Abstract
Doxycycline generally alleviates clinical monocytic ehrlichiosis, but its efficacy in the control of monocytotropic ehrlichial pathogens requires further investigation. In this study, Ehrlichia canis was detected in dogs treated with doxycycline for 14 days and in ticks fed on these dogs, suggesting that treated dogs can remain reservoirs for E. canis.
Canine monocytic ehrlichiosis (CME) is a cosmopolitan disease of dogs that is caused by the tick-borne rickettsia, Ehrlichia canis (20). Ehrlichia spp., like the closely related genus Anaplasma, are biologically transmitted among vertebrates by ticks of the family Ixodidae (8), and Rhipicephalus sanguineus, the “brown dog tick,” is considered the primary vector of E. canis. In addition to its global importance to canine health, this vector-pathogen-vertebrate interaction provides useful transmission and disease models for zoonotic tick-borne bacteria, the majority of which naturally infect dogs.
CME occurs in acute, subclinical, and chronic phases (9, 13, 16, 18, 23). Acute experimental CME often begins approximately 10 days postinfection (dpi) with E. canis and can involve anemia, anorexia, ataxia, conjunctivitis, depression, fever, pancytopenia, ocular discharge, and vomiting, with clinical signs subsiding 20 to 30 dpi and usually followed by a long-term subclinical phase (1, 3, 12, 21). Long-term mild or severe chronic phases of CME can also occur, with recurrent clinical and hematologic signs that include pancytopenia, hemorrhage, monocytosis, lymphocytosis, and weight loss (9).
The tetracyclines are the drugs of choice for the treatment of rickettsial diseases. Oral tetracycline and injectable oxytetracycline both are effective treatments for CME (6, 7, 17), and oral doxycycline was reportedly associated with the lowest incidence of disease recrudescence following treatment (6). Although doxycycline alleviates clinical CME, there are questions regarding the efficacy of this antibiotic for elimination of E. canis. Some reports suggested persistence of infection following doxycycline regimens of naturally and experimentally infected dogs during postacute CME (2, 11, 14, 22). Other reports suggested E. canis clearance after doxycycline treatments of dogs during acute CME (4, 10). Furthermore, the relevance of E. canis persistence after acute CME is questioned due to an unsuccessful attempt to infect R. sanguineus on dogs during subclinical CME (15). However, the utility of antibiotic treatments to minimize the possibility of E. canis transmission to invertebrate hosts should be confirmed.
The objective of this study was to determine if doxycycline treatment would reduce E. canis infection levels sufficiently to prevent acquisition of the pathogen by R. sanguineus nymphs. Four Beagle dogs were experimentally infected with E. canis (Ebony isolate) by tick transmission that was demonstrated in a previous study (5). All of the dogs tested negative by PCR and immunofluorescence assays prior to tick feeding, and transmission was confirmed by seroconversion, mild clinical signs, and PCR-positive buffy coats after feeding PCR-positive ticks on these dogs (5). The dog referred to as AXM, which was not exposed to E. canis, served as a negative control for feeding cohorts of the ticks used in this study (5). All of the dogs were cared for in accordance with a protocol on file with The Ohio State University Institutional Laboratory Animal Care and Use Committee. Canine blood (5 ml) was collected with EDTA, and buffy coats were separated as previously described (19). Buffy coats and ticks were incubated in protein digestion buffer and subjected to phenol-chloroform extraction and ethanol precipitation as reported by Bremer et al. (5), except the digestion buffer contained 2% sodium dodecyl sulfate. PCR assays with primers ECA30-384S and ECA30-583A were exactly as previously described (5).
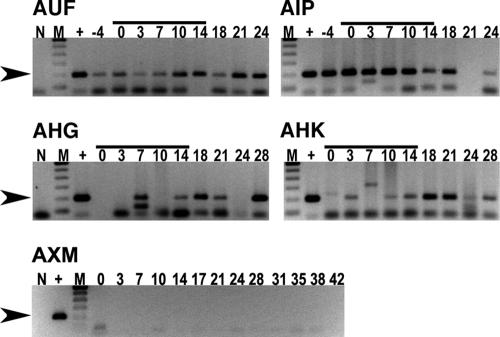
Doxycycline hyclate tablets (100 mg) were administered orally for 14 days (once per day at approximately 10 mg/kg). To the best of our knowledge, this was the first time these dogs received this antibiotic. The numbers of days post-tick attachment in the original study to the first day of doxycycline treatment (day 0 in this study) were 55, 76, 83, and 104 for dogs AHK, AHG, AIP, and AUF, respectively. Semiweekly blood collections were evaluated with the PCR assay through the course of treatment. All of the dogs tested PCR positive for E. canis during doxycycline treatment (Fig. 1), and dogs AIP, AHG, AUF, and AHK tested PCR positive on 3, 4, 4, and 5 posttreatment sample dates, respectively. Dog AXM, which was neither exposed to E. canis nor treated with doxycycline, was PCR negative on each date tested.
FIG. 1.
Detection of E. canis in canine blood after doxycycline treatment. An E. canis-specific PCR assay was used to test buffy coat samples collected from subclinical experimentally infected dogs through the course of doxycycline therapy as described in the text. For each row, no-template reactions (N) served as contamination controls, template DNA (100 pg) collected from E. canis-infected DH82 cells served as a positive control (+), and the numbers 0 to 28 indicate days after initiation of doxycycline therapy. The molecular size standard (M) is a 100-bp ladder (Invitrogen). Doxycycline treatment was initiated after blood collection on day 0 and continued through day 14, which is indicated by the solid bars. Lanes containing the 200-bp amplicon (arrowhead) were considered PCR positive. Dog AXM served as the specific-pathogen-free control, with the numbers 0 to 42 representing days after attachment of uninfected nymphs that were cohorts of those allowed to acquisition feed on the dogs infected with E. canis.
For xenodiagnosis, approximately 200 R. sanguineus nymphs were placed on each dog 7 days after treatment and allowed to feed to repletion and to molt into adults (5, 19). Cohorts of these nymphs were fed on untreated dogs and an uninfected dog as positive and negative controls, respectively. In the first phase of this experiment, nymphs were fed on dogs AHG and AHK after treatment. Equivalent numbers (5 of 10) of resultant male ticks tested PCR positive for E. canis from each dog (Fig. 2A). Nymphs were also fed on dogs AIP and AUF prior to treatment, and additional nymphs were fed on the same dogs after the same doxycycline regimen used in the first experiment. Prior to treatment, 4 and 5 of 10 ticks tested PCR positive for dogs AIP and AUF, respectively, while 10 and 5 of 10 ticks were PCR positive from AIP and AUF, respectively, after treatment (Fig. 2B). Ticks fed on AXM, the negative control, were PCR negative (Fig. 2C).
FIG. 2.
Detection of E. canis in transstadially exposed male R. sanguineus ticks. Nymphal ticks were allowed to feed to repletion on infected dogs before or after doxycycline treatment and allowed to molt into adults, and male ticks were assayed for the presence of E. canis DNA. No-template reactions (N) served as contamination controls, template DNA (100 pg) collected from E. canis-infected DH82 cells served as a positive control (+), and the numbers 1 to 10 represent individual ticks. The molecular size standard (M) is a 100-bp ladder. (A) Nymphs were placed on dogs AHG and AHK 7 days after the treatment ended (day 21). (B) Nymphs were allowed to feed on dogs AIP and AUF prior to doxycycline therapy (pretreatment), and more nymphs were placed on the same dogs 7 days after the treatments ended (posttreatment). (C) Ticks allowed to feed on the uninfected canine control, AXM.
These results confirmed that this doxycycline regimen did not clear E. canis from these dogs and that R. sanguineus nymphs acquired infections that persisted through their molting period. Earlier investigations also suggested persistent infections following doxycycline treatment at similar doses during subclinical CME. Iqbal and Rikihisa (14) reported reisolation of E. canis from three of five experimentally infected dogs with subclinical CME that were treated for 7 days. Harrus et al. (11) reported persistent E. canis infection in one of six dogs after a 42-day regimen. Conversely, other studies suggested clearance of E. canis after doxycycline treatment during acute CME. Breitschwerdt et al. (4) reported successful clearance of infection from eight of eight dogs with acute experimental CME after 14 days of treatment, while Harrus et al. (10) reported successful clearance of infection from five of five dogs after 16 of 60 days of treatment. Taken together, the above-mentioned investigations suggest that the phase of CME could affect the efficacy of doxycycline in clearing E. canis infections.
The current study was unique in that ticks were used to infect and to detect infection of their canine hosts. To our knowledge, this is the first report of tick acquisition (xenodiagnosis) as a means of assessing infection status following antibiotic treatment. Dogs with postacute, subclinical CME were used, because our objective was to determine whether such dogs could be a source of infection for other hosts. In a clinical setting, treatment of dogs undergoing acute or severe chronic forms of CME was more likely in the past, but recent widespread use of serologic tests for exposure to E. canis could lead to treatment of dogs with subclinical or mild, postacute CME. Therefore, further work is warranted to evaluate the efficacy of alternative regimens for elimination of E. canis during different phases of CME and in hosts experimentally infected by different means (e.g., needle inoculation and tick feeding). Vector xenodiagnosis is also a valuable parameter regarding the efficacy of such regimens to interrupt infectious cycles of E. canis and, importantly, zoonotic rickettsial pathogens that infect dogs.
Acknowledgments
We thank Kirsten Boughan for technical assistance during this study. R. sanguineus nymphs were purchased from the Oklahoma State University Medical Entomology Laboratory.
This work was supported by NIH grant AI47932.
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Baneth, G., T. Waner, A. Koplah, S. Weinstein, and A. Keysary. 1996. Survey of Ehrlichia canis antibodies among dogs in Israel. Vet. Rec. 138:257-259. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch, R. C., and R. T. Greene. 1996. Post-therapy antibody titers in dogs with ehrlichiosis: follow-up study on 68 patients treated primarily with tetracycline and/or doxycycline. J. Vet. Intern. Med. 10:271-274. [DOI] [PubMed] [Google Scholar]
- 3.Botros, B. A., M. S. Elmolla, A. W. Salib, C. A. Calamaio, G. A. Dasch, and R. R. Arthur. 1995. Canine ehrlichiosis in Egypt: sero-epidemiological survey. Onderstepoort J. Vet. Res. 62:41-43. [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 42:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer, W. G., J. J. Schaefer, E. R. Wagner, S. A. Ewing, Y. Rikihisa, G. R. Needham, S. Jittapalapong, D. L. Moore, and R. W. Stich. 2005. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet. Parasitol. 131:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codner, E. C., and L. L. Farris-Smith. 1986. Characterization of the subclinical phase of ehrlichiosis in dogs. J. Am. Vet. Med. Assoc. 189:47-50. [PubMed] [Google Scholar]
- 7.Davidson, D. E., Jr., G. S. Dill, Jr., M. Tingpalapong, S. Premabutra, P. L. Nguen, E. H. Stephenson, and M. Ristic. 1978. Prophylactic and therapeutic use of tetracycline during an epizootic of ehrlichiosis among military dogs. J. Am. Vet. Med. Assoc. 172:697-700. [PubMed] [Google Scholar]
- 8.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 9.Ewing, S. A. 1969. Canine ehrlichiosis. Adv. Vet. Sci. Comp. Med. 13:331-353. [PubMed] [Google Scholar]
- 10.Harrus, S., M. Kenny, L. Miara, I. Aizenberg, T. Waner, and S. Shaw. 2004. Comparison of simultaneous splenic sample PCR with blood sample PCR for diagnosis and treatment of experimental Ehrlichia canis infection. Antimicrob. Agents Chemother. 48:4488-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrus, S., T. Waner, I. Aizenberg, and H. Bark. 1998. Therapeutic effect of doxycycline in experimental subclinical canine monocytic ehrlichiosis: evaluation of a 6-week course. J. Clin. Microbiol. 36:2140-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrus, S., T. Waner, I. Aizenberg, J. E. Foley, A. M. Poland, and H. Bark. 1998. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J. Clin. Microbiol. 36:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrus, S., T. Waner, H. Bark, F. Jongejan, and A. W. Cornelissen. 1999. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J. Clin. Microbiol. 37:2745-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal, Z., and Y. Rikihisa. 1994. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J. Clin. Microbiol. 32:1644-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis, G. E., Jr., M. Ristic, R. D. Smith, T. Lincoln, and E. H. Stephenson. 1977. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am. J. Vet. Res. 38:1953-1955. [PubMed] [Google Scholar]
- 16.Neer, T. M., and S. Harrus. 2006. Canine monocytotropic ehrlichiosis and neorickettsiosis (E. canis, E. chaffeensis, E. ruminantium, N. sennetsu, and N. risticii infections), p. 203-216. In C. E. Greene (ed.), Infectious diseases of the dog and cat, 3rd ed. Saunders Elsevier, St. Louis, MO.
- 17.Ogunkoya, A. B., J. B. Adeyanju, and R. Abduhllahi. 1985. Experimental and clinical trials of long acting oxytetracycline in the treatment of canine ehrlichiosis. Vet. Q. 7:158-161. [DOI] [PubMed] [Google Scholar]
- 18.Skotarczak, B. 2003. Canine ehrlichiosis. Ann. Agric. Environ. Med. 10:137-141. [PubMed] [Google Scholar]
- 19.Stich, R. W., Y. Rikihisa, S. A. Ewing, G. R. Needham, D. L. Grover, and S. Jittapalapong. 2002. Detection of Ehrlichia canis in canine carrier blood and in individual experimentally infected ticks with a p30-based PCR assay. J. Clin. Microbiol. 40:540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stich, R. W., J. J. Schaefer, W. G. Bremer, G. R. Needham, and S. Jittapalapong. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen, Ehrlichia canis. Vet. Parasitol, in press. [DOI] [PMC free article] [PubMed]
- 21.Waner, T., S. Harrus, H. Bark, E. Bogin, Y. Avidar, and A. Keysary. 1997. Characterization of the subclinical phase of canine ehrlichiosis in experimentally infected beagle dogs. Vet. Parasitol. 69:307-317. [DOI] [PubMed] [Google Scholar]
- 22.Wen, B., Y. Rikihisa, J. M. Mott, R. Greene, H. Y. Kim, N. Zhi, G. C. Couto, A. Unver, and R. Bartsch. 1997. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 35:1852-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woody, B. J., and J. D. Hoskins. 1991. Ehrlichial diseases of dogs. Vet. Clin. N. Am. Small Anim. Pract. 21:75-98. [DOI] [PubMed] [Google Scholar]