Abstract
Telavancin is a novel bactericidal lipoglycopeptide with multiple mechanisms of action against gram-positive pathogens. The aim of this study was to describe the dynamics of the antimicrobial effect of telavancin against two strains of Staphylococcus aureus (methicillin susceptible and methicillin resistant) in an in vitro kinetic model with simulated human pharmacokinetics. Also, static experiments were performed to determine the rate and extent of killing by telavancin in the presence and absence of human albumin and human serum. Experiments in broth and in nutrient-depleted medium were performed to study the rate and extent of killing by telavancin of bacteria in different growth phases. In the in vitro kinetic model regrowth was noted at 24 h for both strains when exposed to initial concentrations below 5 mg/liter. There was a >3-log10 killing at all concentrations from 0.5× MIC and above at 24 h both in broth and in the presence of 40-g/liter human albumin. In contrast to the methicillin-susceptible strain, the methicillin-resistant strain in 40-g/liter human albumin showed a regrowth at concentrations of 0.5× MIC and 1× MIC at 24 h. At all the other concentrations >3-log10 killing was seen at 24 h. Concordant results were seen in 50% human serum. At a target area under the curve/MIC ratio of 50 (corresponding to the human dose of 10 mg/kg of body weight, administered intravenously), >3-log10 killing was observed at 6 to 8 h. Unlike most antibiotics, telavancin was able to kill both strains in a nongrowing phase.
Staphylococcus aureus is an important cause of both serious community-acquired infections and hospital-acquired infections, and the emergence and spread of methicillin-resistant S. aureus (MRSA) have become an increasing concern worldwide (1, 3, 11, 15). This has led to increasing use of vancomycin and teicoplanin, drugs that are potentially inferior to antistaphylococcal penicillins in terms of terminating S. aureus bacteremia (23). In parts of the United States and in Great Britain approximately 50% of invasive isolates are methicillin resistant (3, 15), and recent reports of vancomycin-intermediate and vancomycin-resistant S. aureus (24) have raised great concern. Telavancin is a new parenteral semisynthetic lipoglycopeptide active against clinically important gram-positive aerobic and anaerobic pathogens (5, 20). In contrast to vancomycin and linezolid, telavancin has been shown to exert a concentration-dependent killing, and previous experimental studies with mice have indicated that the pharmacodynamic index best correlated with antimicrobial effect is the area under the concentration-time curve over 24 h divided by the MIC (AUC/MIC ratio) (7). Animal studies, including studies with a mouse neutropenic thigh infection model and a mouse subcutaneous infection model, have also shown that telavancin exerted a better effect in terms of decrease in log CFU than did vancomycin and linezolid (7). Similar results were found in a neutropenic mouse model of pneumonia (21). Also in this study it was shown that telavancin was superior to vancomycin and linezolid against MRSA at doses simulating human dosage regimens. The protein binding of telavancin in human plasma is high, approximately 92 to 94% (7, 22), and is due to binding to human albumin (7, 22; J. P. Shaw, Theravance, personal communication). Different studies have shown different impacts of the protein binding of telavancin on the activity against bacterial strains. Some in vitro studies have indicated that the efficacy of the drug is minimally affected by the presence of human serum (8a, 9), while others have shown a 5- to 10-fold increase in MIC in the presence of mouse serum and mouse albumin (7). Pharmacokinetic studies of healthy volunteers have shown that telavancin has a linear dose-concentration relationship. In healthy humans a dose of 7.5 mg/kg of body weight results in a maximum concentration in serum (Cmax) of approximately 60 mg/liter with a half-life of between 7 and 9 h (22).
The aim of this study was to study the pharmacodynamics of telavancin in an in vitro kinetic model with simulated human pharmacokinetics against two strains of S. aureus (one methicillin susceptible and one methicillin resistant). Also, static experiments were performed to determine the rate and extent of killing by telavancin in the presence and absence of human albumin and human serum. Experiments in broth and in nutrient-depleted medium were also performed to study the rate and extent of killing by telavancin of bacteria in different growth phases.
(This study was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 16 to 19 December 2005 [15a].)
MATERIALS AND METHODS
Antibiotic.
Telavancin was provided by Theravance Inc., South San Francisco, CA, as a reference powder with known potency. An 0.01-g amount of the drug was dissolved in 10 ml sterile water with the addition of 120 μl of acetate acid. The solution was thereafter sonicated for 90 min to dissolve the substance completely.
Bacterial strains and medium.
Two reference strains of Staphylococcus aureus, ATCC 13709 (methicillin susceptible) and ATCC 33591 (methicillin resistant), were studied. Before the experiments, the strains were grown in Mueller-Hinton (M-H) medium supplemented with 50 mg Ca2+ and 25 mg Mg2+ for 6 to 7 h at 37°C, which yielded an inoculum of approximately 5 × 108 CFU/ml.
Determination of MICs.
The MICs of telavancin for the investigated strains were determined in fluid medium by a macrodilution technique in triplicate on different occasions according to the guidelines of the Clinical and Laboratory Standards Institute. Twofold serial dilutions of the antibiotic were made in broth, inoculated with approximately 5 × 105 CFU of the test strain per ml, and incubated at 37°C for 18 to 24 h. The MICs were defined as the lowest concentrations of the antibiotics that gave no visible growth (14).
Determination of antibiotic concentrations.
A microbiological agar diffusion assay using Bacillus stearothermophilus ATCC 3032 as the test organism was developed to measure the concentrations of telavancin. Samples and standards were applied into agar wells containing a standardized inoculum of spore suspension and were allowed to prediffuse at 22°C for 24 h, after which the plates were incubated at 55°C overnight. Three parallel determinations were made both for each of the samples and for the standards. The limit of detection was 1 mg/liter.
In vitro kinetic model.
The pharmacodynamics of telavancin for the investigated strains was studied in an in vitro kinetic model described before (6, 10, 16-19). The model consists of a spinner flask with a 0.45-μm filter membrane and a prefilter fitted in between the upper and the lower parts in order to prevent bacterial dilution. A magnetic stirrer ensures homogenous mixing of the culture and prevents membrane pore blockage. In one of the side arms of the culture vessel, a silicon membrane is inserted to enable repeated sampling. The other arm is connected by thin plastic tubing to a vessel containing fresh medium. The medium is removed from the culture flask, through the filter, at a constant rate with a pump. Fresh sterile medium is sucked into the flask at the same rate by the negative pressure built up inside the culture vessel. The antibiotic is added to the vessel and eliminated at a constant rate according to the first-order kinetics C = Co × e−kt, where Co is the initial antibiotic level, C is the antibiotic level at the time t, k is the rate of elimination, and t is the time elapsing since the addition of antibiotic. The apparatus is placed in a thermostatic room at 37°C during the experiments. Before the experiments, the culture vessel is filled with broth and bacteria are added at a starting inoculum of 5 × 105 CFU/ml.
Experiments. (i) Determination of the rate and extent of killing by telavancin in the presence and absence of human albumin or human serum.
Tubes containing 4 ml of M-H broth, 4 ml of M-H broth with 40 g/liter of purified human albumin without free fatty acids (Sigma lot no. 09K7604), and 4 ml of M-H broth with inactivated 50% human serum were inoculated with a suspension of the test strains with a final bacterial count of approximately 5 × 105 CFU/ml. Telavancin was added to the tubes at concentrations of 0.5, 1, 2.5, 5, 10, 25, and 50× MIC. The tubes were thereafter incubated at 37°C. Samples were withdrawn at various times (0, 3, 6, 12, and 24 h) and, when necessary, diluted in phosphate-buffered saline (PBS). At least three dilutions of each sample were spread onto blood agar plates and incubated at 37°C, and the colonies were counted after 24 h. The limit of detection of the method was 5 × 101 CFU/ml. All experiments were performed in duplicate.
(ii) Determination of the rate and extent of killing by telavancin in broth and in nutrient-depleted medium.
The strains were diluted in M-H broth to approximately 105 CFU/ml and incubated for 6 hours. At this time, when the cultures were in logarithmic growth phase, the cultures were divided into four tubes. One tube served as a growth control in M-H broth. To the second tube telavancin was added at a concentration of 10× MIC. The third and fourth tubes of the culture were transferred to PBS and incubated for 3 additional hours, after which telavancin was added at a concentration of 10× MIC to one tube and the other served as a growth control in PBS. Samples were withdrawn and seeded for viable counts as described above at 0, 3, 6, 9, 12, and 24 h. All the experiments were performed in duplicate.
(iii) Pharmacodynamics of telavancin in the in vitro kinetic model.
Before the experiments, the culture vessel was filled with M-H broth and bacteria were added at a starting inoculum of approximately 5 × 105 CFU/ml. Telavancin was added at concentrations of 1.0, 2.0, 5, 10, 20, and 40 mg/liter, and the flow rate was adjusted to give a half-life of approximately 8 h. Samples were withdrawn at various times (0, 2, 4, 6, 8, 12, and 24 h) and seeded as described above. The experiments were performed in duplicate for each bacterial strain and antibiotic concentration.
RESULTS
MICs.
The MICs of telavancin for both the investigated strains were 1 mg/liter with the macrodilution technique. This is in accordance with the findings of other authors who found the same MICs (7) or one dilution-step lower for the same strains with the microdilution technique (20).
Antibiotic concentrations.
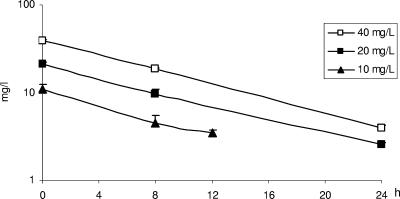
The time-concentration profiles of telavancin at different initial concentrations are shown in Fig. 1 and were as expected. The half-life was between 8 and 9 h.
FIG. 1.
Concentrations of telavancin in the in vitro kinetic model after initial doses shown by the symbols (10 mg/liter at 24 h, not done). Values shown are means of three experiments.
Rate and extent of killing by telavancin in the presence and absence of human albumin or human serum.
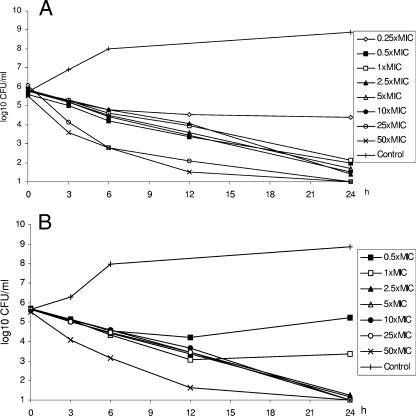
A concentration-dependent killing was noted for the methicillin-susceptible strain when grown in broth. Regrowth occurred at 0.25× MIC; at all other concentrations there was a >3-log killing over 24 h. However, when the strain was grown in 40-g/liter human albumin, no concentration-dependent killing was noted except at the highest concentration (50× MIC). As in broth a >3-log killing was noted at all concentrations (0.25× MIC was not tested) at 24 h.
In contrast to the methicillin-susceptible strain, the resistant strain showed regrowth in 40-g/liter human albumin at concentrations of 0.5× MIC and 1× MIC at 24 h. At all the other concentrations a >3-log killing was seen at 24 h. No concentration-dependent killing was noted for this strain in broth or in albumin except at the highest concentrations (Fig. 2A and B). The two strains behaved in similar ways in 50% human serum. Complete killing was noted at all concentrations except for a slight regrowth at 24 h at the concentration of the MIC for the methicillin-resistant strain (data not shown).
FIG. 2.
Killing curves for telavancin against S. aureus 33591 (MRSA) in broth (static experiments) (A) and in 40-g/liter human albumin (static experiments) (B). Values shown are means of two experiments.
Rate and extent of killing by telavancin in broth and in nutrient-depleted medium during different bacterial growth phases.
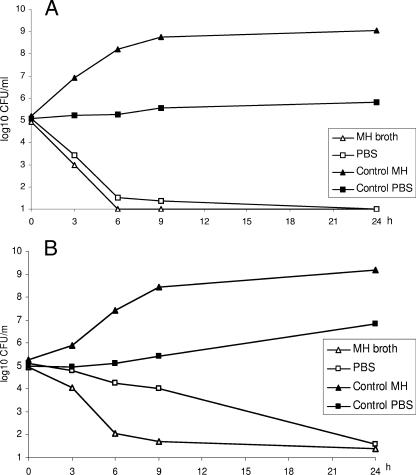
A complete killing was noted for the methicillin-susceptible strain at 6 h in both broth and PBS (Fig. 3A). For the methicillin-resistant strain the killing rate in broth was higher than that in PBS (Fig. 3B). However, complete killing was noted in both media at 24 h.
FIG. 3.
Killing curves for telavancin at static concentrations of 10× MIC against growing and nongrowing S. aureus ATCC 13709 (methicillin susceptible) (A) and S. aureus 33591 (MRSA) (B). Symbols: ▴, control in broth; ▪, control in PBS; ▵, 10× MIC in broth; □, 10× MIC in PBS. Values shown are means of two experiments.
Pharmacodynamics of telavancin in the in vitro kinetic model.
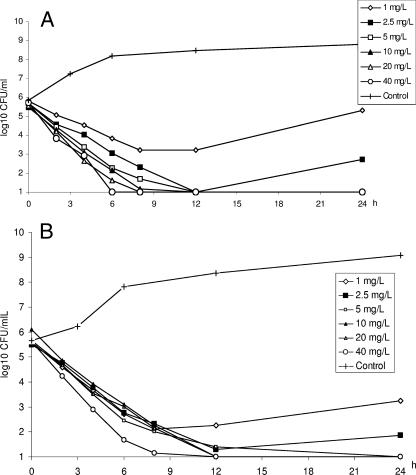
As observed in the static experiments, a concentration-dependent killing was noted for the methicillin-susceptible strain but only between 1 and 5 mg/liter. The lowest Cmax which gave no regrowth was 5 mg/liter, corresponding to an AUC/MIC ratio of 50. Maximal killing was obtained at a Cmax of 40 mg/liter with an AUC/MIC ratio of 404. In the experiments where the strain was exposed to an initial concentration of 2.5 mg/liter, regrowth occurred at 24 h. At a Cmax of 1 mg/liter, an initial killing was noted, but at 24 h the inoculum was similar to that of the starting inoculum (Table 1; Fig. 4A). For the methicillin-resistant strain, a similar pattern was shown. The lowest Cmax which gave no regrowth was 5 mg/liter, and maximal killing was obtained at 40 mg/liter. No concentration-dependent killing was noted between these concentrations (Table 2; Fig. 4B).
TABLE 1.
Antibacterial effect of telavancin against S. aureus ATCC 13709 at different time points in the in vitro kinetic model
| Time point (h) |
S. aureus level (Δlog10 0-24 CFU/ml) at telavancin concn (mg/liter):
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2.5 | 5 | 10 | 20 | 40 | |
| 2 | −0.8 | −1.1 | −1.2 | −1.2 | −1.3 | −1.9 |
| 4 | −1.3 | −1.6 | −2.3 | −2.4 | −2.9 | −2.8 |
| 6 | −2.0 | −2.6 | −3.4 | −3.3 | −3.9 | −4.7 |
| 8 | −2.6 | −3.4 | −4.0 | −4.3 | −4.6 | −4.7 |
| 12 | −2.6 | −4.7 | −4.7 | −4.5 | −4.6 | −4.7 |
| 24 | −0.5 | −2.9 | −4.7 | −4.5 | −4.6 | −4.7 |
FIG. 4.
Killing curves for telavancin in the in vitro kinetic model against S. aureus ATCC 13709 (methicillin susceptible) (A) and S. aureus 33591 (MRSA) (B). Values shown are means of two experiments.
TABLE 2.
Antibacterial effect of telavancin against S. aureus ATCC 33591 at different time points in the in vitro kinetic model
| Time point (h) |
S. aureus level (Δlog10 0-24 CFU/ml) at telavancin concn (mg/liter):
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2.5 | 5 | 10 | 20 | 40 | |
| 2 | −1.0 | −1.2 | −0.9 | −1.2 | −1.0 | −1.2 |
| 4 | −1.9 | −1.7 | −2.1 | −2.2 | −2.1 | −2.7 |
| 6 | −2.9 | −2.7 | −3.1 | −3.0 | −2.6 | −3.9 |
| 8 | −3.5 | −3.2 | −3.6 | −3.8 | −3.5 | −4.5 |
| 12 | −3.3 | −4.2 | −4.2 | −5.1 | −4.7 | −4.6 |
| 24 | −2.6 | −3.6 | −4.6 | −5.1 | −4.7 | −4.6 |
DISCUSSION
Telavancin is a semisynthetic derivative of vancomycin that has been shown to be more active than vancomycin in vitro and in animal models (5, 8, 20, 21), probably due to the presence of a hydrophobic side chain on the vancosamine sugar of the molecule that interferes with critical membrane functions (8). Generally it is believed that it is the free (non-protein-bound) drug that is microbiologically active (13, 26). However, Kaniga et al. could in the early development of telavancin show that human inactivated serum had no effect on the bactericidal activity of telavancin against staphylococci (8a). In another study, the presence of human serum had modest effects on the MICs (increase of one- to fourfold) of telavancin against glycopeptide-nonsusceptible staphylococci (9). In contrast, the presence of mouse serum or albumin increased the MIC of telavancin 5- to 10-fold (7). In our killing experiments, there was a >3-log10 killing at 24 h for the methicillin-susceptible strain both in broth and in 40-g/liter human albumin at concentrations of 0.5× MIC and above. (The MICs were determined with the macrodilution technique. However, the results were identical to those for the microdilution technique [7].) The only difference seen between the two media was that regrowth was noted at 24 h at the concentrations of 0.5× MIC and the MIC in the presence of human albumin but not in broth for the methicillin-resistant strain. Results concordant with those for albumin were seen in 50% human serum for both strains. Complete killing was noted at all concentrations except for a slight regrowth at 24 h at the MIC for the methicillin-resistant strain.
In contrast to vancomycin, telavancin has been considered to act as a concentration-dependent agent, and previous experimental studies with mice have indicated that the pharmacodynamic index best correlated with antimicrobial effect is the AUC/MIC ratio (7). What magnitude of this index must be reached to achieve different pharmacodynamic targets has not yet been fully explored. In our experiments, the lowest Cmax that gave no regrowth at 24 h was 5 mg/liter, corresponding to an AUC/MIC ratio of 50. This corresponds to the drug exposure following the human dose of 10 mg/kg once daily (Cmax of 5.2 mg/liter and an AUC from 0 to 24 h of 51 μg·h/ml [free drug] [22]). At this exposure, >3-log killing was noted at 6 to 8 h against both the methicillin-susceptible and the methicillin-resistant strains of S. aureus.
A possible explanation for the apparent minimal effect of human albumin or human serum is that telavancin, unlike other glycopeptides, not only inhibits the peptidoglycan synthesis but also operates, at least in part, through a membrane effect that is less affected by the extent of protein binding (8, 9). Similar behavior has been noted with daptomycin. Cha and Rybak showed, in an in vitro pharmacodynamic model, that although delayed, the extent of killing of MRSA by daptomycin was unaltered in albumin-supplemented broth (2). Daptomycin also acts on the cytoplasmic membrane and causes a modification of the membrane potential.
Another unusual finding was that telavancin had the potential to kill nongrowing bacteria. This is in accordance with the findings of Gander et al., who in a biofilm model could show that telavancin exhibited substantial antimicrobial activity against staphylococcal biofilms, including glycopeptide-intermediate S. aureus strains (4). Also, in a rabbit endocarditis model, it was shown that telavancin reduced mean aortic vegetation titers by 4.7 log10 CFU/g after 4 days of treatment in comparison to 3.4 log10 for vancomycin. However, this difference was not statistically significant (12). Very few antibiotics have a bactericidal effect on nongrowing bacteria; the carbapenems are one exception (16, 25).
In conclusion, in the in vitro kinetic model the lowest Cmax giving no regrowth was 5 mg/liter, corresponding to an AUC/MIC ratio of 50. Maximal killing was obtained at a Cmax of 40 mg/liter with an AUC/MIC ratio of 404. No concentration-dependent killing was observed for either of the two strains between the concentrations of 5 and 40 mg/liter. In the static experiments, human albumin and human serum had little influence on the bacterial killing effect of telavancin. Unlike most antibiotics, telavancin was able to kill both strains in a nongrowing phase.
Acknowledgments
This study was supported in part by a grant from Theravance Inc., South San Francisco, CA.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Bell, J. M., J. D. Turnidge, and SENTRY APAC Participants. 2002. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY Antimicrobial Surveillance Program, 1998-1999. Antimicrob. Agents Chemother. 46:879-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha, R., and M. J. Rybak. 2004. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 54:259-262. [DOI] [PubMed] [Google Scholar]
- 3.European Antimicrobial Resistance Surveillance System. Annual report 2004. http://www.earss@rivm.nl.
- 4.Gander, S., A. Kinnaird, and R. Finch. 2005. Telavancin: in vitro activity against staphylococci in a biofilm model. J. Antimicrob. Chemother. 56:337-343. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrell, and H. T. Fernandez. 2004. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against gram-positive species and Corynebacterium spp. Antimicrob. Agents Chemother. 48:2149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson, I., E. Löwdin, I. Odenholt, and O. Cars. 2001. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45:2436-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J.-P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Kaniga, K., D. Johnson, T. Wu, D. Debabov, K. Krause, J. Pace, D. Higgins, B. Christensen, and K. Judice. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-1632. [DOI] [PMC free article] [PubMed]
- 9.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 10.Löwdin, E., I. Odenholt, S. Bengtsson, and O. Cars. 1996. Pharmacodynamic effects of sub-MICs of benzylpenicillin against Streptococcus pyogenes in a newly developed in vitro kinetic model. Antimicrob. Agents Chemother. 40:2478-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 12.Madrigal, A. G., L. Basuino, and H. F. Chambers. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3163-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601-607. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.National Nosocomial Infections Surveillance System. August 2003. Report: data summary from January 1992 through June 2003. Division of Healthcare Quality Promotion, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. http://www.cdc.gov.
- 15a.Odenholt, I., O. Cars, and E. Löwdin. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-457, p. 19.
- 16.Odenholt, I., E. Löwdin, and O. Cars. 1997. Comparative in vitro pharmacodynamics of BO-2727, meropenem and imipenem against Gram-positive and Gram-negative bacteria. Clin. Microbiol. Infect. 3:73-81. [DOI] [PubMed] [Google Scholar]
- 17.Odenholt, I., E. Löwdin, and O. Cars. 2003. In vitro studies of the pharmacodynamics of teicoplanin against Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecium. Clin. Microbiol. Infect. 9:930-937. [DOI] [PubMed] [Google Scholar]
- 18.Odenholt, I., O. Cars, and E. Löwdin. 2004. Pharmacodynamic studies of amoxicillin against Streptococcus pneumoniae: comparison of a new pharmacokinetically enhanced formulation (2000 mg twice daily) with standard dosage regimens. J. Antimicrob. Chemother. 54:1062-1066. [DOI] [PubMed] [Google Scholar]
- 19.Odenholt, I., and O. Cars. 2006. Pharmacodynamics of moxifloxacin and levofloxacin against Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli: simulation of human plasma concentrations after intravenous dosage in an in vitro kinetic model. J. Antimicrob. Chemother. 58:960-965. [DOI] [PubMed] [Google Scholar]
- 20.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes, N., R. Skinner, K. Kaniga, K. M. Krause, J. Shelton, G. P. Obedencio, A. Gough, M. Conner, and S. S. Hegde. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw, J. P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegman-Igra, Y., P. Reich, R. Orni-Wasserlauf, D. Schwartz, and M. Giladi. 2005. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand. J. Infect. Dis. 37:572-578. [DOI] [PubMed] [Google Scholar]
- 24.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancester, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. B. Band, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 25.Tuomanen, E. 1986. Phenotypic tolerance: the search for β-lactam antibiotics that kill nongrowing bacteria. Rev. Infect. Dis. 8(Suppl. 3):279-291. [DOI] [PubMed] [Google Scholar]
- 26.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]