Abstract
Fluoroquinolones are poorly active against enterococci. Recently, plasmid-borne resistance to fluoroquinolones due to the qnr gene was reported in members of the Enterobacteriaceae family. The gene encodes a pentapeptide repeat protein that protects DNA gyrase from inhibition by fluoroquinolones. We have identified in the genome of Enterococcus faecalis V583 a qnr-like gene, named E. faecalis qnr (qnrE. faecalis), encoding a putative pentapeptide repeat protein that shares 25% identity with Qnr. To assess its potential role in the intrinsic resistance of E. faecalis to fluoroquinolones, qnrE. faecalis was inactivated in E. faecalis JH2-2 by insertion of the thermosensitive vector pG1KT. This strain was then complemented with qnrE. faecalis cloned in the multicopy plasmid pORI23. The effects of its overexpression were also studied. Inactivation of the qnrE. faecalis gene resulted in twofold decreases in the MICs of ofloxacin and ciprofloxacin. When the gene was complemented or overexpressed, MICs of fluoroquinolones increased four- to nine-fold, leading to MICs of ofloxacin and ciprofloxacin equal to 32 μg/ml and 8 μg/ml, respectively. The E. faecalis Qnr (QnrE. faecalis) protein was produced and purified. QnrE. faecalis protein protected Escherichia coli DNA gyrase from inhibition by ofloxacin. The qnrE. faecalis gene was then introduced into E. coli DH10B, Staphylococcus aureus RN4220, and Lactococcus lactis IL-1419 to study its heterologous expression. MICs of the various fluoroquinolones tested increased 4- to 16-fold, showing that QnrE. faecalis conferred resistance to fluoroquinolones in various bacterial backgrounds. Overexpression of qnrE. faecalis in enterococci or mobilization of the gene to other bacterial species may be anticipated as a possible new mechanism for fluoroquinolone resistance.
Fluoroquinolones are synthetic antibacterial agents that show potent activity against gram-negative bacteria, including members of the Enterobacteriaceae family and staphylococci. More recently, fluoroquinolones such as gatifloxacin, levofloxacin, and moxifloxacin that have increased activity against Streptococcus pneumoniae have been developed. As a result of their wide-spectrum activity, fluoroquinolones are used to treat a great variety of infections, including urinary tract infections, osteomyelitis, enteric infections, and respiratory tract infections; however, they are of limited interest in the treatment of enterococcal infections. Enterococci are important nosocomial pathogens that cause serious life-threatening infections, including bacteremia, endocarditis, and meningitidis (15). Fluoroquinolones show poor or moderate activity against this bacterial genus, as the MIC of ofloxacin for the reference strain Enterococcus faecalis ATCC 29212 is 2 to 4 μg/ml, in contrast to 0.03 to 0.06 μg/ml for the reference strain Escherichia coli ATCC 25922 (8).
Targets of quinolones are DNA gyrase and topoisomerase IV that are tetrameric A2B2 enzymes encoded by the gyrA and gyrB and the parC and parE genes, respectively. DNA gyrase catalyzes the negative supercoiling of DNA essential for transcription initiation and chromosome condensation. Topoisomerase IV is implicated in DNA decatenation. The fluoroquinolone targets in gram-negative bacteria are different than those in gram-positive bacteria. In gram-negative organisms, the primary target is DNA gyrase, whereas in gram-positive organisms, it seems to be topoisomerase IV, as reported for Staphylococcus aureus (4). However, in S. pneumoniae, the primary target seems to depend on a specific fluoroquinolone (17). In enterococci, the primary target is still unclear. Some studies have shown that a single mutation in the gyrA gene was associated with fluoroquinolone resistance (11, 23). Data from Kanematsu et al. (10) were in favor of topoisomerase IV as the primary target, since a single mutation in the parC gene was sufficient to obtain a low level of E. faecalis resistance to fluoroquinolones. According to a recent study by Oyamada et al. (16), the primary target of fluoroquinolones in Enterococcus faecium would depend on the structure of the compound tested. The weak activity of quinolones against enterococci has not been clearly explained and might hypothetically be due to a low affinity of the gyrase of this bacterial genus for quinolones. The extensive use of fluoroquinolones has lead to a rapid development of bacterial resistance. This resistance is due mainly to chromosomal mutations in genes encoding quinolone targets, DNA gyrase, and topoisomerase IV, located predominantly in the quinolone resistance-determining region. Decreased accumulation into the bacteria by mutations in the regulatory genes of outer-membrane proteins or efflux pumps was also reported. The first plasmid-borne resistance was described in 1998 in a strain of Klebsiella pneumoniae isolated in Alabama (14). This strain harbored a plasmid, pMG252, which conferred resistance when transferred to a recipient strain. This plasmid contained a new gene, qnr, responsible for the resistance (24). Since then, several proteins belonging to the Qnr protein family have been described in enterobacteria: QnrA, QnrB (9), and QnrS (5). These proteins are characterized by tandem pentapeptide repeats organized in the consensus sequence (A/C) (D/N) (L/F) (S/R) (G/R) (2, 19, 24). QnrA is a 218-amino-acid protein that protects DNA gyrase (24, 25) and topoisomerase IV (26) from inhibition by fluoroquinolones by decreasing their ability to bind DNA. Proteins similar to Qnr seem to be present in the genome of various microorganisms, and some are implicated in the resistance to DNA gyrase inhibitors, such as McbG or MfpA. McbG is a component of the system that protects bacteria synthesizing microcin B17 from self-inhibition. This microcin is a peptide that blocks DNA replication and can inhibit DNA gyrase supercoiling by stabilizing the cleavage complex (6, 27). MfpA is a Mycobacterium smegmatis protein that interacts with DNA gyrase in a dimeric form (7). The inactivation and the overexpression of mfpA gene are implicated in variations of ciprofloxacin MICs (27).
We detected the presence of a homologue of the qnr gene in the genome of E. faecalis V583 (18), and we assessed its potential role in the intrinsic resistance of enterococci to fluoroquinolones.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains were routinely grown at 37°C in tryptone soy (TS) broth or agar (Bio-Rad, Marnes-la-Coquette, France), except when noted. TS agar medium was supplemented with 10% horse blood for Lactococcus lactis. Media for the selection of transformants contained kanamycin (20 and 500 μg/ml) and/or erythromycin (5 and 500 μg/ml) and/or chloramphenicol (5 and 20 μg/ml). E. faecalis JH2-2, E. coli DH10B and BL21(DE3), L. lactis IL-1419, and S. aureus RN4220 were used as recipient strains in transformation experiments.
Antimicrobial susceptibility.
MICs of fluoroquinolones for the different strains were determined in three independent experiments by the broth microdilution method in Mueller-Hinton broth (Bio-Rad), as recommended by the CA-SFM (http://www.sfm.asso.fr; last release, January 2007). For E. faecalis JH2-2, the dilutions tested were 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 16, 32, 64, and 128 μg/ml for ofloxacin and 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 3, 4, 8, 16, 32, and 64 μg/ml for ciprofloxacin. For heterologous expression experiments, MICs of ofloxacin, ciprofloxacin, levofloxacin, sparfloxacin, and moxifloxacin were determined by a standard twofold dilution method.
PCR conditions.
Oligonucleotide primers used in this study are listed in Table 1. The PCR consisted of 30 cycles of denaturation (94°C, 30 s), annealing (50°C, 30 s), and extension (72°C, 30 s to 1 min). E. faecalis JH2-2 DNA was extracted by an Instagen Matrix kit (Bio-Rad) as recommended by the manufacturer.
TABLE 1.
Oligonucleotide primers
| Target | Target size (bp) | Primer name | Nucleotide sequence (5′-3′)a | Restriction site |
|---|---|---|---|---|
| qnrE. faecalis | 731 | qnr-1F | AATTGATGGATCCTTTGAAAATC | BamHI |
| 731 | qnr-1R | TAAGAAAGAGTCGACAGAACCAG | SalI | |
| Internal fragment of qnrE. faecalis | 459 | qnr-2F | AAATCATTTGGATCCGCAGATTG | BamHI |
| 459 | qnr-2R | GTCAAGCCTGCAGAGGTGTTTTG | PstI | |
| Chloramphenicol acetyltransferase | 713 | CAT-F1 | AATTCACTGTCGACAAAAATTTAGG | SalI |
| 713 | CAT-R3 | AAAACTGCAGAGTCGGCATTATCTC | PstI | |
| qnrE. faecalis | 696 | qnrX-F | CCTACTCCATGGATGAAACCTTTG | NcoI |
| 696 | qnrX-R2 | AAAATCTCGAGTGTAATCACCAAACC | XhoI |
Restriction sites are underlined.
Identification of a homologue of the qnr gene in E. faecalis JH2-2.
A BLAST analysis of the genomic sequence of E. faecalis V583 was performed (http://www.ncbi.nih.gov/BLAST; accession number AE016830). A homologue of the qnr gene was identified at the locus EF0905 and named E. faecalis qnr (qnrE. faecalis). This gene was amplified with specific primers qnr-1F and qnr-1R (Table 1) from E. faecalis JH2-2 DNA and sequenced (Ceq 8000; Beckmann Coulter, Villepinte, France) for comparison with the E. faecalis V583 qnrE. faecalis gene.
Inactivation of qnrE. faecalis.
A fragment internal to the qnrE. faecalis gene was amplified from E. faecalis JH2-2 DNA with specific primers qnr-2F and qnr-2R (Table 1). After purification on Microspin S400 columns (Amersham Biosciences, Orsay, France), this fragment was digested with the BamHI and SalI enzymes (Amersham), cloned into the thermosensitive vector pG1KT, and introduced into the electrocompetent E. coli DH10B strain. Plasmid pG1KT was a derivative of the thermosensitive shuttle plasmid pG+host5 (conferring resistance to erythromycin) containing a promoterless and terminatorless kanamycin resistance cassette AphA-3 (12). The transformants were selected on medium containing 500 μg/ml erythromycin. Proper qnrE. faecalis gene placement in the recombinant plasmid was confirmed by specific PCR. This plasmid was introduced into electrocompetent E. faecalis JH2-2. The transformants were selected on medium containing kanamycin, 500 μg/ml, and erythromycin, 5 μg/ml, after incubation at 42°C, a temperature that promotes plasmid integration in the chromosomal qnrE. faecalis gene by homologous recombination. The spontaneous loss of pG1KT was obtained by daily subculture in TS broth at 42°C without any antimicrobial agent. The derivatives susceptible to erythromycin and kanamycin were tested for their susceptibilities to ofloxacin and ciprofloxacin.
Overexpression of the qnrE. faecalis gene and complementation of the qnr gene-inactivated strain.
The entire gene was amplified with the qnr-1F and qnr-1R primers from E. faecalis JH2-2 and digested with SalI enzyme. The promoterless pC194 chloramphenicol-acetyl-transferase (cat) gene was then amplified from the plasmid pBT1 (3) with specific primers CAT-F1 and CAT-R3 and digested by SalI. The two fragments were ligated with the T4 DNA ligase (New England Biolabs, Ipswich, MA), and the fusion was amplified with the qnr-1F and CAT-R3 primers. The resulting 1,444-bp fragment was digested with BamHI and PstI enzymes and cloned into the multicopy plasmid pORI23 under the control of a L. lactis strong promoter (21). The chloramphenicol resistance of the transformants was therefore the indicator of the fusion expression. The recombinant plasmid was introduced into the electrocompetent E. faecalis JH2-2 strain inactivated for the qnrE. faecalis gene in order to complement this derivative. The transformants were selected on medium containing kanamycin, 500 μg/ml, erythromycin, 5 μg/ml, and chloramphenicol, 20 μg/ml. The recombinant plasmid was also introduced into the electrocompetent E. faecalis JH2-2 strain to study the effects of qnrE. faecalis overexpression. The transformants were selected on medium containing erythromycin, 5 μg/ml, and chloramphenicol, 20 μg/ml.
Production and purification of QnrE. faecalis protein.
The entire qnrE. faecalis gene was amplified with specific primers qnrX-F and qnrX-R2 from E. faecalis JH2-2 DNA. After it was digested with NcoI and XhoI, the DNA segment was ligated into expression vector pET28a+ (Apha-3) (Novagen, Nottingham, United Kingdom), placing the qnrE. faecalis gene under the control of a phage T7 promoter associated with a lac gene operator, and adding coding sequence for a C-terminal His6 tag. The construct was verified by gel electrophoresis of the recombinant plasmid and PCR specific of the insert. This plasmid was introduced into the E. coli BL21(DE3) strain that synthesizes a T7 phage RNA polymerase, and the transformants were selected on medium containing kanamycin, 20 μg/ml. The production and the purification of QnrE. faecalis protein were realized as described previously (1, 24). Briefly, the strain was incubated in 50 ml of Mueller-Hinton broth with kanamycin, 20 μg/ml, at 30°C until the optical density at 600 nm reached 0.6. Then, isopropyl-β-d-thiogalactopyranoside was added at a final concentration of 1 mM, and the culture was incubated 4 h further, until cells were harvested by centrifugation for 20 min at 10,000 × g and stored at −20°C before protein isolation. The purification was realized on nickel nitriloacetate columns (Ni-NTA; QIAGEN, Courtaboeuf, France), as recommended by the manufacturer. The next steps were carried out at 4°C. The pellet was resuspended in 1 ml of lysis buffer (50 mM NaH2PO4-300 mM NaCl-10 mM imidazole [pH 8.0]), sonicated, and centrifuged for 20 min at 10,000 × g. The supernatant (600 μl) was charged on Ni-NTA columns and washed four times with 600 μl of wash buffer (50 mM NaH2PO4-300 mM NaCl-20 mM imidazole [pH 8.0]). Four elutions followed, two elutions with 250 mM imidazole and then two elutions with 325 mM imidazole (25). All centrifugations were performed at 700 × g for 2 min at 4°C. Samples were dialyzed immediately in Slide-A-lyzer 3.5 K (Pierce, Rockford, IL) for 18 h at 4°C in 50 mM Tris-HCl (pH 7.5). After dialysis, 10% glycerol was added, and the samples were stored at −20°C. Protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie blue, and protein final concentration was determined by using a protein assay kit (Bio-Rad).
DNA gyrase assay.
The DNA supercoiling assay was realized in the presence of the relaxed plasmid pBR322 (Topogen, Marne la Vallée, France) with purified E. coli DNA gyrase (John Innes Enterprises, Norwich, United Kingdom), as recommended by the manufacturer. One unit of DNA gyrase was incubated with 0.5 μg of relaxed pBR322 in a reaction volume of 30 μl at 37°C for 30 min in incubation buffer (Tris-HCl 35 mM [pH 7.5], 24 mM KCl, 4 mM MgCl2, 2 mM dithiothreitol, 1.8 mM spermidine, 1 mM ATP, 6.5% glycerol, and 0.1 mg/ml albumin). The different concentrations of ofloxacin tested (0, 0.25, 0.5, 1, 5, and 10 μg/ml) were incubated with the reaction mixture for 1 h at 25°C and for 30 min at 37°C. The resulting topoisomers of pBR322 were resolved by running a 1% agarose gel stained with ethidium bromide for 16 h at 3.5 V/cm that was visualized under UV light. In experiments using QnrE. faecalis, the dialyzed protein extract was first preincubated with DNA gyrase for 1 h at 25°C, before ofloxacin was added to the reaction mixture.
Heterologous expression.
To assess the effects of the qnrE. faecalis gene expression in other bacterial species, the recombinant plasmid pORI23::qnr-cat was introduced into electrocompetent strains E. coli DH10B, S. aureus RN4220, and L. lactis IL-1419. The transformants were selected on medium containing erythromycin, 500 μg/ml, and chloramphenicol, 20 μg/ml, for the E. coli DH10B strain and on medium containing erythromycin, 5 μg/ml, and chloramphenicol, 5 μg/ml, for the S. aureus RN4220 and L. lactis IL-1419 strains. The MICs of ofloxacin, ciprofloxacin, sparfloxacin, levofloxacin, and moxifloxacin were determined as described above.
RESULTS AND DISCUSSION
Characterization of the QnrE. faecalis protein.
The BLAST analysis of the complete genome of E. faecalis V583 revealed a homologue of the qnrA gene at locus EF0905 (positions 871,088 to 871,723). This gene putatively encoded a 211-amino-acid pentapeptide repeat protein that shared 25% identity and 40% similarity with that of QnrA. Homology was also found with the other Qnr proteins and with other proteins implicated in resistance to DNA gyrase inhibitors, such as McbG and MfpA (Table 2). No sequence characteristic of the presence of a mobile element was found in the close vicinity of the qnrE. faecalis gene in E. faecalis V583.
TABLE 2.
Percentage of identity and similarity between QnrE. faecalis and homologous proteins
| Protein | GenBank accession no. | % of protein identity (similarity) relative to QnrE. faecalisa
|
||||
|---|---|---|---|---|---|---|
| QnrB | QnrS | McbG | MfpA | QnrE. faecalis | ||
| QnrA | AAL60061 | 43 (59) | 59 (74) | 21 (40) | 24 (36) | 25 (40) |
| QnrB | ABC86904 | 44 (63) | 21 (43) | 25 (34) | 25 (39) | |
| QnrS | BAD88776 | 21 (35) | 25 (36) | 20 (36) | ||
| McbG | CAA30724 | 25 (42) | 23 (40) | |||
| MfpA | 2BM7_C | 22 (38) | ||||
Percentages of similarity are in parentheses.
The homologue of the qnrA gene, named qnrE. faecalis, was amplified from E. faecalis JH2-2 with specific primers and sequenced. The analysis of the deduced amino acid sequence revealed a putative protein that shared 99% identity with the E. faecalis V583 protein. Only two amino acids differed: the proline at position 40 of the E. faecalis V583 sequence was replaced by a serine in the E. faecalis JH2-2 sequence, and the valine at position 44 was replaced by an isoleucine.
The QnrE. faecalis protein had a calculated size of 24.25 kDa and could be organized in 42 pentapeptide repeats that formed two distinct domains of 9 and 33 pentapeptides each, separated by a single asparagine (Fig. 1). In this hypothetical configuration, 21% of the residues in the first position were alanine or cysteine, 20% in the second position were aspartate or asparagine, and 64% in the third position were leucine or phenylalanine. This observation is consistent with the description of the consensus sequence made by Tran and Jacoby (24): (A/C) (D/N) (L/F) X X, where X could be any amino acid. Moreover, 17% of the residues in the fourth position were serine or arginine, and 14% in the fifth position were glycine or arginine, which corresponds to the consensus sequence proposed later by Poirel et al. (19): (A/C) (D/N) (L/F) (S/R) (G/R). Taken together, these observations confirmed that the QnrE. faecalis protein belonged to the Qnr family. In addition, the C terminus of QnrE. faecalis presented more similarity than the N terminus with members of the pentapeptide repeat family, which was mentioned by Bateman et al. (2) as a general characteristic of this family.
FIG. 1.
Hypothetical structure of the QnrE. faecalis protein. The amino acid sequence of QnrE. faecalis was divided into pentapeptide repeats organized in two domains of 9 and 33 units each and connected by a single asparagine. The conserved amino acid residues according to the consensus sequence (A/C) (D/N) (L/F) (S/R) (G/R) are in bold, and the most characteristic pentapeptide units are underlined.
Among proteins that interact with DNA gyrase, MfpA is the only one that has been crystallized (7). Structure studies showed that this protein formed a right-handed beta-helical structure and displayed size, shape, and electrostatic similarities to DNA. This capacity to mimic DNA explains both the inhibitory effect of the protein on DNA gyrase and the fluoroquinolone resistance. A similar mechanism could be suspected for the Qnr proteins, including QnrE. faecalis.
Role of the qnrE. faecalis gene.
The chromosomal qnrE. faecalis gene was inactivated in the E. faecalis JH2-2 strain by homologous recombination using an internal fragment of this gene cloned into the pG1KT thermosensitive vector. The resistance of the transformants to kanamycin at a nonpermissive temperature was the indicator of the qnrE. faecalis gene inactivation. Gene inactivation resulted in a twofold decrease in MICs of ofloxacin and ciprofloxacin for E. faecalis JH2-2. This weak difference was repeatedly observed in three independent experiments and also at 42°C in the presence of kanamycin (500 μg/ml) incorporated into the agar to prevent spontaneous plasmid excision (Table 3). However, given the crossing-over technique that we used, we cannot fully exclude the occurrence of spontaneous excision of the plasmid in a subpopulation with an intact qnr gene that could lead to an underestimation of the effect of the qnr inactivation. After the loss of pG1KT by spontaneous excision and plasmid curing at a nonpermissive temperature, the MICs returned to their original values.
TABLE 3.
Activities of ofloxacin and ciprofloxacin for derivatives of E. faecalis JH2-2
| E. faecalis derivative | Characteristic | MIC (μg/ml)
|
|
|---|---|---|---|
| Ofloxacin | Ciprofloxacin | ||
| JH2-2 | Control | 3 | 1.5 |
| JH2-2ΩpG1KT | qnr inactivation | 1.5 | 0.75 |
| JH2-2ΔpG1KT | Spontaneous loss of pG1KT | 3 | 1.75 |
| JH2-2/pORI23 | Control | 3.5 | 2 |
| JH2-2ΩpG1KT/pORI23::qnr-cat | qnr complementation | 32 | 8 |
| JH2-2/pORI23::qnr-cat | qnr overexpression | 32 | 8 |
The complementation experiment was realized by cloning the qnrE. faecalis gene under the control of the strong promoter of pORI23 and by introducing the recombinant plasmid into the E. faecalis JH2-2 strain inactivated for this gene. A promoterless gene, cat, was cloned as a reporter just downstream from the qnrE. faecalis gene, allowing expression of chloramphenicol resistance by the recombinant strains. The MICs of ofloxacin and ciprofloxacin for the control E. faecalis JH2-2 strain containing the plasmid pORI23 alone were 3.5 μg/ml and 2 μg/ml, respectively. When the recombinant plasmid pORI23::qnr-cat was introduced in the JH2-2ΩpG1KT strain, the MICs of ofloxacin and ciprofloxacin increased to 32 and 8 μg/ml, respectively, which corresponds to a 4- to 9-fold increase (Table 3). The introduction of the recombinant plasmid pORI23::qnr-cat in E. faecalis JH2-2 led to a similar increase in MICs of fluoroquinolones (ofloxacin MIC of 32 μg/ml and ciprofloxacin MIC of 8 μg/ml). The multiplication factor of quinolone MICs was similar to that resulting from the expression of the qnrA gene in E. coli J53 (14). However, in this case, the MIC increase was not sufficient to confer resistance in E. coli (MIC of ciprofloxacin, 0.25 μg/ml), since the wild-type strain displayed low quinolone MICs. Although the role of QnrA alone seemed to be marginal to conferring resistance, it can supplement resistance due to mutated quinolone target enzymes, efflux pump activation, or deficiencies in outer-membrane porin channels (13) and facilitate selection of higher resistance by mutation (22). In the case of E. faecalis JH2-2, the overexpression of the qnrE. faecalis gene was sufficient to confer resistance to ciprofloxacin and ofloxacin.
Overall, these experiments indicate a role for the qnrE. faecalis gene in the intrinsic resistance of E. faecalis JH2-2 to fluoroquinolones.
Protection of DNA gyrase by the QnrE. faecalis protein.
The qnrE. faecalis gene was inserted into the pET28a+ expression vector, adding a C-terminal His6 tag that allowed purification of the complex with nickel nitriloacetate columns. The 25-kDa QnrE. faecalis protein was purified to electrophoretic homogeneity using 325 mM imidazole elutions (data not shown). Protein concentration was measured at 1.9 μM in the third elution fraction and at 0.9 μM in the fourth elution fraction.
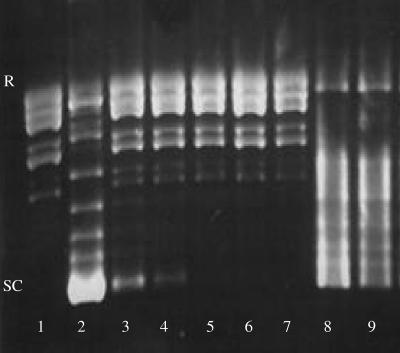
The supercoiling activity of DNA gyrase was evaluated by its ability to form supercoiled isomers from relaxed pBR322 DNA. A series of topoisomers was visualized by agarose gel electrophoresis, with the most supercoiled isomer having the greatest mobility (Fig. 2, lanes 1 and 2). The presence of ofloxacin in the reaction mixture inhibited DNA gyrase supercoiling activity: supercoiled isomers disappeared proportionally to ofloxacin concentration (Fig. 2, lanes 3 to 7). Surprisingly, the electrophoretic pattern of supercoiled isomers was different when DNA gyrase was incubated with the QnrE. faecalis protein, suggesting that the enzyme activity might be modified by the presence of the Qnr protein (Fig. 2, lane 8). When a 0.9 μM QnrE. faecalis-His6 tag was preincubated with DNA gyrase and then 1 μg/ml ofloxacin added, the migration profile was similar to that with Qnr alone, showing that ofloxacin no longer inhibited supercoiling (Fig. 2, lane 9). This effect was not observed when ofloxacin was replaced by buffer or in the absence of a preincubation step (data not shown). In conclusion, the effect of QnrE. faecalis on quinolone susceptibility could be explained by a mechanism of DNA gyrase protection similar to that proposed for QnrA.
FIG. 2.
QnrE. faecalis protein protects E. coli DNA gyrase from inhibition by ofloxacin. Reaction mixtures contained 0.5 μg of pBR322 (lanes 1 to 9), 1 U of E. coli DNA gyrase (lanes 2 to 9), ofloxacin (lane 3 contained 0.25 μg/ml; lane 4, 0.5 μg/ml; lane 5, 1 μg/ml; lane 6, 5 μg/ml; lane 7, 10 μg/ml; and lane 9, 1 μg/ml), and a QnrE. faecalis-His6 tag (lanes 8 and 9, 0.9 μM). SC, supercoiled form; R, relaxed form.
Heterologous expression of the qnrE. faecalis gene.
The effect of qnrE. faecalis gene expression on fluoroquinolones MICs was evaluated in various genetic backgrounds, using two gram-positive organisms, S. aureus and L. lactis, and one gram-negative organism, E. coli, by introducing the recombinant plasmid pORI23::qnr-cat into electrocompetent cells. The control strains contained pORI23 alone. The expression of the qnrE. faecalis gene in S. aureus RN4220 led to fourfold increases in the MICs of ofloxacin, ciprofloxacin, levofloxacin, and moxifloxacin and an eightfold increase for sparfloxacin, compared to that of the control strain S. aureus RN4220/pORI23 (Table 4). The effects were similar in L. lactis IL-1419: fourfold increases occurred for the different fluoroquinolones, except for an eightfold increase for sparfloxacin. In E. coli DH10B, for which the fluoroquinolones MICs are very low (from 0.002 to 0.008 μg/ml), the expression of the qnrE. faecalis gene also led to marked increases in MICs, 8-fold for ofloxacin, levofloxacin, and moxifloxacin and 16-fold for ciprofloxacin and sparfloxacin, compared to that of the control strain E. coli DH10B/pORI23. Therefore, the effect of the QnrE. faecalis protein on quinolone susceptibility was not confined to the original E. faecalis species but extended to other studied gram-positive and gram-negative organisms. Therefore, mobilization of the gene to other bacterial genera might be an efficient way for the acquisition of quinolone resistance, as previously reported for the naturally occurring qnrA gene of Shewanella algae, which was the ancestor of the plasmid-borne qnrA gene in enterobacteria (20).
TABLE 4.
Heterologous expression of qnrE. faecalis
| Strain | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|
| OFX | CIP | LVX | SPX | MOX | |
| Staphylococcus aureus RN4220 | 0.5 | 0.5 | 0.25 | 0.125 | 0.064 |
| S. aureus RN4220/pORI23 | 0.5 | 0.5 | 0.25 | 0.125 | 0.064 |
| S. aureus RN4220/pORI23::qnr-cat | 2 | 2 | 1 | 1 | 0.25 |
| Lactococcus lactis IL-1419 | 1 | 2 | 0.5 | 0.5 | 0.25 |
| L. lactis IL-1419/pORI23 | 1 | 2 | 0.5 | 0.5 | 0.25 |
| L. lactis IL-1419/pORI23::qnr-cat | 4 | 8 | 2 | 4 | 1 |
| Escherichia coli DH10B | 0.008 | 0.002 | 0.008 | 0.002 | 0.002 |
| E. coli DH10B/pORI23 | 0.016 | 0.002 | 0.008 | 0.004 | 0.002 |
| E. coli DH10B/pORI23::qnr-cat | 0.125 | 0.032 | 0.125 | 0.064 | 0.032 |
OFX, ofloxacin; CIP, ciprofloxacin; LVX, levofloxacin; SPX, sparfloxacin; MOX, moxifloxacin.
Acknowledgments
We thank Alexandra Aubry for help with DNA gyrase assays.
We also thank the Fondation pour la Recherche Médicale for financial support.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Aubry, A., X. S. Pan, L. M. Fisher, V. Jarlier, and E. Cambau. 2004. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob. Agents Chemother. 48:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman, A., A. G. Murzin, and S. A. Teichmann. 1998. Structure and distribution of pentapeptide repeats in bacteria. Protein Sci. 7:1477-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruckner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. Mol. Microbiol. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferrero, L., B. Cameron, B. Manse, D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 13:641-653. [DOI] [PubMed] [Google Scholar]
- 5.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heddle, J. G., S. J. Blance, D. B. Zamble, F. Hollfelder, D. A. Miller, L. M. Wentzell, C. T. Walsh, and A. Maxwell. 2001. The antibiotic microcin B17 is a DNA gyrase poison: characterisation of the mode of inhibition. J. Mol. Biol. 307:1223-1234. [DOI] [PubMed] [Google Scholar]
- 7.Hegde, S. S., M. W. Vetting, S. L. Roderick, L. A. Mitchenall, A. Maxwell, H. E. Takiff, and J. S. Blanchard. 2005. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 308:1480-1483. [DOI] [PubMed] [Google Scholar]
- 8.Hoogkamp-Korstanje, J. A. 1997. In-vitro activities of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J. Antimicrob. Chemother. 40:427-431. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanematsu, E., T. Deguchi, M. Yasuda, T. Kawamura, Y. Nishino, and Y. Kawada. 1998. Alterations in the GyrA subunit of DNA gyrase and the ParC subunit of DNA topoisomerase IV associated with quinolone resistance in Enterococcus faecalis. Antimicrob. Agents Chemother. 42:433-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korten, V., W. M. Huang, and B. E. Murray. 1994. Analysis by PCR and direct DNA sequencing of gyrA mutations associated with fluoroquinolone resistance in Enterococcus faecalis. Antimicrob. Agents Chemother. 38:2091-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalioui, L., E. Pellegrini, S. Dramsi, M. Baptista, N. Bourgeois, F. Doucet-Populaire, C. Rusniok, M. Zouine, P. Glaser, F. Kunst, C. Poyart, and P. Trieu-Cuot. 2005. The SrtA sortase of Streptococcus agalactiae is required for cell wall anchoring of proteins containing the LPXTG motif, for adhesion to epithelial cells, and for colonization of the mouse intestine. Infect. Immun. 73:3342-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Martínez, L., A. Pascual, I. Garcia, J. Tran, and G. A. Jacoby. 2003. Interaction of plasmid and host quinolone resistance. J. Antimicrob. Chemother. 51:1037-1039. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Martínez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 15.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oyamada, Y., H. Ito, K. Fujimoto, R. Asada, T. Niga, R. Okamoto, M. Inoue, and J. Yamagishi. 2006. Combination of known and unknown mechanisms confers high-level resistance to fluoroquinolones in Enterococcus faecium. J. Med. Microbiol. 55:729-736. [DOI] [PubMed] [Google Scholar]
- 17.Pan, X. S., and L. M. Fisher. 1999. Streptococcus pneumoniae DNA gyrase and topoisomerase IV: overexpression, purification, and differential inhibition by fluoroquinolones. Antimicrob. Agents Chemother. 43:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 19.Poirel, L., A. Liard, J. M. Rodriguez-Martinez, and P. Nordmann. 2005. Vibrionaceae as a possible source of qnr-like quinolone resistance determinants. J. Antimicrob. Chemother. 56:1118-1121. [DOI] [PubMed] [Google Scholar]
- 20.Poirel, L., J. M. Rodriguez-Martinez, H. Mammeri, A. Liard, and P. Nordmann. 2005. Origin of plasmid-mediated quinolone resistance determinant qnrA. Antimicrob. Agents Chemother. 49:3523-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Que, Y. A., J. A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Martinez, J. M., C. Velasco, A. Pascual, I. Garcia, and L. Martinez-Martinez. 2006. Correlation of quinolone resistance levels and differences in basal and quinolone-induced expression from three qnrA-containing plasmids. Clin. Microbiol. Infect. 12:440-445. [DOI] [PubMed] [Google Scholar]
- 23.Tankovic, J., F. Mahjoubi, P. Courvalin, J. Duval, and R. Leclercq. 1996. Development of fluoroquinolone resistance in Enterococcus faecalis and role of mutations in the DNA gyrase gyrA gene. Antimicrob. Agents Chemother. 40:2558-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran, J. H., and G. A. Jacoby. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. USA 99:5638-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 49:3050-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamble, D. B., D. A. Miller, J. G. Heddle, A. Maxwell, C. T. Walsh, and F. Hollfelder. 2001. In vitro characterization of DNA gyrase inhibition by microcin B17 analogs with altered bisheterocyclic sites. Proc. Natl. Acad. Sci. USA 98:7712-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]