apes that use the same methods and testing procedures. This would allow for a direct assessment of the time course of the evolution of lateralized hand use in great apes as well as provide important information on the role of ecological and morphological factors on the expression of laterality.
One purpose of this study was to comparatively assess hand preferences for a coordinated bimanual task in a sample of gorillas and orangutans for comparison with previously published findings in chimpanzees. Hand preference data for coordinated bimanual tasks are virtually absent in the literature for orangutans and relatively unstudied in gorillas, with the exception of one study in 44 wild mountain gorillas (Byrne & Byrne, 1991) and one study in 10 captive lowland gorillas (Fagot & Vauclair, 1988). Clearly, additional data are needed in these two great ape species for comparison with the more commonly studied chimpanzee. Using a task designed to assess hand preferences for coordinated bimanual actions (referred to as the tube task), Hopkins and colleagues (Hopkins, 1995; Hopkins & Cantalupo, in press; Hopkins et al., 2001) have previously found that captive chimpanzees exhibit population-level right-handedness. The tube task has been shown to be sensitive to individual differences in hand use (i.e., the majority of subjects show significant hand preferences) and is stable as indicated by significant test–retest correlations, r(106) = .55, p < .01, in hand use that have exceeded 5 years of testing (see Hopkins et al., 2001). Therefore, the tube task appears to be a reliable, sensitive measure of hand use and a fair test for comparative assessment because all of the species can perform the task without any explicit training.
In addition to the aim of collecting additional hand preference data in great apes, we aimed to explore the significant differences among orangutans, gorillas, and chimpanzees in terms of their positional behavior and role differentiation of the hands associated with foraging (see Byrne, Corp, & Byrne, 2001; Tuttle, 1986). For example, mountain gorillas, and to a lesser extent lowland gorillas, feed while seated and use their hands in a coordinated manner (Byrne & Byrne, 1991; Byrne et al., 2001; Parnell, 2001; Remis, 1999). In contrast, orangutans, and to a lesser extent chimpanzees, forage in the trees with one hand while posturally supporting themselves with the opposite hand (Cant, 1992; Doran, 1993; Marchant & McGrew, 1996). The variation in positional behavior during feeding in great apes provides an excellent context to test, to some extent, the postural origin theory of handedness (MacNeilage et al., 1987). The postural origin theory proposes that arboreal species are more likely to exhibit left-hand preferences for manual actions because the right hand is used for postural support, in the form of vertical clinging, while the left is used for ballistic grasping. According to MacNeilage et al. (1987), for more terrestrial primates, the right hand has evolved the function of manipulation because it is released from its function of postural support for vertical clinging. If this theory is applied to the natural positional behavior of great apes, then orangutans should be more left-handed than either gorillas or chimpanzees because they are more arboreal in terms of habitat use (see Tuttle, 1986). Because gorillas are considered to be the most terrestrial great ape, the postural origin theory would propose that they would be more right-handed. Chimpanzees should fall somewhere in between orangutans and gorillas because they occupy both arboreal and terrestrial niches.
Method
Subjects
The primary subjects were 31 lowland gorillas (Gorilla gorilla) and 19 orangutans (Pongo pygmaeus). Of the 31 gorillas, there were 13 males and 18 females. For the orangutans, there were 8 females and 11 males. The sex, age, and rearing history of each subject can be seen in Table 1. Twenty of the gorillas were housed at Zoo Atlanta, Atlanta, Georgia, and the remaining 11 gorillas were housed at the Lincoln Park Zoo, Chicago, Illinois. Nine of the orangutans were housed at the Yerkes National Primate Research Center (YNPRC) at Emory University, Atlanta, Georgia, and the remaining 10 orangutans were housed at Zoo Atlanta.
Table 1.
Descriptive Data for the Gorilla and Orangutan Samples
| Subject | Sex | Rearing | Age
(years) |
#L | #R | z | Handedness |
|---|---|---|---|---|---|---|---|
| Gorillas | |||||||
| Jasiri | M | Captive, MR, ZA | 3 | 8 | 46 | 5.17 | R |
| Kidogo | M | Captive, MR, ZA | 3 | 55 | 30 | −2.71 | L |
| Bengati | M | Captive, MR, LP | 4 | 0 | 13 | 3.60 | R |
| Charlie | M | Captive, MR, ZA | 5 | 76 | 83 | 0.55 | A |
| Jelani | M | Captive, MR, LP | 5 | 81 | 31 | −4.72 | L |
| Kudzoo | M | Captive, MR, ZA | 7 | 150 | 1 | −12.13 | L |
| Stadi | M | Captive, MR, ZA | 10 | 39 | 64 | 2.46 | R |
| Taz | M | Captive, MR, ZA | 12 | 101 | 31 | −6.09 | L |
| Kekla | M | Captive, MR, ZA | 12 | 9 | 81 | −7.58 | L |
| Carlos | M | Wild caught, ZA | 30 | 1 | 112 | 10.44 | R |
| Frank | M | Wild caught, LP | 38 | 25 | 50 | 2.88 | R |
| Ivan | M | Wild caught, ZA | 39 | 33 | 0 | −5.74 | L |
| Ozoum | M | Wild caught, ZA | 40 | 44 | 16 | −3.61 | L |
| Sukari | F | Captive, MR, ZA | 3 | 9 | 40 | 4.43 | R |
| Olympia | F | Captive, MR, ZA | 5 | 37 | 83 | 4.20 | R |
| Mumbali | F | Captive, HR, LP | 5 | 74 | 5 | −7.76 | L |
| Madini | F | Captive, MR, LP | 6 | 56 | 26 | −3.31 | L |
| Rollie | F | Captive, HR, LP | 6 | 96 | 0 | −9.80 | L |
| Kashata | F | Captive, MR, ZA | 8 | 33 | 117 | 6.86 | R |
| Tabibu | F | Captive, HR, LP | 10 | 63 | 38 | −2.49 | L |
| Mia Moja | F | Captive, MR, ZA | 12 | 113 | 113 | −0.07 | A |
| Bahati | F | Captive, MR, LP | 12 | 25 | 23 | −0.29 | A |
| Makari | F | Captive, HR, LP | 15 | 48 | 41 | −0.74 | A |
| Kuchi | F | Captive, MR, ZA | 17 | 73 | 50 | −2.07 | L |
| Machi | F | Captive, MR, ZA | 25 | 72 | 46 | −2.39 | L |
| Debbie | F | Wild caught, LP | 36 | 52 | 71 | 1.71 | R |
| Paki | F | Wild caught, ZA | 38 | 8 | 91 | 8.34 | R |
| Choomba | F | Wild caught, ZA | 38 | 15 | 76 | 6.39 | R |
| Banga | F | Wild caught, ZA | 37 | 42 | 80 | 5.47 | R |
| Shamba | F | Wild caught, ZA | 42 | 0 | 43 | 6.56 | R |
| Helen | F | Wild caught, LP | 44 | 42 | 80 | 3.44 | R |
|
| |||||||
| Orangutans | |||||||
| Lokan | M | Captive, MR, YP | 4 | 23 | 0 | −4.79 | L |
| Mentubar | M | Captive, HR, YP | 6 | 91 | 47 | −3.74 | L |
| Sulango | M | Captive, MR, ZA | 9 | 94 | 10 | −8.24 | L |
| Solok | M | Captive, HR, YP | 10 | 20 | 38 | 2.36 | R |
| Jantan | M | Captive, MR, ZA | 13 | 50 | 11 | −4.99 | L |
| Minyak | M | Captive, HR, YP | 13 | 113 | 49 | −5.02 | L |
| Molek | M | Captive, MR, YP | 16 | 113 | 151 | 2.33 | R |
| Gelar | M | Captive, MR, YP | 17 | 88 | 60 | −2.30 | L |
| Chantak | M | Captive, HR, ZA | 25 | 128 | 0 | −11.31 | L |
| Loklok | M | Captive, MR, YP | 25 | 30 | 329 | 15.78 | R |
| Allen | M | Captive, MR, ZA | 31 | 35 | 17 | −2.50 | L |
| Miri | F | Captive, HR, ZA | 10 | 94 | 44 | −4.26 | L |
| Penari | F | Captive, MR, YP | 15 | 100 | 43 | −4.76 | L |
| Madu | F | Captive, HR, ZA | 19 | 120 | 47 | −5.64 | L |
| Daisy | F | Captive, HR, ZA | 21 | 49 | 25 | −2.79 | L |
| Hati | F | Captive, HR, ZA | 24 | 43 | 24 | −2.32 | L |
| Biji | F | Captive, MR, ZA | 32 | 114 | 26 | −7.44 | L |
| Tupa | F | Wild caught, YP | 37 | 183 | 94 | −5.35 | L |
| Sibu | F | Wild caught, ZA | 44 | 20 | 116 | 8.23 | R |
Note. Mother-reared subjects were those reared by their biological, conspecific mother for the duration of their development. Human-reared subjects were those that were brought to a nursery setting and raised by humans. Most of the subjects arrived in the nursery setting within 3 days of birth. The longest any subject stayed with its biological mother prior to human intervention was approximately 4 months. Subjects with z scores greater than 1.64 or less than −1.64 were classified as right- and left-handed, respectively. #L = number of times the subject used its left hand in the tube task; #R = number of times the subject used its right hand in the tube task; M = male; F = female; Captive = captive born; Wild caught = wild caught or unknown origin, presumably wild caught; MR = mother reared; HR = human reared; ZA = Zoo Atlanta; LP = Lincoln Park Zoo; YP = Yerkes National Primate Research Center; R = right-handed; L = left-handed; A = ambiguously handed.
The rearing histories of the subjects were classified two ways. First, subjects were classified as being either wild caught (n = 12) or captive born (n = 38). Second, within the captive-born cohort, subjects were classified as being either mother reared (n = 26) or human reared (n = 12). Mother-reared subjects were those that were raised by their biological, conspecific mother. Human-reared subjects were those that were removed from their biological mother prior to adolescence and raised by humans.
To facilitate comparison between the findings in gorillas and orangutans with findings previously reported in chimpanzees, we used a matched-design approach in selecting chimpanzee subjects for comparison with the other great apes. Over 200 chimpanzees from the YNPRC have been tested on the tube task; thus, finding matches for the gorilla and orangutan subjects was not difficult. Specifically, for gorillas and orangutans, a chimpanzee from the YNPRC that matched each subject on the basis of sex, rearing history, and age was identified and included for subsequent comparison. Forty-four of the subjects were unique in that they matched 1 and only 1 orangutan or gorilla subject. Six subjects were matches for both 1 orangutan and 1 gorilla. Thus, for species comparisons that were not matched-design approaches, these subjects were presented only once in the chimpanzee group (see below).
Procedure
Hand preference was assessed using the tube task, a task designed to elicit coordinated bimanual actions (see Figure 1). The procedure for this task has been described in detail elsewhere (Hopkins et al., 2001). Briefly, peanut butter is smeared on the inside edges of polyvinyl chloride (PVC) tubes approximately 15.0 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths but rather must use their fingers to remove the substrate. For the 10 gorilla subjects from the Lincoln Park Zoo, peanut butter was not used as the food incentive, but rather a banana-flavored paste that was part of their diet was used as the substrate. Like the peanut butter, the paste had a very strong adhesive quality and easily stuck to the inside of the PVC pipe. The PVC tubes were handed to the subjects in their home cages, and a focal sampling technique was used to collect individual data from each subject. The hand and finger used to extract the peanut butter was recorded as either right or left by the experimenter. Each time the subjects reached into the tube with their finger, extracted peanut butter, and brought it to their mouth, the hand used was recorded as left or right.
Figure 1.

Orangutan engaged in the bimanual tube task.
Data were collected until the subjects dropped the tube, stopped extracting peanut butter for a period of 10 s, or returned the PVC pipe to the experimenter. The 10-s limit did not include instances in which the subjects were locomoting with the PVC pipe. Rather, this time limit was specific to instances in which they had the PVC pipe in hand, were stationary in positional behavior, and were not attempting to feed (usually because of the absence of any remaining peanut butter).
All but 4 subjects were tested on four occasions, and specific attention was paid to the hand used to take the tube by the subject. Specifically, for two tests, the subject was required to take the tube with its left hand. For the remaining two tests, the subject was required to take the tube with its right hand. The order of presentation of the tubes to either the left or right hand was randomized across subjects. For the remaining 4 subjects (all young apes whose mothers would take their tubes from them), two test sessions were obtained with the hand taking the tube counterbalanced in each case. Most of the subjects received two test sessions per day and were tested on 2 consecutive days. A 5- to 10-min interval separated each test session, during which time the PVC pipes were retrieved from the subjects, cleaned, and refilled with peanut butter. For a smaller sample of subjects, all four test sessions were conducted in 1 day. Most of these subjects were housed in circumstances or involved in other research projects that limited the time available to access them for data collection.
Data Analysis
Hand preferences were characterized several different ways in this study. First, a handedness index (HI) was calculated for each of the four test sessions and for all of the test sessions combined (ΣHI) by subtracting the number of left bouts from the number of right bouts and dividing by the total number of responses. The handedness indices varied on a continuum from −1.0 to 1.0 with positive values reflecting right-hand preferences and negative values reflecting left-hand preferences. The absolute value of the handedness index reflects the magnitude of asymmetry in hand use. Larger values reflect stronger preference in hand use. Second, a mean handedness index (MHI) was derived by averaging the four handedness index values across the four test sessions. This was done to rule out the possibility that the ΣHI score could be skewed on the basis of different frequencies in right- and left-hand use within a given test session.
Third, on the basis of the total left- and right-hand frequencies, z scores were used to evaluate whether the hand preferences of individual subjects deviated significantly from chance. This is the procedure most frequently used in the nonhuman primate literature (see Hopkins, 1999). Subjects with z scores greater than 1.64 or less than −1.64 were classified as right- and left-handed, respectively. These critical values were adopted because they were the same as those used in previous studies (Hopkins, 1995). All other subjects were classified as ambiguously handed. For all inferential analyses, alpha was set at p < .05.
Results
Consistency in Hand Use
Handedness indices were calculated for each of the four tests, and these values were correlated to evaluate consistency in hand use across tests. This analysis was restricted to the orangutan and gorilla data collected in this study because the chimpanzee findings have been previously reported (Hopkins et al., 2001). The correlation coefficients between each test can be seen in Table 2. As was found in previous studies in chimpanzees (Hopkins et al., 2001), there were significantly positive correlation coefficients across tests indicating that the preferences were stable over time. This indicates that hand preference was stable and consistent across test sessions at the individual level. The MHI and ΣHI scores were also significantly positively correlated, r(48) = .98, p < .01, indicating that the two ways in which overall hand use was characterized were consistent across subjects. Because the correlation was very high, subsequent analyses focused only on the ΣHI score to minimize the number of statistical tests performed and vulnerability to Type I error.
Table 2.
Correlation Coefficients for the Four Tests of Hand Use
Note. df = 48.
p < .01.
Species Comparison
In the initial analysis, an analysis of variance (ANOVA) was performed with species serving as the independent variable. The ΣHI was the dependent variable. A significant main effect for species was found, F(2, 94) = 5.09, p < .02. Tukey's post hoc analysis indicated that the mean ΣHI scores for chimpanzees and gorillas were significantly higher than the score for the orangutans. There was no significant difference between the HI scores of gorillas and chimpanzees. The mean ΣHI score for each species can be seen in Figure 2.
Figure 2.
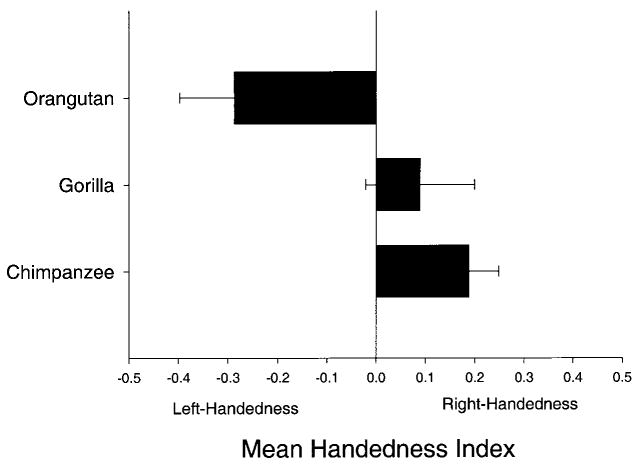
Mean overall handedness index scores (± SE) for chimpanzees, gorillas, and orangutans.
Given that there were differences in variables such as age and rearing history among subjects in each of the three species, two matched-design correlated t tests were performed to further elucidate any difference in handedness between species. For the orangutan and gorilla samples, a chimpanzee subject that matched each subject on the basis of sex, rearing history, and age was identified from the sample of chimpanzees housed at the YNPRC. The ΣHI scores from the chimpanzees were then compared with the scores of the gorillas and orangutans using paired t tests. The chimpanzee ΣHI scores were significantly higher than the orangutan scores (chimpanzee, M = .307, vs. orangutan, M = −.287), t(18) = 4.03, p < .01, but not the gorilla scores (chimpanzee, M = .141, vs. gorilla, M = .089), t(30) = 0.47, ns. Overall, these results are largely consistent with the between-group subject analyses, both of which indicate significantly higher handedness index scores for chimpanzees and gorillas compared with orangutans.
On the basis of the individual z scores, the distribution of left-handed, ambiguously handed, and right-handed subjects varied significantly between species. For the orangutans, there were 15 left-handed, 4 right-handed, and 0 ambiguously handed subjects. Among the gorillas, there were 15 right-handed, 12 left-handed, and 4 ambiguously handed subjects. Lastly, in the chimpanzee sample, there were 29 right-handed, 10 left-handed, and 7 ambiguously handed subjects. Comparison of the distribution of hand preference as a function of the species revealed a significant interaction, χ2(4, N = 92) = 24.77, p < .01. Subsequent comparisons in the number of left- and right-handed subjects within each species indicated that the orangutans were significantly left-handed (z = −2.53, p < .05) and the chimpanzees were significantly right-handed (z = 3.04, p < .01). The gorillas did not show population-level handedness (z = 0.58, ns).
Species differences in strength of hand preference were compared using an ANOVA with species serving as the independent variable. The absolute value of the ΣHI score was the dependent variable. No significant main effects or interactions were found. The mean absolute values in hand preference for gorillas, chimpanzees, and orangutans were .535, .417, and .488, respectively. This indicates that the degree of asymmetry elicited by the tube task at the individual level was comparable between species.
Potential Effects of Rearing History and Sex
In previous studies in chimpanzees using the tube task, rearing effects and sex differences have not been found, but these are relevant subject variables to consider (see Hopkins, 1995; Hopkins et al., 2001). In the gorilla sample in this study, there were 10 wild-caught and 21 captive-born subjects. For the orangutans, there were 2 wild-born (both females) and 17 captive-born subjects. Given the disparity in sample sizes for rearing history in the orangutans, no statistical analyses could be performed. For the gorillas, an analysis of covariance was performed with rearing history serving as the between-group independent variable and the ΣHI serving as the dependent variable. Age served as the covariate. No significant effect of rearing was found, despite a relatively large difference in ΣHI scores between captive-born (M = −.159) and wild-caught (M = .610) subjects.
In terms of sex differences, independent samples t tests in the gorilla and orangutan samples failed to reveal any significant differences between male and female apes. For the gorillas, the mean handedness scores for male and female subjects were .029 and .133, respectively. In the orangutan samples, the mean handedness scores for male and female apes were −.301 and −.256, respectively.
Discussion
The results of this study indicate that there is phylogenetic discontinuity among great apes in hand preferences for a coordinated bimanual task. Chimpanzees and gorillas were significantly more right-handed than orangutans. In terms of the species differences in directional biases of hand preference, there are several potential explanations for these findings. First, it may be that right-handedness is a recently evolved trait and only emerged within the past 5–6 million years in Pongid evolution. This would explain the prevalence of right-handedness in the genus Pan and not in Gorilla or Pongo. One problem with this conclusion is that population-level right-handedness has been found for the tube task in Old (Westergaard & Suomi, 1996; but see Westergaard, Champoux, & Suomi, 1997) and New World monkeys (Spinozzi, Castornina, & Truppa, 1998; but see Westergaard & Suomi, 1996). Thus, population-level handedness may have evolved on more than one occasion in primate evolution. A second limitation of this conclusion is that Pongo showed population-level left-handedness rather than a lack of asymmetry. In other words, the Pongo data should have looked more like the Gorilla findings if population-level right-handedness evolved from a symmetrically based system.
A second explanation for the species differences in hand preference is that ecological and biomechanical factors have selected for the differential expression of handedness in these three species. This explanation is partly consistent with the postural origins theory of handedness proposed by MacNeilage et al. (1987). Specifically, the orangutan data are consistent with the postural origins theory in that the most arboreal ape showed the highest degree of left-handedness; however, the gorilla data did not strongly support the postural origins theory because they were predicted to have the strongest degree of right-handedness and, in fact, failed to reveal population-level right-handedness. However, it is important to remember that the sample sizes are relatively small in this study, and this is problematic because population-level handedness is not as robust of a phenomenon as is reported in human populations. With the collection of additional data in gorillas for the tube task, population-level right-handedness will likely emerge because the mean handedness score will remain relatively constant whereas the error term will decrease with increasing sample size (see Hopkins, 1999, for a discussion).
A third explanation may lie in the potential role that early mother–infant interactions play in the development of handedness in different species (see Damerose & Vauclair, 2002, for a review). Hopkins, Bard, Jones, and Bales (1993) have proposed that cradling bias by the mother or nipple preferences by the infant during the early days of life may differentially stimulate the development of hand use in the developing offspring. Hopkins et al. (1993) showed that female chimpanzees that cradled their infants on the left had offspring that developed right-hand preferences. In contrast, female chimpanzees that cradled their infants on the right had offspring that developed left-hand preferences. Manning and Chamberlain (1990) and Manning, Heaton, and Chamberlain (1994) have reported that great apes show a left-side cradling bias, although in their sample the effects were most prevalent for chimpanzee and gorilla females compared with orangutans. Specifically, in chimpanzees, 16 females showed a left-side bias and 4 showed a right-side bias. For gorillas, 13 showed a left-side bias and 2 showed a right-side bias. In orangutans, 4 showed a left-side bias and 4 showed a right-side bias. More recently, Rogers and Kaplan (1996) reported maternal cradling biases in 4 wild orangutan females, with 3 displaying a right-side bias and 1 exhibiting a left-side bias. Assuming that the cradling bias of the female has an inverse relationship with the development of the infant's hand preference, the comparative data on cradling bias in great apes are consistent with a greater expression of left-handedness in the orangutans compared with the gorillas and chimpanzees.
Lastly, it is possible that the orangutans differ from the gorillas and chimpanzees in terms of their assignment of dominant and subordinate roles of the hands in the execution of bimanual actions. Dominant hand use for the tube task is operationally defined as the hand that is used for removing the peanut butter from the PVC pipe. Of course, this is an arbitrary definition assigned by the experimenters, and maybe in the case of the orangutans, they use their dominant hand for holding the PVC pipe rather than for the removal of the peanut butter. Whether this is true cannot be easily discerned from purely behavioral studies, but how behavioral asymmetries correlate with neuroanatomical or neurofunctional asymmetries may be a way to test this hypothesis. Assuming that the hand preferences manifest by the tube task correlate with some neuroanatomical regions, it would be predicted that the orangutan hand preference would positively correlate with a particular brain region whereas the opposite correlation would be found for gorillas and chimpanzees. In contrast, if the behavioral differences in hand preference found for the orangutans reflect a difference in neural organization, then it can be predicted that the pattern of brain asymmetry would be opposite for orangutans contrasted with gorillas and chimpanzees. The existing comparative data on neuroanatomical asymmetries in great apes do not suggest that orangutans are overtly different from other apes (LeMay, 1985), but some recent studies have indicated that the primary motor cortex of orangutans is quite different than chimpanzees and gorillas at the morphological level (Hopkins & Pilcher, 2001; Semendeferi, Damasio, Frank, & Van Hoesen, 1997). With the recent application of neural imaging techniques in nonhuman primates, including apes, this hypothesis seems testable in the not too distant future.
Neither rearing history (at least in the gorillas) nor sex had a significant influence on hand preference. Given the limited sample sizes, these results should be viewed as tentative and warrant further investigation. Notwithstanding, it should be emphasized that differences in the rearing histories of the animals cannot explain the reported differences between species in hand preference because the analyses were conducted on subjects that were matched on the basis of rearing history, sex, and age. Thus, the t tests comparing the chimpanzees with both the gorillas and orangutans are the most compelling results, in terms of species differences, because subject variables were controlled for in the analysis (see the Results section).
In conclusion, our findings indicate differences in directional biases of hand use for coordinated bimanual actions in great apes. Orangutans showed a significant left-hand bias, gorillas showed a trend toward right-handedness, and chimpanzees exhibited a population-level right-hand bias. The tube task proved to be a sensitive measure of hand preference in all three ape species, in that nearly all of the subjects showed a significant hand preference. Thus, it does not appear that the findings can be explained by differences in the sensitivity of the tube task for measuring hand use. Rather, the differences in expression of hand use for the tube task in great apes may reflect presently unknown ecological or biomechanical factors. Further studies in larger samples of great apes using a larger array of behavioral measures will contribute substantially to researchers' understanding of the evolution of handedness in primates, including humans.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RR-00165, NS-36605, NS-42867, and HD-38051. We are immensely grateful for the helpful assistance of the care staff at Zoo Atlanta and Lincoln Park Zoo in the management of the apes that enabled data collection.
Contributor Information
William D. Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, Atlanta, Georgia, and Department of Psychology, Berry College
Tara S. Stoinski, Zoo Atlanta, Atlanta, Georgia
Kristen E. Lukas, Lincoln Park Zoo, Chicago
Stephen R. Ross, Lincoln Park Zoo, Chicago
Michael J. Wesley, Division of Psychobiology, Yerkes National Primate Research Center
References
- Bradshaw J, Rogers LJ. The evolution of lateral asymmetries, language, tool use and intellect. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Corp N, Byrne JM. Manual dexterity in the gorilla: Bimanual and digit role differentiation in a natural task. Animal Cognition. 2001;4:347–361. doi: 10.1007/s100710100083. [DOI] [PubMed] [Google Scholar]
- Cant JGH. Positional behavior and body size of arboreal primates: A theoretical framework for field studies and an illustration of its application. American Journal of Physical Anthropology. 1992;88:273–283. doi: 10.1002/ajpa.1330880302. [DOI] [PubMed] [Google Scholar]
- Christel MI. Catarrhine primates grasping small objects: Techniques and hand preferences. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current primatology: Behavioral neuroscience, physiology and reproduction. Vol. 4. Strasbourg, France: Universite Louis Pasteur; 1994. pp. 37–49. [Google Scholar]
- Damerose E, Vauclair J. Posture and laterality in human and non-human primates: Asymmetries in maternal handling and the infants' early motor asymmetries. In: Rogers LJ, Andrew RJ, editors. Comparative vertebrate lateralization. Oxford, England: Oxford University Press; 2002. pp. 306–362. [Google Scholar]
- Doran DM. Sex differences in adult chimpanzee positional behavior. The influence of body size on locomotion and posture. American Journal of Physical Anthropology. 1993;91:99–115. doi: 10.1002/ajpa.1330910107. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Handedness and bimanual coordination in the lowland gorilla. Brain, Behavior and Evolution. 1988;12:89–95. doi: 10.1159/000116536. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Heestand J. Unpublished doctoral dissertation. University of Washington; Seattle: 1986. Behavioral lateralization in four species of ape. [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 55 years since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preference of chimpanzees (Pan troglodytes)? An empirical study and reply to Palmer. American Journal of Physical Anthropology. 2002 doi: 10.1002/ajpa.10170. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of a left hemisphere advantage in motor skill. Journal of Experimental Psychology: General. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Fernandez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. Journal of Comparative Psychology. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. A review of findings. International Journal of Primatology. 1993;14:1–25. [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area using magnetic resonance imaging: The left hemisphere is larger in great apes. Behavioral Neuroscience. 2001;115:1159–1164. [PMC free article] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. pp. 107–124. [Google Scholar]
- LeMay M. Asymmetries of the brains and skulls of nonhuman primates. In: Glick SD, editor. Cerebral lateralization in nonhuman species. New York: Academic Press; 1985. pp. 223–245. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Manning JT, Chamberlain AT. The left-sided cradling preference in great apes. Animal Behaviour. 1990;39:1224–1227. [Google Scholar]
- Manning JT, Heaton R, Chamberlain AT. Left-sided cradling: Similarities and differences between apes and humans. Journal of Human Evolution. 1994;26:77–83. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- Olson DA, Ellis JE, Nadler RD. Hand preferences in captive gorillas, orangutans, and gibbons. American Journal of Primatology. 1990;20:83–94. doi: 10.1002/ajp.1350200203. [DOI] [PubMed] [Google Scholar]
- Parnell RJ. Hand preference for food processing in wild western lowland gorillas (Gorilla gorilla gorilla) Journal of Comparative Psychology. 2001;115:365–375. [PubMed] [Google Scholar]
- Remis MJ. Tree structure and sex differences in arboreality among western lowland gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. Primates. 1999;40:383–396. [Google Scholar]
- Rogers LJ, Andrews RJ. Comparative vertebrate lateralization. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans. Animal Behaviour. 1996;51:13–25. [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: A volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. Journal of Human Evolution. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences in unimanual and coordinated-bimanual tasks by tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. International Journal of Primatology. 1995;16:17–34. [Google Scholar]
- Tuttle RH. Apes of the world. Their social behavior, communication, mentality and ecology. Park Ridge, NJ: Noyes; 1986. [DOI] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Welles J. A comparative study of manual prehension in Anthropoids. Saugetierlaundliche Mitteilungen. 1976;24:24–37. [Google Scholar]
- Westergaard GC, Champoux M, Suomi SJ. Hand preference in infant rhesus macaques (Macaca mulatta) Child Development. 1997;68:387–393. doi: 10.1111/j.1467-8624.1997.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Bipedal posture and hand preference in humans and other primates. Journal of Comparative Psychology. 1998;112:56–63. doi: 10.1037/0735-7036.112.1.55. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]


