Abstract
Previously, it has been shown that the polysaccharide chitosan inhibits the growth of gram-positive bacteria. In this study, chitosan malate was evaluated in broth and thin-film cultures for its effect on the growth and exotoxin production of toxic shock syndrome (TSS)-inducing Staphylococcus aureus (five strains, three producing TSS toxin 1 and one each producing enterotoxin B or C) and group A streptococci (three strains producing streptococcal pyrogenic exotoxin A). Also, the compound was evaluated in a rabbit subcutaneous Wiffle ball model for its ability to prevent S. aureus and group A streptococcal induction of TSS. Finally, chitosan malate was evaluated for its ability to prevent TSS and necrotizing fasciitis in rabbits after subcutaneous inoculation with microbes. Chitosan malate inhibited both bacterial growth and, at sub-growth-inhibitory concentrations, the production of exotoxins, in both broth and thin-film cultures. Rabbits treated with chitosan malate in implanted Wiffle balls were protected from prior challenge with TSS-inducing S. aureus compared to animals not receiving chitosan malate (P < 0.001) and group A streptococci (P < 0.005). Chitosan malate protected rabbits from the development of streptococcal TSS with necrotizing fasciitis (P < 0.01). The data suggest that use of this growth- and toxin-inhibitory compound may be able to reduce the severity of S. aureus and group A streptococcal mucous membrane and trauma-associated skin infections.
Staphylococcus aureus and group A streptococci induce a variety of illnesses as a result of their abilities to initiate mucous membrane infections and cause postsurgical or other trauma-associated infections, subsequent to having breaks in the skin (14, 17, 33). Two of these illnesses are staphylococcal and streptococcal toxic shock syndrome (TSS), which is associated with superantigen (SAg) exotoxins made primarily locally (S. aureus) (17) or both locally and systemically (group A streptococci) (33), but usually having systemic effects (17, 33). The SAg exotoxins causing or associated with TSS include TSS toxin-1 (TSST-1), the staphylococcal enterotoxins (SEs), and the streptococcal scarlet fever toxins (referred to as streptococcal pyrogenic exotoxins or SPEs) (17, 33). The toxins capable of causing TSS belong to a large family of pyrogenic toxin SAgs, based on their shared biological activities and structures, despite having variable primary amino acid sequence similarity (17, 33). The biological properties include pyrogenicity, enhancement of lethal endotoxin shock, and ability to stimulate T-lymphocyte proliferation dependent on the composition of the variable part of the β chain of the T-cell receptor (superantigenicity) (17, 33). The abilities of the toxins to cause TSS result from the massive release of cytokines from both macrophages and T lymphocytes (12, 15).
In addition to causing serious TSS-related illnesses, recent strains of both S. aureus and group A streptococci are associated with development of other significant skin infections that may lead to large abscesses, necrotizing fasciitis, and sepsis without TSS. Community-associated methicillin-resistant S. aureus (MRSA) strains have been associated with large numbers of serious skin abscesses (5, 9-11, 19, 33, 36). Lung infections by related strains have been associated with necrotizing pneumonia and TSS symptoms (5, 6, 8-10, 13). Through breaks in the skin, whether from cuts, pressure sores, or other trauma, group A streptococcal strains may lead to sepsis and necrotizing fasciitis with or without TSS (33, 36).
Many of the SAgs, including TSST-1 and SEs B and C, are expressed under partial control of the global regulatory DNA elements, which include two-component systems such as accessory gene regulator (AgrA)-AgrC (20) and staphylococcal respiratory response (Srr)A-SrrB (40) in S. aureus. These two-component systems are part of quorum-sensing mechanisms or global regulators influenced by oxygen, CO2, pH, and glucose (20, 21). Differential regulation of exotoxin production dependent on the environmental conditions surrounding the microbe suggests that it may be possible to identify agents that downregulate exotoxin production in the host, even if the microbe cannot be effectively killed. This is particularly important in light of recent increases in antimicrobial resistance of organisms such as S. aureus. In previous studies it has been shown that production of TSST-1 and other gram-positive exotoxins can be inhibited by the antibiotic clindamycin at sub-growth-inhibitory antibiotic concentrations (32, 34, 35). Further, it has been shown that glycerol monolaurate, a signal transduction inhibitor proposed for use on mucosal and skin surfaces, significantly inhibits production of a variety of exotoxins made by staphylococci and streptococci, including SAgs, and B. anthracis at the level of transcription (22, 31, 39).
In the present study, chitosan malate has been evaluated for its ability to inhibit S. aureus and group A streptococcal growth and the production of SAgs in vitro and was evaluated in vivo in rabbit models of TSS and necrotizing fasciitis for its ability to alter the induction of illness. Chitosan is a polysaccharide derived from the chitin of shellfish that has been used on humans in bandages, primarily to stem bleeding, and is well known to have the ability to absorb toxic chemicals from aqueous environments and exhibit antimicrobial properties against both gram-positive and gram-negative pathogens (3, 23, 37). However, studies have not evaluated chitosan for the ability to interfere with exotoxin production independent of effects on bacterial growth. The data in the present study show that chitosan malate inhibits bacterial growth, as previously reported, but to an even greater extent inhibits exotoxin production by staphylococci and streptococci. In vivo, chitosan malate is not grossly toxic to rabbits but is highly effective in reducing the ability of S. aureus and group A streptococci to cause serious illnesses.
MATERIALS AND METHODS
Bacteria.
The S. aureus strains used in these studies include MN8 (TSST-1+ methicillin-sensitive S. aureus; Tri-State TSS Study isolate representative of 75% of menstrual TSS strains), 587 (TSST-1+ methicillin-sensitive S. aureus; CDC menstrual TSS isolate), MNPa (TSST-1+ MRSA menstrual TSS isolate), MN Ho (SEB+ MRSA nonmenstrual TSS isolate), and MN Don (SEC1+ MRSA nonmenstrual TSS isolate). These organisms are maintained in the laboratory in the lyophilized state and are of low passage. Group A streptococcal strains used included strain 594, T253cured(T12), and a recent M3 streptococcal TSS isolate (MNBi). The strains are maintained in the laboratory in the lyophilized state and are producers of SPE A.
In vitro broth cultures.
Variable amounts of chitosan malate (generously provided by 3M Company, St. Paul, MN) were solubilized in 25 ml of growth medium (beef heart dialysate in 125-ml Erlenmeyer flasks) (0 to 7.0 mg/ml, final chitosan malate concentrations in broths) and tested for their ability to alter bacterial growth by plate counting and production of SAgs by enzyme-linked immunosorbent assay (ELISA) for staphylococcal SAgs and quantitative Western immunoblotting for SPE A (1, 26). In these experiments, the staphylococcal cultures were incubated with aeration (200 rpm, gyratory shaker; New Brunswick Scientific Company, New Brunswick, NJ) at 37°C in a standard incubator. Group A streptococcal strains were cultured at 37°C in the presence of 7% CO2 with stirring just sufficient to maintain the organisms in suspension. In some cultures the production of delta hemolysin was assessed by competition ELISA as a measure of agr activity and the possible effect of chitosan malate on signal transduction. Delta-hemolysin was synthesized at the University of Minnesota Microchemical facility for use in these studies, verified as having the correct composition and biological activity, and used to hyperimmunize rabbits; the immunoglobulin G fraction in the rabbit serum was collected by 33% ammonium sulfate precipitation. Chitosan malate (final concentrations of 0 to 3.5 mg/ml) did not interfere with ELISA and Western immunoblot procedures for the detection of known concentrations of SAgs and delta-hemolysin.
In vitro thin-film cultures.
Variable amounts (0 to 7.0 mg/ml, final concentration) of chitosan malate were added to thin-film cultures approximately 3 mm in depth to allow sufficient aeration for production of staphylococcal exotoxins as described by Yarwood and Schlievert (41). Briefly, this consisted of placing thin films of bacteria, both S. aureus and group A streptococci, with or without chitosan malate, in 1-ml volumes on 4-cm-by-4-cm squares of polyester mesh in petri dishes, followed by stationary incubation at 37°C in the presence of 7% CO2. Samples were removed at selected times for CFU determination and SAg quantification.
In vivo rabbit tests.
All in vivo experiments were performed in accordance with requirements established by the University of Minnesota IACUC. Past experience (more than 20 years) has established that when rabbits fail to exhibit escape behavior and fail to right themselves, they uniformly succumb to SAgs. Thus, according to IACUC guidelines, these represent the criteria for premature euthanasia of animals during experimentation; lethality in these studies included both animals that succumbed and a small number of animals that were prematurely euthanized.
Chitosan malate alone (7.0 mg/kg administered as 7 mg/ml) was tested for its capacity to induce fever (28) and cause lethality in Dutch belted rabbits when given intravenously in phosphate-buffered saline (PBS; 0.005 M NaPO4 [pH 7.2], 0.15 M NaCl). Animals were monitored for 4 h for fever development with use of rectal thermometers and 7 days for lethality and other obvious signs of toxicity (diarrhea, labored breathing, and ruffled fur on the face). At the completion of experimentation, the animals’ organs were examined for grossly observable abnormalities.
Wiffle golf balls were individually implanted subcutaneously in the flanks of Dutch belted rabbits (either sex, 2 to 3 kg) (27). The animals were allowed to heal for 8 weeks, during which time capsules formed around the Wiffle balls. At that time, the Wiffle balls contained 30 ml of sterile serous fluid, 6 ml of which was removed by using syringes. Portions (2 ml) of S. aureus strain MN8 or group A streptococcal strain T253cured(T12) at approximately 109 CFU/ml were injected directly into the Wiffle balls in each animal. The bacterial strains were prepared by culture just until the stationary phase was achieved in beef heart medium (2), with one wash with PBS, and resuspended to the desired concentration in fresh beef heart medium. Immediately after receiving the bacteria, the animals received 4 ml of chitosan malate (7.0 mg/ml) in fresh beef heart medium; control animals received fresh beef heart medium alone. The administered chitosan malate concentration corresponds to approximately 900 μg of chitosan malate/ml (final concentration) in the 30-ml Wiffle balls; chitosan malate was given subsequent to the microbes to ensure that the organisms were not killed as a result of immediate mixing with an antimicrobial concentration of chitosan malate. The animals were monitored daily for 1 week for signs of TSS, including fever, diarrhea, reddening of mucous membranes and ears, weight loss, and death.
Prior to injection of S. aureus and group A streptococci and on day 2 postinjection, the temperatures of the rabbits were determined. Past experience has indicated that peak fever responses of rabbits to challenge with SAg-producing organisms in Wiffle balls occurs 1 to 2 days postinjection.
Finally, group A streptococcal strain MNBi was suspended to 5 × 108 CFU/ml in fresh beef heart medium, and 20 ml was directly injected subcutaneously into the flanks of Dutch belted rabbits (27). Subsequently, chitosan malate (3 ml of 7 mg/ml) in fresh beef heart medium or fresh beef heart medium alone was injected into the same site as the microbes. Animals were monitored for 24 h for signs of TSS with necrotizing fasciitis (evidenced by the flanks of the animals turning black in color and sloughing).
Statistics.
The Fisher exact test was used to determine differences in live versus dead animals in the in vivo challenge experiments. Student t test analysis of unpaired, normally distributed data was used for other statistical analyses.
RESULTS
Chitosan inhibits bacterial growth and SAg production in vitro.
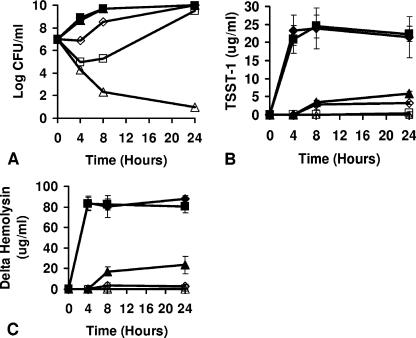
Broth cultures of S. aureus MN8 were tested for inhibition of bacterial growth by chitosan malate (final concentrations in 25-ml broths ranged from 0 to 7.0 mg/ml). The cultures of S. aureus MN8 (Fig. 1A) exhibited delayed growth in cultures containing chitosan malate at concentrations of 1.0 and 3.5 mg/ml compared to growth of the organism in the absence of chitosan malate or in the presence of chitosan malate at 0.1 and 0.5 mg/ml. However, the organism grew to the same CFU/ml in the absence of chitosan malate or in the presence of 0.1-, 0.5-, 1.0-, and 3.5-mg/ml final concentrations by 24 h. Chitosan malate (7.0 mg/ml, final concentration) was bactericidal for S. aureus MN8 by 4 h (3 log decrease in CFU/ml), with continued killing across the entire 24-h period (>6 log CFU/ml decrease after 24 h). Similar effects of chitosan malate on S. aureus growth were seen with the use of two additional TSST-1-positive S. aureus (strains 587 and MN Pa); the two strains grew to the same extent as their respective untreated cultures by 24 h in the presence of 0.1 to 3.5 mg of chitosan malate/ml, with chitosan malate at 7.0 mg/ml being bactericidal (Table 1). In addition, similar effects were observed with a strain that produces SEB (MN Ho) and a strain that produces SEC1 (MN Don); both strains grew to the same extent as their respective controls by 24 h in the presence of 0.1 to 3.5 mg of chitosan malate/ml, with 7.0 mg/ml being bactericidal (Table 1).
FIG. 1.
Chitosan malate inhibits the growth of S. aureus MN8 (A), TSST-1 production (B), and delta-hemolysin production (C) in broth cultures. S. aureus inoculum size was 1.0 × 107 CFU/ml. Cultures were incubated at 37°C, with shaking (200 rpm), in beef heart medium. Symbols: ⧫, chitosan malate at 0 mg/ml; ▪, chitosan malate at 0.1 mg/ml; ▴, chitosan malate at 0.5 mg/ml; ⋄, chitosan malate at 1.0 mg/ml; □, chitosan malate at 3.5 mg/ml; ▵, chitosan malate at 7.0 mg/ml.
TABLE 1.
Chitosan malate inhibits growth of S. aureus and, independently, SAg and delta-hemolysin production in broth cultures
| S. aureus strain (SAg) | Chitosan malate concn (mg/ml) | Log CFU/ml at 24 h | Mean concn (μg/ml) ± SDa
|
|
|---|---|---|---|---|
| SAg | Delta-hemolysin | |||
| 587 (TSST-1) | 0 | 9.9 | 5.8 ± 0.7 | 58 ± 12 |
| 0.5 | 9.9 | 1.6 ± 0.9 | 22 ± 3.6 | |
| 1.0 | 9.8 | 0.8 ± 0.2 | 2.0 ± 1.3 | |
| 3.5 | 9.3 | 0.2 ± 0.1 | 0.5 ± 0.3 | |
| 7.0 | <1.0 | <0.06 | <0.01 | |
| MN PA (TSST-1) | 0 | 9.4 | 36 ± 5.7 | 41 ± 5.3 |
| 0.5 | 9.4 | 18 ± 3.1 | 24 ± 1.7 | |
| 1.0 | 9.4 | 9.7 ± 1.0 | 4.0 ± 1.5 | |
| 3.5 | 8.9 | 0.6 ± 0.2 | 0.05 ± 0 | |
| 7.0 | <1.0 | <0.06 | <0.01 | |
| MN Ho (SEB) | 0 | 9.7 | 79 ± 5.5 | 61 ± 4.4 |
| 0.5 | 9.7 | 52 ± 3.5 | 45 ± 3.5 | |
| 1.0 | 9.7 | 10 ± 5.7 | 5.0 ± 1.5 | |
| 3.5 | 9.6 | 0.6 ± 0.2 | 0.6 ± 0.4 | |
| 7.0 | <1.0 | <0.06 | <0.01 | |
| MN Don (SEC) | 0 | 9.8 | 50 ± 6.0 | 54 ± 7.2 |
| 0.5 | 9.8 | 22 ± 2.3 | 23 ± 7.5 | |
| 1.0 | 9.8 | 8.7 ± 1.2 | 5.0 ± 2.1 | |
| 3.5 | 9.8 | 2.3 ± 1.2 | 2.0 ± 1.0 | |
| 7.0 | <1.0 | <0.06 | <0.01 | |
The P values for all chitosan malate cultures were <0.01 compared to untreated controls. The SAg and delta-hemolysin concentrations were determined by ELISA.
In the same broth cultures, the production of TSST-1 by S. aureus MN8 was also significantly altered by chitosan malate (Fig. 1B). Cultures of strain MN8 contained approximately 25 μg of TSST-1/ml after 4 h or more of culture in the absence of chitosan malate or in the presence of 0.1 mg of chitosan malate (the P value being not significantly different from cultures with no chitosan malate)/ml. In contrast, chitosan malate at 0.5, 1.0, and 3.5 mg/ml significantly inhibited the production of TSST-1 at 4, 8, and 24 h (P < 0.001), even if there was no inhibition of bacterial growth (for example, 24 h). As expected, chitosan malate (7.0 mg/ml) completely inhibited the production of TSST-1, concurrent with the inhibition of bacterial growth. The production of TSST-1 by S. aureus strains 587 and MN Pa was comparably inhibited by chitosan malate concentrations of 0.5 mg/ml (72 and 50% reductions at 24 h), 1.0 mg/ml (86 and 73% reductions), and 3.5 mg/ml (97 and 98% reductions), despite not interfering with staphylococcal growth at 24 h (Table 1). The production of SEB by strain MN Ho and of SEC1 by strain MN Don were also inhibited by chitosan malate concentrations of 0.5 mg/ml (34 and 56% reductions), 1.0 mg/ml (87 and 83% reductions), and 3.5 mg/ml (99 and 95% reductions) (Table 1). For all organisms, chitosan malate at 7.0 mg/ml inhibited the production of these exotoxins to levels below detection by the respective strains, concurrent with the inhibition of bacterial growth. The production of delta-hemolysin by S. aureus MN8 was comparably significantly inhibited by chitosan malate concentrations of 0.5 to 7.0 mg/ml compared to controls lacking chitosan malate or containing a 0.1-mg/ml concentration of chitosan malate (Fig. 1C) (for example, P < 0.001 for chitosan malate concentrations of 0.5, 1.0, 3.5, and 7.0 mg/ml at 24 h compared to control without added chitosan malate). The production of delta-hemolysin by strains 587, MN PA, MN Ho, and MN Don was also significantly inhibited by chitosan malate concentrations of 0.5 to 7.0 mg/ml (Table 1). For example, chitosan malate at 1.0 and 3.5 mg/ml did not inhibit bacterial growth by 24 h compared to untreated controls but caused a >90% reduction in delta-hemolysin production by strains 587, MN PA, MN Ho, and MN Don.
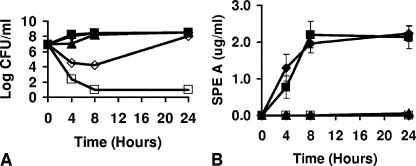
In broth cultures, group A streptococcal strain 594 exhibited delayed growth in the presence of chitosan malate at 0.5 and 1.0 mg/ml (final concentrations) compared to cultures that either contained no chitosan malate or contained chitosan malate at 0.1 mg/ml, but the cultures recovered to control levels by 24 h (Fig. 2A). Chitosan malate (3.5 mg/ml, final concentration) was bactericidal for strain 594 by 4 h with continued killing for the entire 24-h period. Two other group A streptococcal strains were comparably delayed or inhibited in growth by chitosan malate [strains T253cured(T12) and MNBi]. The two strains grew to the same extent as their respective untreated cultures by 24 h in the presence of chitosan malate at 0.1 to 1.0 mg/ml (final concentration), with chitosan malate at 3.5 mg/ml being bactericidal (data not shown).
FIG. 2.
Chitosan malate inhibits growth of group A streptococcal strain 594 (A) and SPE A production (B) in broth cultures. The group A streptococcal inoculum size was 107 CFU/ml. Cultures were incubated at 37°C, with shaking (200 rpm), in beef heart medium. Symbols: ⧫, chitosan malate at 0 mg/ml; ▪, chitosan malate at 0.1 mg/ml; ▴, chitosan malate at 0.5 mg/ml; ⋄, chitosan malate at 1.0 mg/ml; □, chitosan malate at 3.5 mg/ml.
In these same broth cultures, chitosan malate at 0.5 and 1.0 mg/ml (final concentrations) inhibited the production of SPE A by group A streptococcal strain 594, even when growth inhibition was not observed (Fig. 2B) (P < 0.001). Chitosan malate (3.5 mg/ml) inhibited SPE A production, concurrent with the inhibition of bacterial growth. Chitosan malate (0.1 mg/ml) did not affect SPE A production compared to the control lacking chitosan malate (the P value not being significantly different). Similar effects on SPE A production by chitosan malate were seen with group A streptococcal strains T253cured(T12) and MNBi. For example, the production of SPE A by strains T253cured(T12) and MNBi was comparably inhibited by chitosan malate concentrations of 0.5 mg/ml (97 and 98% reductions at 24 h) and 1.0 mg/ml (98 and 99% reductions), despite not interfering with streptococcal growth at 24 h (P < 0.001).
The major use of chitosan malate in preventing or treating bacterial infections may be as an agent incorporated into wound dressings, for example, in the management of trauma, bed sores, or burn-associated infections. In such uses, chitosan malate will most likely have to exert its effects on bacteria growing as thin films in or on human tissues. The ability of chitosan malate to affect growth and the production of exotoxins of S. aureus MN8 and group A streptococcal strain 594 as cultured in vitro as thin films was assessed after 24 h.
Chitosan malate at 3.5 and 7.0 mg/ml (final concentrations) was bactericidal for S. aureus MN8 (Fig. 3A) when the organism was grown in thin films for 24 h. Similarly, the compound at 3.5 mg/ml (final concentration) was bactericidal for group A streptococcal strain 594 (Fig. 4A) when cultured in thin films for 24 h. Chitosan malate at 0, 0.1, 0.5, and 1.0 mg/ml (final concentrations) did not inhibit the growth of S. aureus MN8 (Fig. 3A); concentrations of 0, 0.1, 0.5, and 1.0 mg/ml also did not inhibit the growth of group A streptococcal strain 594 (Fig. 4A). Chitosan malate inhibited the production of TSST-1 at final concentrations of 0.5 and 1.0 mg/ml (Fig. 3B) and the production of SPE A (Fig. 4B) at final concentrations of 0.5 and 1.0 mg/ml, despite not inhibiting bacterial growth. Chitosan malate at 3.5 and 7.0 mg/ml also inhibited TSST-1 production and at 3.5 mg/ml inhibited SPE A production by the organisms, a finding consistent with the bactericidal activity of the compound at these concentrations. Chitosan 0.1 mg/ml did not inhibit exotoxin production by either S. aureus MN8 or group A streptococcus 594.
FIG. 3.
Chitosan malate inhibits growth (A) and TSST-1 production (B) of S. aureus MN8 at 24 h when cultured in thin films. The inoculum size was 107 CFU/ml.
FIG. 4.
Chitosan malate inhibits the growth (A) and SPE A production (B) of group A streptococcus 594 at 24 h when cultured in thin films. The inoculum size was approximately 107 CFU/ml.
Chitosan inhibition of TSS and necrotizing fasciitis in rabbits.
Chitosan malate at concentrations as high as 7.0 mg/kg, given as 1 ml/kg dissolved in PBS, was not pyrogenic (the average change in temperature of three rabbits was <0.5°C over 4 h) and not lethal in rabbits (three animals/group) after intravenous challenge. In addition, the rabbits showed no visible signs of illness as a result of the challenge when observed for 1 week postinjection, and the animals’ organs appeared normal upon completion of experimentation.
Groups of rabbits received subcutaneously implanted Wiffle golf balls. The animals were allowed to heal and encapsulate the Wiffle balls for 8 weeks postimplantation. At that time, 6 ml of serous fluid were removed from each Wiffle ball. The rabbits were subsequently challenged with S. aureus MN8 (109/2-ml volume of fresh beef heart medium) and subsequently with either 4 ml of chitosan malate (28 mg total) in fresh beef heart medium or beef heart medium alone. Chitosan malate provided significant protection to the animals treated with S. aureus MN8 (12 of 13 surviving) compared to only 2 of 10 non-chitosan malate-treated control animals surviving (Table 2) (P < 0.001). All animals showed fevers on day 2 after injection, whether chitosan malate treated (the average change in temperature was 1.1°C) or not (the average change in temperature was 2.3°C), but the fevers were significantly lower in the chitosan malate-treated rabbits (P < 0.001 [Student t test]). Except for one animal, the chitosan malate-treated rabbits did not develop other signs of TSS; all control, non-chitosan malate-treated animals developed signs of TSS.
TABLE 2.
Chitosan malate inhibits TSS in Wiffle ball models of staphylococcal (S. aureus strain MN8) and streptococcal TSS [streptococcal strain T253cured(T12)] and necrotizing fasciitis (streptococcal strain MNBi) in a rabbit subcutaneous injection model
| Model | Presence (+) or absence (−) of chitosan malate | Bacterium | No. of rabbits alive/total no. tested | Pa |
|---|---|---|---|---|
| Wiffle ball | - | S. aureus | 2/10 | |
| + | S. aureus | 12/13 | <0.001 | |
| - | Streptococcus sp. | 3/10 | ||
| + | Streptococcus sp. | 10/10 | <0.005 | |
| Necrotizing | - | Streptococcus sp. | 0/5 | |
| fasciitis | + | Streptococcus sp. | 5/5 | <0.01 |
Compared to animals not treated with chitosan malate.
Similar in vivo studies were performed with use group A streptococcal strain T253cured(T12) administered in the presence or absence of chitosan malate. Rabbits were administered the group A streptococcal strain in Wiffle balls (109 CFU/2-ml volume) and then 4 ml of chitosan malate (28 mg) in beef heart medium or beef heart medium alone and subsequently monitored for TSS and lethality (Table 2). Animals that received streptococci and chitosan malate survived the challenge (0 of 10 succumbed), whereas 7 of 10 rabbits receiving streptococci in the absence of chitosan malate succumbed (P < 0.005). All rabbits developed fevers on day 2 postinjection, whether chitosan malate treated (the average temperature was 1.4°C) or not (the average temperature was 2.4°C), but rabbits that received chitosan malate showed significantly lower fever responses than animals not receiving chitosan malate (P < 0.001 [Student t test]). The rabbits that received chitosan malate did not show other signs of TSS, whereas all non-chitosan malate-treated rabbits showed signs of TSS, whether or not they succumbed.
In a final set of studies, rabbits were inoculated subcutaneously with group A streptococcal strain MNBi, an M3 isolate from a case of streptococcal TSS, followed by chitosan malate (21 mg in 3 ml of beef heart medium) or 3 ml of beef heart medium alone (Table 2). Rabbits that received streptococci with chitosan malate survived the subcutaneous challenge and did not develop necrotizing fasciitis (0 of 5 succumbed). In contrast, rabbits that received streptococci but not chitosan malate developed streptococcal TSS with necrotizing tissue damage (necrotizing fasciitis) and succumbed in less than 24 h (5 of 5) (P < 0.01 compared to chitosan malate-treated rabbits).
DISCUSSION
Several studies have shown previously that physical and chemical factors significantly affect the production of pyrogenic toxin SAgs by S. aureus and group A streptococci (20, 21, 40, 42). For example, anaerobic conditions and pHs below 6 to 6.5 significantly inhibit the production of TSST-1 (29). Similarly, the antibiotic clindamycin inhibits the production of TSST-1 at concentrations well below those necessary to inhibit bacterial growth (32). Finally, it has been shown that components of tampons, such as surfactants and lubricants, have significant effects both on bacterial growth and on toxin production. For example, cetiol strongly inhibits the production of several SAgs (30). Similarly, glycerol monolaurate, proposed for the addition to tampons, inhibits the production of TSST-1, other staphylococcal exotoxins, and exotoxins of group A streptococci (31). In contrast, Pluronic L-92 at high concentrations appears to stimulate the production of exotoxins (25).
The studies described above suggest that it may be possible to identify agents particularly for use in wound dressings that inhibit the production of staphylococcal and streptococcal exotoxins, including SAgs, or other virulence factors and thus reduce the risk of TSS and other types of infections, even if microbial growth is not inhibited. Such agents have become increasingly important for addition to medical devices since microorganisms, such as S. aureus, are becoming increasingly antibiotic resistant. In the present study, chitosan malate was evaluated for the ability to inhibit both bacterial growth and, independently, exotoxin production. This compound is well characterized to inhibit bacterial growth (3, 23, 37). The present study confirmed these findings but also showed that chitosan malate is able to inhibit exotoxin production by S. aureus and group A streptococci at sub-growth-inhibitory concentrations.
There has been an increase in the use of occlusive wound dressings, presumably due to the increased speed of healing and reduced pain and scarring associated with their use (3, 16, 18, 43). The use of these dressings raises concern over their effect on bacterial colonization or infection at the wound site. For example, Mertz et al. noted that S. aureus, the pathogen most often isolated from skin infections, was able to survive and proliferate well in occlusive dressing-covered wounds (18). This raises the possibility that staphylococcal TSS, related streptococcal illnesses, or other infections may occur in association with the use of such dressings (7, 38). In light of these findings, studies have begun evaluating the ability of agents, such as silver, added to dressings to interfere with microbial growth (3, 4, 24). Such wound dressings have large numbers of possible applications, including the management of postsurgical infections, infections associated with pressure sores and burns, war trauma infections, and infections associated with diabetes, severe invasive streptococci, and skin infections due to community-associated MRSA.
In the present study, it was shown that chitosan malate, an agent that could be added to wound dressings, inhibited both bacterial growth and exotoxin production in vitro and prevented TSS and necrotizing fasciitis in rabbit models. It has long been known that chitosan inhibits the growth of gram-positive and gram-negative bacteria, with greater antimicrobial activity against gram-positive agents (3, 23, 37). This observation was confirmed here. However, the present study extends these findings by demonstrating that sub-growth-inhibitory concentrations of chitosan malate also interfere with exotoxin production, much the same as has previously been noted for clindamycin and glycerol monolaurate (31, 32). In addition, the present study clearly shows that chitosan malate at sub-growth-inhibitory concentrations prevents the development of both staphylococcal and streptococcal TSS in a Wiffle ball abscess model and the development of streptococcal TSS with necrotizing fasciitis in a direct subcutaneous injection model.
Chitosan malate is a polysaccharide comprised of repeating units of glucosamine. The mechanism underlying its growth- and toxin-inhibitory effects remains unknown but may be related to its strong charge. The polymeric size and charge suggest the agent may inhibit exotoxin production by effects on the microbial surface and thus by interfering with signal transduction. A similar mechanism of action has been proposed for glycerol monolaurate's inhibition of exotoxin production at sub-bacterial-growth-inhibitory concentrations (22). Both S. aureus and group A streptococci have large numbers of two-component systems that allow them to sense and respond to the external environment through signal transduction. One of the staphylococcal two-component systems is AgrA-AgrC, which senses small peptides in the external environment (20). When AgrA-AgrC is activated, a regulatory RNA, termed RNA III, is made that leads to exotoxin production. RNA III also encodes delta-hemolysin. The present study shows that delta-hemolysin production was inhibited by the same concentrations of chitosan malate that prevented the production of TSST-1 and staphylococcal enterotoxins. It is thus possible that chitosan malate exerts its exotoxin-inhibitory effects by altering two-component systems, such as AgrA-AgrC. However, further studies are needed to test this hypothesis thoroughly.
The present study has shown that chitosan malate has the ability to inhibit exotoxin production by gram-positive bacteria at chitosan malate concentrations that are not overtly toxic to rabbits, and in vivo the compound has the ability to prevent serious staphylococcal and streptococcal illnesses. Further studies are required to assess the human safety of chitosan malate use, but these studies suggest the compound may be useful as an additive to wound dressings for the management of infections.
Acknowledgments
This study was supported by Public Health Service research grant HL-36611 from the National Heart, Lung, and Blood Institute.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Blake, M. S., K. H. Johnston, G. J. Russell-Jones, and E. C. Gotschlich. 1984. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 136:175-179. [DOI] [PubMed] [Google Scholar]
- 2.Blomster-Hautamaa, D. A., and P. M. Schlievert. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 165:37-43. [DOI] [PubMed] [Google Scholar]
- 3.Burkatovskaya, M., G. P. Tegos, E. Swietlik, T. N. Demidova, A. P. Castano, and M. R. Hamblin. 2006. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 27:4157-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso, D. M., K. N. Foster, S. A. Blome-Eberwein, J. A. Twomey, D. N. Herndon, A. Luterman, P. Silverstein, J. R. Antimarino, and G. J. Bauer. 2006. Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J. Burn Care Res. 27:298-309. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. JAMA 282:1123-1125. [PubMed] [Google Scholar]
- 6.Daum, R. S., T. Ito, K. Hiramatsu, F. Hussain, K. Mongkolrattanothai, M. Jamklang, and S. Boyle-Vavra. 2002. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J. Infect. Dis. 186:1344-1347. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, A. J., M. P. Dixon, and J. B. Dixon. 2006. Randomized clinical trial of the effect of applying ointment to surgical wounds before occlusive dressing. Br. J. Surg. 93:937-943. [DOI] [PubMed] [Google Scholar]
- 8.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 9.Fey, P. D., B. Said-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C. Davis, B. N. Kreiswirth, and P. M. Schlievert. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus.Antimicrob. Agents Chemother. 47:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 11.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 12.Kotzin, B. L., D. Y. Leung, J. Kappler, and P. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 13.Kravitz, G., D. J. Dries, M. L. Peterson, and P. M. Schlievert. 2005. Purpura fulminans due to Staphylococcus aureus.Clin. Infect. Dis. 40:941-947. [DOI] [PubMed] [Google Scholar]
- 14.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 15.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 16.Marshall, D. A., P. M. Mertz, and W. H. Eaglstein. 1990. Occlusive dressings: does dressing type influence the growth of common bacterial pathogens? Arch. Surg. 125:1136-1139. [DOI] [PubMed] [Google Scholar]
- 17.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 18.Mertz, P. M., D. A. Marshall, and W. H. Eaglstein. 1985. Occlusive wound dressings to prevent bacterial invasion and wound infection. J. Am. Acad. Dermatol. 12:662-668. [DOI] [PubMed] [Google Scholar]
- 19.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 20.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429-1449. [DOI] [PubMed] [Google Scholar]
- 21.Pragman, A. A., and P. M. Schlievert. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42:147-154. [DOI] [PubMed] [Google Scholar]
- 22.Projan, S. J., S. Brown-Skrobot, P. M. Schlievert, F. Vandenesch, and R. P. Novick. 1994. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabea, E. I., M. E. Badawy, C. V. Stevens, G. Smagghe, and W. Steurbaut. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457-1465. [DOI] [PubMed] [Google Scholar]
- 24.Scanlon, E., T. Karlsmark, D. J. Leaper, K. Carter, P. B. Poulsen, K. Hart-Hansen, and T. W. Hahn. 2005. Cost-effective faster wound healing with a sustained silver-releasing foam dressing in delayed healing leg ulcers: a health-economic analysis. Int. Wound J. 2:150-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlievert, P. M. 1996. Effect of Merocel vaginal sponge on growth of Staphylococcus aureus and production of toxic shock syndrome-associated toxins. J. Am. Coll. Surg. 183:19-24. [PubMed] [Google Scholar]
- 26.Schlievert, P. M. 1988. Immunochemical assays for toxic shock syndrome toxin-1. Methods Enzymol. 165:339-344. [DOI] [PubMed] [Google Scholar]
- 27.Schlievert, P. M., A. P. Assimacopoulos, and P. P. Cleary. 1996. Severe invasive group A streptococcal disease: clinical description and mechanisms of pathogenesis. J. Lab. Clin. Med. 127:13-22. [DOI] [PubMed] [Google Scholar]
- 28.Schlievert, P. M., K. M. Bettin, and D. W. Watson. 1978. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc. Soc. Exp. Biol. Med. 157:472-475. [DOI] [PubMed] [Google Scholar]
- 29.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 30.Schlievert, P. M., D. A. Blomster, and J. A. Kelly. 1984. Toxic shock syndrome Staphylococcus aureus: effect of tampons on toxic shock syndrome toxin 1 production. Obstet. Gynecol. 64:666-671. [PubMed] [Google Scholar]
- 31.Schlievert, P. M., J. R. Deringer, M. H. Kim, S. J. Projan, and R. P. Novick. 1992. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 36:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlievert, P. M., and J. A. Kelly. 1984. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J. Infect. Dis. 149:471. [DOI] [PubMed] [Google Scholar]
- 33.Schlievert, P. M., M. Y. Kotb, and D. L. Stevens. 2000. Streptococcal superantigens: streptococcal toxic shock syndrome, p. 25-39. In M. W. Cunningham and R. S. Fujinami (ed.), Effects of microbes on the immune system. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 34.Stevens, D. L., A. E. Bryant, and S. P. Hackett. 1995. Antibiotic effects on bacterial viability, toxin production, and host response. Clin. Infect. Dis. 20(Suppl. 2):S154-S157. [DOI] [PubMed] [Google Scholar]
- 35.Stevens, D. L., A. E. Gibbons, R. Bergstrom, and V. Winn. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 158:23-28. [DOI] [PubMed] [Google Scholar]
- 36.Stevens, D. L., M. H. Tanner, J. Winship, R. Swarts, K. M. Ries, P. M. Schlievert, and E. Kaplan. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 37.Takai, K., T. Ohtsuka, Y. Senda, M. Nakao, K. Yamamoto, J. Matsuoka, and Y. Hirai. 2002. Antibacterial properties of antimicrobial-finished textile products. Microbiol. Immunol. 46:75-81. [DOI] [PubMed] [Google Scholar]
- 38.Trop, M., G. Zobel, S. Roedl, H. M. Grubbauer, and G. Feierl. 2004. Toxic shock syndrome in a scald burn child treated with an occlusive wound dressing. Burns 30:176-180. [DOI] [PubMed] [Google Scholar]
- 39.Vetter, S., T. J. Tripp, and P. M. Schlievert. 2005. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis.Antimicrob. Agents Chemother. 49:1302-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarwood, J. M., J. K. McCormick, and P. M. Schlievert. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus.J. Bacteriol. 183:1113-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarwood, J. M., and P. M. Schlievert. 2003. Quorum sensing in staphylococcus infections. J. Clin. Investig. 112:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zitelli, J. 1987. Wound healing for the clinician. Adv. Dermatol. 2:243-267. [PubMed] [Google Scholar]