Abstract
Homodimerization of histatin-derived peptides generally led to improved bactericidal activity against Staphylococcus aureus in vitro. In vivo, monomers and dimers were equally active in killing bacteria in mice with a soft tissue infection. Altogether, these peptides are promising compounds for the development of novel therapeutics against infections with drug-resistant bacteria.
The rapid emergence of microbial resistance makes the development of novel antibiotic agents urgent. Antimicrobial peptides, characterized by a positive charge and amphipathic structure, are considered promising candidates, as they display high therapeutic indices in vitro (16). One putative active domain of histatin 5 (Dh5: residues 11 to 24) was used as scaffold for the design of peptides Dhvar4 (increased amphipathicity) and Dhvar5 (reduced amphipathicity) with improved potency in vitro and improved resistance to proteolytic degradation (14, 5, 10, 4). P-113 (residues 4 to 15 of histatin 5) is active against several clinically important pathogens in vitro and in vivo in animals (7, 9, 2, 3).
In this study we examined the effect of dimerization of Dh5, Dhvar4, Dhvar5, and P-113 on the antimicrobial potency in vitro and in mice with Staphylococcus aureus infections. The rationale behind using homodimers of antimicrobial peptides is that multiple peptides line up together in a dose-dependent fashion so that dimerization would lead to an increase in reaction rates and thus to a significant potentiation (11). Peptides and their homodimers were synthesized as described previously (12, 15) and are listed in Table 1. S. aureus ATCC 25923 was obtained from the American Type Culture Collection (Rockville, MD). S. aureus strain 2141 (multidrug-resistant S. aureus [MRSA]) is a multidrug-resistant clinical isolate (1). In the in vitro assay, 1 × 106/ml S. aureus organisms in phosphate buffer supplemented with 1% (wt/vol) tryptic soybean broth (Difco, Becton Dickinson Microbiology Systems, Sparks, MD) were exposed to various amounts of peptides for 2 h at 37°C, and the number of viable microorganisms was assessed microbiologically using DST plates as described by Welling et al. (18). Data are expressed as 50% lethal concentrations (LC50), i.e., in vitro as the concentration required reducing the viable bacteria by 50% compared to incubations without peptide. Histatin 5, Dh5, P-113, Dhvar4, and Dhvar5 killed both S. aureus and MRSA. Histatin 5 and Dhvar4 were the most potent peptides (Table 2). The dimers of Dh5, Dhvar4, and Dhvar5 were 4, 11, and 128 times as potent as the respective monomers against S. aureus and 14, 8, and 43 times as potent against MRSA. For P-113, dimerization led to a decrease in bactericidal potency.
TABLE 1.
Characteristics of peptides used in this study
| Peptide | Sequence | Mass (Da) | <μ>a | <H>b | Net charge |
|---|---|---|---|---|---|
| Histatin 5 | DSHAKRHHGYKRKFHEKHHSHRGY | 3,036 | 0.09 | −0.55 | +12 |
| Dh5 | KRKFHEKHHSHRGY | 1,847 | 0.15 | −0.61 | +8 |
| Dh5 dimer | α,ε-(KRKFHEKHHSHRGY)2K-amide | 3,803 | −0.61 | +18 | |
| Dhar4 | KRLFKKLLFSLRKY | 1,840 | 0.44 | −0.34 | +6 |
| Dhvar4 dimer | α,ε-(KRLFKKLLFSLRKY)2K-amide | 3,789 | −0.35 | +14 | |
| Dhvar5 | LLLFLLKKRKKRKY | 1,847 | 0.06 | −0.41 | +7 |
| Dhvar5 dimer | α,ε-(LLLFLLKKRKKRKY)2K-amide | 3,803 | −0.42 | +16 | |
| P-113 | AKRHHGYKRKFH | 1,565 | 0.22 | −0.58 | +8 |
| P-113 dimer | α,ε-(AKRHHGYKRKFH)2K-amide | 3,239 | −0.58 | +18 |
Mean hydrophobic moments, a measure for amphipathicity, calculated assuming α-helical conformation of the peptides.
Mean hydrophobicity. For the dimers, the hydrophobicity of the lysine-amide spacer was considered similar to that of glutamine (−0.69) or asparagine (−0.64), which resulted in values that did not differ significantly from those of the respective monomers.
TABLE 2.
Bactericidal activities of the peptides for S. aureus and MRSA
| Peptide | LC50a
|
|||
|---|---|---|---|---|
| In vitro
|
In vivo
|
|||
| S. aureus | MRSA | S. aureus | MRSA | |
| Histatin 5 | 8.3 | 2.8 | 1.0 | 3.5 |
| Dh5 | 35.0 | 112.5 | 2.0 | 1.0 |
| Dh5 dimer | 8.4 | 8.2 | 1.5 | 2.0 |
| Dhvar4 | 4.2 | 3.1 | 1.0 | 1.5 |
| Dhvar4 dimer | 0.5 | 0.4 | 1.0 | 1.0 |
| Dhvar5 | 51.0 | 17.3 | 2.0 | 3.0 |
| Dhvar5 dimer | 0.4 | 0.4 | 5.0 | 2.0 |
| P-113 | 12.5 | 18.6 | ND | 3.0 |
| P-113 dimer | 49.8 | 38.9 | ND | 3.0 |
Concentration required to reduce viable counts by 50% compared to incubations without peptide (in vitro) (μM) or to reduce viable counts by 50% compared to untreated mice (in vivo) (μmol/g of infected tissue). In vitro results are means from at least three sets of independent experiments; in vivo results are means from at least three independent animals per peptide concentration.
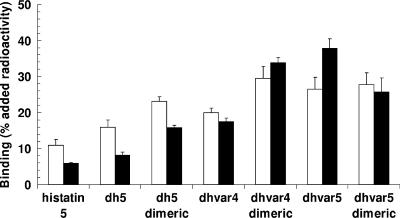
Binding of equal amounts of 99mTc-labeled peptides to bacteria was assessed at 4°C as described previously (18). In short, labeled peptide was incubated with 2 × 107 viable bacteria for 1 h, and the radioactivity bound to the bacterial pellet was determined. Data are expressed as the percentage of total added 99mTc activity/2 × 107 CFU. With exception of Dhvar5, the dimers of all peptides showed significantly (P < 0.01) higher binding than their respective monomers (Fig. 1). Notably, for this peptide the effect of dimerization on the bactericidal effect was the most pronounced. In the in vivo studies, performed in compliance with the Dutch laws related to the conduct of animal experiments, pathogen-free male Swiss mice (22 to 30 g; Broekman Institute, Someren, The Netherlands) were infected with 0.1 ml saline containing 0.5 to 2.0 × 107 CFU (18). After 18 h, animals were given intravenous injections of various amounts of peptide. The animals were kept for another 24 h and sacrificed, and the number of viable bacteria was determined microbiologically (1). Histatin 5, Dh5, P-113, Dhvar4, and Dhvar5 killed both strains of bacteria, but the range of bactericidal potencies was less pronounced than that determined in vitro. Histatin 5 and Dhvar4 stood out as more potent than the other peptides. In contrast with the in vitro experiments, dimerization had little if any effect on the bactericidal activity in vivo (Table 2). The high in vivo activity is surprising. In a pilot experiment in vitro using phosphate-buffered saline instead of a low-phosphate buffer to better simulate physiological conditions, we found that the activities of all peptides were virtually abolished at higher ionic strengths. Furthermore, in vivo these bacteria exist as multicellular complexes (biofilms), in which form they are far more resistant to antimicrobial peptides than the cultured monocellular planktonic bacteria used in the in vitro assays (8). A possible explanation is that the activity of this kind of peptide in vivo is not a reflection of their cytolytic activity expressed in vitro but the result of their immunomodulatory properties (17, 13, 6). Apparently, these are not influenced by dimerization.
FIG. 1.
In vitro binding of 99mTc-labeled histatins to S. aureus (open bars) and MRSA (closed bars). Values are expressed as percentages (means plus standard errors of the means) of the radioactivity in the pellet containing bacteria (2 × 107 CFU) compared to the total added radioactivity.
Scintigraphic imaging after intravenous injection of mice with technetium-99m-radiolabeled peptides (2 to 5 MBq) revealed that the peptides were rapidly removed (biological half-life between 9 to 39 min) from the circulation (19, 18). Most of the radioactivity accumulated initially in the kidneys, after which it collected in the urinary bladder (Table 3). It was observed at later intervals that radioactivity that had accumulated in the liver and spleen also rapidly decreased (data not shown). Only a small amount (0.5 to 0.9% of the injected dose) accumulated at the infected thigh muscles.
TABLE 3.
Biodistribution of the radiolabeled peptides in vivo
| Peptide | Half-life (min) | % of injected dose (mean ± SD) in:
|
||||
|---|---|---|---|---|---|---|
| Urinary bladder | Kidneys | Spleen | Liver | Infected muscle | ||
| Histatin 5 | 9.7 ± 4.7 | 25.9 ± 7.3 | 23.8 ± 4.8 | 1.4 ± 0.9 | 8.7 ± 5.7 | 0.5 ± 0.2 |
| Dh5 | 17.7 ± 4.3 | 30.7 ± 7.5 | 21.0 ± 4.1 | 2.1 ± 0.6 | 9.4 ± 1.9 | 0.4 ± 0.1 |
| Dh5 dimer | 8.6 ± 2.6 | 26.8 ± 2.3 | 10.5 ± 0.8 | 3.2 ± 0.1 | 9.0 ± 0.7 | 0.5 ± 0.02 |
| Dhvar4 | 28.6 ± 5.7 | 23.8 ± 6.1 | 24.9 ± 4.2 | 3.1 ± 1.5 | 10.7 ± 2.1 | 0.5 ± 0.1 |
| Dhvar4 dimer | 34.0 ± 4.7 | 17.0 ± 0.2 | 29.1 ± 4.4 | 4.0 ± 1.2 | 19.9 ± 0.4 | 0.8 ± 0.04 |
| Dhvar5 | 38.8 ± 4.1 | 14.6 ± 4.8 | 26.0 ± 5.8 | 10.8 ± 2.7 | 12.8 ± 4.2 | 0.9 ± 0.2 |
| Dhvar5 dimer | 23.2 ± 6.7 | 18.8 ± 1.8 | 28.3 ± 7.6 | 3.1 ± 0.5 | 16.7 ± 5.4 | 0.6 ± 0.1 |
| P-113 | ND | ND | ND | ND | ND | ND |
| P-113 dimer | ND | ND | ND | ND | ND | ND |
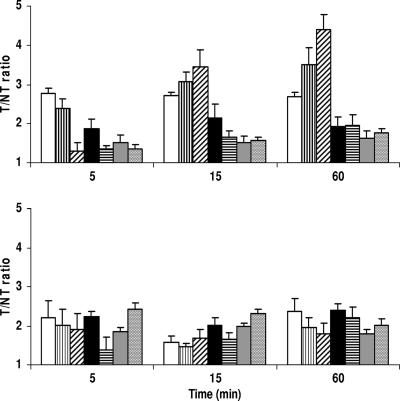
The accumulation of peptides varied, with ratios of accumulation in infected thigh muscle (target [T]) to accumulation in the contralateral noninfected thigh muscle (nontarget [NT]) between 1.6 and 4.4 for mice infected with S. aureus (Fig. 2). The highest T/NT ratios were calculated for monomeric (3.5 ± 0.4) and dimeric (4.4 ± 0.4) peptides of Dh5. For mice infected with MRSA, T/NT ratios for all peptides ranged between 1.8 and 2.4 (Fig. 2). We do not have a conclusive explanation for the differences in T/NT ratios observed for radiolabeled Dh5 and its dimer between S. aureus and MRSA.
FIG. 2.
Accumulation of 99mTc-labeled histatin peptides (open bars, histatin 5; vertically striped bars, Dh5; diagonally striped bars, dimeric Dh5; black bars, Dhvar4; horizontally striped bars, dimeric Dhvar4; gray bars, Dhvar5; stippled bars, dimeric Dhvar5) in thigh muscles of mice infected with S. aureus (top) or MRSA (bottom). T/NT ratios were determined at the indicated intervals as described in the text.
Apparently, there is no clear correlation between bacterial binding, ionic strength sensitivity, and bactericidal activity in vitro on the one hand and biodistribution data, clearance kinetics, and bactericidal activity in vivo on the other. It is evident that extrapolation of in vitro findings to the observations in animals is extremely difficult. Peptide properties determined in vitro, such as pore-forming and membrane-disrupting properties, secondary structure, and state of aggregation, may be different in vivo. The presence of many potentially synergistic compounds and an active immune system in live animals is another complicating factor. Considering that potentially antimicrobial peptides act in vivo through other molecular mechanisms than in vitro, we think that a critical reappraisal of the predictive value of data obtained in vitro for actual activity in vivo may be imperative. Despite the uncertainty as to how these peptides exert their potent antimicrobial activity in vivo, our data clearly show that they may be considered as promising new therapeutics to fight infections with resistant bacteria.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Brouwer, C. P. J. M., S. J. P. Bogaards, M. Wulferink, M. P. Velders, and M. M. Welling. 2006. Synthetic peptides derived from human antimicrobial peptide ubiquicidin accumulate at sites of infections and eradicate (multi-drug resistant) Staphylococcus aureus in mice. Peptides 27:2585-2591. [DOI] [PubMed] [Google Scholar]
- 2.Cirioni, O., A. Giacometti, R. Ghiselli, F. Orlando, W. Kamysz, G. D'Amato, F. Mocchegiani, J. Lukasiak, C. Silvestri, V. Saba, and G. Scalise. 2004. Potential therapeutic role of histatin derivative P-113D in experimental rat models of Pseudomonas aeruginosa sepsis. J. Infect. Dis. 190:356-364. [DOI] [PubMed] [Google Scholar]
- 3.Giacometti, A., O. Cirioni, W. Kamysz, G. D'Amato, C. Silvestri, M. S. Del Prete, A. Licci, A. Riva, J. Lukasiak, and G. Scalise. 2005. In vitro activity of the histatin derivative P-113 against multidrug-resistant pathogens responsible for pneumonia in immunocompromised patients. Antimicrob. Agents Chemother. 49:1249-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenink, J., A. L. A. Ruissen, D. Lowies, W. van 't Hof, E. C. I. Veerman, and A. V. Nieuw Amerongen. 2003. Degradation of antimicrobial histatin-variant peptides in Staphylococcus aureus and Streptococcus mutans. J. Dent. Res. 82:753-757. [DOI] [PubMed] [Google Scholar]
- 5.Helmerhorst, E. J., W. van 't Hof, E. C. I. Veerman, I. Simoons-Smit, and A. V. Nieuw Amerongen. 1997. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 326:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khine, A. A., L. Del Sorbo, R. Vaschetto, S. Voglis, E. Tullis, A. S. Slutsky, G. P. Downey, and H. B. Zhang. 2006. Human neutrophil peptides induce interleukin-8 production through the P2Y(6) signaling pathway. Blood 107:2936-2942. [DOI] [PubMed] [Google Scholar]
- 7.Paquette, D. W., J. P. Fiorellini, G. Martuscelli, R. J. Oringer, T. H. Howell, J. R. McCullough, D. S. Reasner, and R. C. Williams. 1997. Enantiospecific inhibition of ligature-induced periodontitis in beagles with topical (S)-ketoprofen. J. Clin. Periodontol. 24:521-528. [DOI] [PubMed] [Google Scholar]
- 8.Pray, L., and M. Anderson. 2003. Microbial multicellularity. Scientist 17:20-23. [Google Scholar]
- 9.Rothstein, D. M., P. Spacciapoli, L. T. Tran, T. Xu, F. D. Roberts, M. Dalla Serra, D. K. Buxton, F. G. Oppenheim, and P. Friden. 2001. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother. 45:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruissen, A. L. A., J. Groenink, E. J. Helmerhorst, E. Walgreen-Weterings, W. van 't Hof, E. C. I. Veerman, and A. V. Nieuw Amerongen. 2001. Effects of histatin 5 and derived peptides on Candida albicans. Biochem. J. 356:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Situ, H., H. Tsai, and L. A. Bobek. 1999. Construction and characterization of human salivary histatin-5 multimers. J. Dent. Res. 78:690-698. [DOI] [PubMed] [Google Scholar]
- 12.Tam, J. P. 1988. Synthetic peptide vaccine design-synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 85:5409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjabringa, G. S., D. K. Ninaber, J. W. Drijfhout, K. F. Rabe, and P. S. Hiemstra. 2006. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immun. 140:103-112. [DOI] [PubMed] [Google Scholar]
- 14.Troxler, R. F., G. D. Offner, T. Xu, J. C. Vanderspek, and F. G. Oppenheim. 1990. Structural relationship between human salivary histatins. J. Dent. Res. 69:2-6. [DOI] [PubMed] [Google Scholar]
- 15.van 't Hof, W., P. C. Driedijk, M. Van den Berg, A. G. Beck Sickinger, G. Jung, and R. C. Aalberse. 1991. Epitope mapping of the Dermatophagoides pteronyssinus house dust mite major allergen Der p II using overlapping synthetic peptides. Mol. Immunol. 28:1225-1232. [DOI] [PubMed] [Google Scholar]
- 16.van 't Hof, W., E. C. I. Veerman, E. J. Helmerhorst, and A. V. Nieuw Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597-619. [DOI] [PubMed] [Google Scholar]
- 17.Welling, M. M., P. S. Hiemstra, M. T. van den Barselaar, A. Paulusma-Annema, P. H. Nibbering, E. K. J. Pauwels, and W. Calame. 1998. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J. Clin. Investig. 102:1583-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welling, M. M., S. Mongera, A. Lupetti, H. S. Balter, V. Bonetto, U. Mazzi, E. K. J. Pauwels, and P. H. Nibbering. 2002. Radiochemical and biological characteristics of Tc-99m-UBI 29-41 for imaging of bacterial infections. Nucl. Med. Biol. 29:413-422. [DOI] [PubMed] [Google Scholar]
- 19.Welling, M. M., A. Paulusma-Annema, H. S. Balter, E. K. J. Pauwels, and P. H. Nibbering. 2000. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur. J. Nucl. Med. 27:292-301. [DOI] [PubMed] [Google Scholar]