Abstract
Morbidity and mortality from malaria remain unacceptably high among young children in sub-Saharan Africa. Intermittent preventive treatment in infancy (IPTi) involves the administration of antimalarials alongside routine vaccinations and might be an option in malaria control. In an area of intense, perennial malaria transmission in northern Ghana, 1,200 children received IPTi with sulfadoxine-pyrimethamine or placebo at approximately 3, 9, and 15 months of age. Children were followed up until 24 months of age to assess morbidity and adverse events. During the intervention period (3 to 18 months of age), IPTi reduced the incidences of malaria and severe anemia by 22.5% (95% confidence interval, 12 to 32%) and 23.6% (95% confidence interval, 4 to 39%), respectively, and reduced hospitalizations and episodes of asymptomatic parasitemia by one-third. Protection was pronounced in the first year of life and not discernible in the second. The malaria-protective effect was largely confined to a period of 1 month after sulfadoxine-pyrimethamine treatments. Following the intervention, protection against asymptomatic parasitemia persisted. In contrast, a significant rebound of severe malaria, predominantly severe malarial anemia, occurred among children having received IPTi. Although the treatment was generally well tolerated, one case of moderately severe skin reaction followed sulfadoxine-pyrimethamine treatment. IPTi reduces malaria and anemia in infants in northern Ghana. Extension of IPTi into the second year of life by administering a dose at 15 months of age provided no substantial benefit beyond a 1-month prophylactic effect. Although this simple intervention offers one of the few available malaria-preventive measures for regions where malaria is endemic, the observed rebound of severe malaria advises caution and requires further investigation.
Worldwide, an estimated 300 to 600 million cases of malaria occur per year, killing one to three million individuals, mainly young African children. In these, severe anemia is a leading manifestation. Malaria control in sub-Saharan Africa largely builds on the concept of early diagnosis and treatment. Preventive approaches such as insecticide-treated nets, indoor residual spraying of insecticides, or vaccinations are either underutilized or not operational. A major obstacle in malaria control in sub-Saharan Africa lies in the limited access of the majority of the population to formal health structures providing accurate treatment or prevention means (4, 5). Intermittent preventive treatment in infancy (IPTi) constitutes an approach to bridge this gap by taking advantage of the well-established infrastructure of the Expanded Program on Immunization (EPI). Targeting the most vulnerable group, i.e., infants and young children, IPTi stands for the administration of a curative dose of an antimalarial at the time children receive their routine vaccinations, regardless of whether a child is parasitemic or not. Here, sulfadoxine-pyrimethamine (SP) offers the advantage of single-dose, observed treatment.
Continuous chemoprophylaxis of children in regions of endemicity reduces malaria and severe anemia, outpatient attendance, hospital admissions, and mortality (11). However, concerns about interference with protective immunity, emerging drug resistance, cost, and delivery problems have made this control strategy unpopular for large-scale use (35). In Tanzanian infants, chemoprophylaxis halved the incidences of malaria and severe anemia in infancy, but following the intervention, rates increased, suggesting that the acquisition of immunity had been impaired (20). IPTi was initially tested in Tanzania as an approach to avoid such a rebound effect. Administering SP alongside EPI vaccinations at ages 2, 3, and 9 months reduced the incidences of malaria and anemia by >50% without evidence of a rebound. Conversely, some degree of protection was found to persist into the second year of life (29, 30). In three subsequent trials of IPTi with SP using slightly different treatment schedules, 20 to 25% protection against malaria episodes was observed. Only in one trial was anemia prevented. A rebound of high-density parasitemia in the postintervention period was found in one study, and a rebound of anemia was found in another (6, 17, 18).
IPTi might be a promising tool in malaria control; however, large-scale implementation requires further evidence of efficacy and safety as well as evaluation of various treatment schemes. In particular, an additional drug application might be useful to extend protection into the second year of life. Here, we report the results of a randomized, placebo-controlled trial from northern Ghana in which IPTi with SP was administered at ages 3, 9, and 15 months.
MATERIALS AND METHODS
Study site and design.
The study was conducted between March 2003 and July 2005 at the periurban, primary Bulpeila Health Centre in Tamale, the capital of Ghana's northern region. Despite a population of 350,000, the city has a rural character and is scattered over a vast area. Subsistence farming and small-scale trade are the main income sources. Climate and vegetation are savanna type with rains from May to October and a dry season from November to April. Malaria in the region is hyperendemic with perennial transmission and modest seasonal variation (10); entomologic data are not available. At the time the study was carried out, bed net usage was low (3%) (9), and malaria control consisted mainly of treatment with chloroquine, which achieved cure rates of <50% (24). SP treatment in Tamale fails in 14% and 28% of children with uncomplicated malaria within 2 and 4 weeks of follow-up, respectively (23). More than half of the children with severe malaria admitted to the Tamale hospital show severe anemia (22).
This was a randomized, double-blind, placebo-controlled trial of the impact of IPTi administered alongside routine EPI vaccinations at approximately 3, 9, and 15 months of age (trial registration URL: http://clinicaltrials.gov, NCT00168948). Primary outcomes were incidences of all and of first or only episodes of malaria and severe anemia during the intervention period and the proportion of children experiencing adverse events (AEs). The intervention period was defined as 3 to 18 months of age, i.e., including 3 months after the last dose, to account for potential sustainable effects and to adapt to previously published studies (6, 17, 18, 29). A potential rebound effect was examined starting from 1 month after the last dose, i.e., after the prophylactic effect of SP was expected to have ceased (34). An external audit was performed by the IPTi Consortium (28). Informed written consent was obtained from the participants' parent(s). The study protocol was reviewed and approved by the Ethics Committee, University for Development Studies, Tamale, Ghana.
Enrollment and follow-up.
Meetings were held with health authorities and community leaders to explain the purpose and procedures of the study. Inclusion criteria were parental informed consent and permanent residence in the study area; exclusion criteria were conditions requiring hospital admission, signs of hepatic or renal dysfunction, and reported allergy to sulfa-containing drugs. Children were enrolled when receiving their second dose of pentavalent vaccine (diphtheria-pertussis-tetanus-Haemophilus influenzae type b-hepatitis B virus), assigned unique identity numbers, and issued study passes. Allocation of children to SP or placebo was done individually by block randomization in groups of 10; SP and placebo were provided in individual, prelabeled blister packs. The study team and caretakers of children were blinded to the treatment regimen. The randomization and drug code lists were kept by an individual not involved in the analysis of the study. SP and placebo (Roche, Switzerland) were indistinguishable. Children received half a tablet of SP (125/6.25 mg of sulfadoxine and pyrimethamine, respectively, per kg of body weight) or placebo when they received diphtheria-pertussis-tetanus-Haemophilus influenzae type b-hepatitis B virus dose 2, measles, and yellow fever vaccinations. Study drugs were crushed, dissolved in water, and administered by staff who observed the children for at least 30 min following treatment. If vomiting occurred, treatment was repeated.
The individual follow-up schedule consisted of the three treatment visits at 3, 9, and 15 months of age and regular review visits at 6, 12, 18, 21, and 24 months of age (±4 weeks each). Field workers visited the participants' home 2 days preceding a regular visit to remind parents. For passive case detection, parents were instructed to bring their children to the health center in case of any health problem. In addition, field workers performed monthly checkup visits at the participants' homes. Passive case detection was affected by a civil conflict which involved a state of emergency and changing curfews during the follow-up period (until August 2004). Consequently, children occasionally could not attend the health center or hospital after late afternoon hours.
At each scheduled visit, children were clinically examined, a standardized medical history was obtained, and a venous blood sample was collected. A history of fever within the past 48 h was recorded when voluntarily reported. Blood samples were collected at unscheduled visits in the case of fever or a history of fever or when requested by the clinician.
Asexual malaria parasites were counted against 500 white blood cells on Giemsa-stained thick blood films, and hemoglobin (Hb) was measured by a HemoCue (Angelholm, Sweden) photometer. Malaria was defined as parasitemia of any density plus fever (axillary temperature, ≥37.5°C) or a voluntarily reported history of fever within 48 h of presentation to the clinic and severe malaria according to WHO criteria (36). Severe anemia was defined as Hb of <7 g/dl (32). The rainy season was defined as those months in which precipitation exceeded 90 mm (Food and Agriculture Organization, Aquastat Climate Information Tool [http://www.fao.org/ag/agl/aglw/aquastat/gis/index3.stm]). Anthropometric indices were calculated on the basis of the National Centre for Health Statistics reference data set (7). Malaria was treated under observation with artesunate (Plasmotrim; Mepha, Switzerland) at a dose of 4 mg/kg (double dose on first day) for 5 days. Iron supplementation was initiated at an Hb of <8 g/dl. Other diseases were treated according to Ghana Health Service guidelines (12). Treatment was free of charge.
Data management and statistical analysis.
Data were double entered, and discrepancies were resolved by consulting the case record form. Consistency checks were performed, and data were cleaned before locking and code breaking. Statistical analysis was adjusted to the analytical plan of the IPTi Consortium to facilitate subsequent meta-analysis (unpublished data).
Sample size estimation extrapolated the data provided by Schellenberg et al. (29). A sample size of 1,000 children was estimated to detect 25% reductions in the rates of malaria episodes and severe anemia in the treatment group compared to the placebo group. Analysis was carried out on an intention-to-treat basis including all randomized children. Person-time at risk started with recruitment and ended with 24 months of age, withdrawal, or death. Following an episode of malaria, anemia, parasitemia, or hospitalization, children were regarded as not being at the respective risk for 21 days. Incidence density ratios were produced by negative binomial regression, and protective efficacy (PE) and 95% confidence intervals (95% CIs) were calculated. PEs for first or only clinical episodes were compared by means of Poisson regression and a robust standard error estimator provided by the Stata software program (StataCorp LP). For analysis of postdose effects, a month was defined as 35 days to allow for active case detection and the prophylactic effect of SP (34). Kaplan-Meier survival estimates were used to produce time-to-event analyses for the first or only episodes of malaria and severe anemia and compared by log-rank tests.
RESULTS
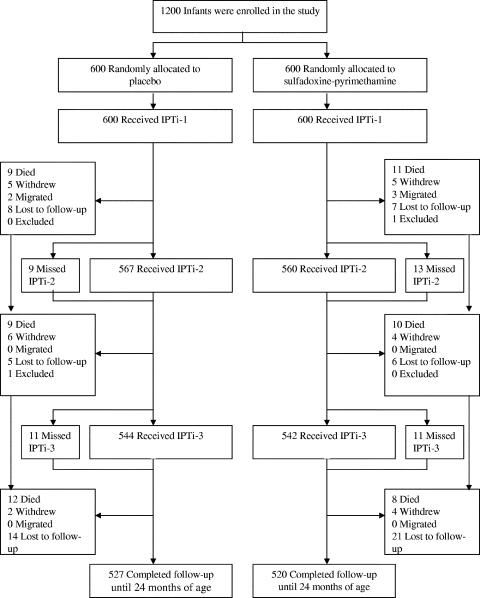
Between March and September 2003, 1,200 infants were randomized to receive SP or placebo. The two groups were similar in terms of baseline characteristics, age at the time of drug administration, number of withdrawals, and completeness of follow-up until 24 months of age (87.3% [Table 1; Fig. 1]). Of the recruited children, 89.2% received all three doses of SP or placebo, and 4.6%, 4.8%, and 1.4% received dose 1 only, doses 1 and 2, and doses 1 and 3, respectively.
TABLE 1.
Baseline characteristics
| Parameter | Placebo group (n = 600) | SP group (n = 600) |
|---|---|---|
| Female sex, % (no.) | 50.0 (300) | 50.5 (297) |
| Dagomba ethnic group, % (no.) | 96.5 (579) | 94.8 (569) |
| Household size, no. of persons (mean ± SD) | 12.2 ± 7.5 | 12.6 ± 9.1 |
| Wt, kg (mean ± SD) | 5.60 ± 0.93 | 5.58 ± 0.87 |
| Mid-upper-arm circumference, cm (mean ± SD) | 14.12 ± 1.35 | 14.10 ± 1.39 |
| z-score (mean ± SD) | ||
| Wt for age | −0.29 ± 1.28 | −0.32 ± 1.23 |
| Wt for length | −0.73 ± 1.92 | −0.83 ± 1.90 |
| Length for age | −0.08 ± 1.80 | −0.04 ± 1.72 |
| Fever, % (no.) | 4.8 (26) | 4.4 (24) |
| History of fever in preceding 48 h, % (no.) | 17.2 (103) | 17.4 (104) |
| Parasitemia, % (no.) | 3.8 (23) | 1.8 (11) |
| Geometric mean parasite density (no. of parasites/μl) | 7,556 | 7,096 |
| Hb, g/dl (mean ± SD) | 10.15 ± 1.12 | 10.32 ± 1.12 |
| Age (mo) at treatment visit (mean ± SD) | ||
| 1 | 2.36 ± 0.48 | 2.33 ± 0.48 |
| 2 | 8.06 ± 0.59 | 8.06 ± 0.58 |
| 3 | 14.30 ± 0.47 | 14.29 ± 0.46 |
FIG. 1.
Trial profile.
The PE of IPTi during the intervention period (3 to 18 months of age) against all episodes of malaria was 22% (Table 2), and in terms of the risk of first or only episode, it was 14.7% (95% CI, 6.5 to 22.2; SP, 335/600 children; placebo, 392/599; P = 0.001). For severe anemia, the respective PEs were 24% and 22.8% (95% CI, 7.2 to 35.7; SP, 147/600 children; placebo, 190/599; P = 0.006). In addition, IPTi significantly reduced the incidences of asymptomatic parasitemia, high-parasite-density malaria, hospitalizations, and borderline, outpatient attendance. No effect was observed on nonmalarial fevers, severe malaria, or mortality (Table 2).
TABLE 2.
Frequency of main outcomes during intervention period (3 to 18 months of age)
| Outcome | Placebo group
|
SP group
|
% PE (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|---|
| No. of events | PYARa | Incidence density (no. of events/PYAR) | No. of events | PYAR | Incidence density (no. of events/PYAR) | |||
| Malaria | ||||||||
| All episodes | 729 | 627.0 | 1.16 | 571 | 630.3 | 0.91 | 22.5 (11.8-31.9) | <0.0001 |
| Episodes with ≥5,000 parasites/μl | 393 | 646.4 | 0.61 | 324 | 644.6 | 0.50 | 17.7 (2.0-30.9) | 0.03 |
| Severe malaria | 15 | 668.2 | 0.02 | 12 | 662.6 | 0.02 | 19.3 (−72.4-62.2) | 0.58 |
| Anemia | ||||||||
| Hb of <7 g/dl | 283 | 641.2 | 0.44 | 215 | 639.1 | 0.34 | 23.6 (4.1-39.1) | 0.02 |
| Hb of <7 g/dl plus asymptomatic parasitemia | 164 | 659.6 | 0.25 | 108 | 657.0 | 0.16 | 34.0 (13.1-49.9) | 0.003 |
| Other | ||||||||
| Hospitalizationb | 240 | 655.2 | 0.37 | 166 | 653.7 | 0.25 | 31.3 (2.9-51.4) | 0.03 |
| Outpatient attendance | 2,294 | 536.8 | 4.27 | 2,140 | 540.0 | 3.96 | 7.2 (−0.5-14.4) | 0.07 |
| Asymptomatic parasitemia | 389 | 646.6 | 0.60 | 260 | 648.3 | 0.40 | 33.7 (21.1-44.3) | <0.0001 |
| Nonmalarial fever | 285 | 652.6 | 0.44 | 269 | 647.7 | 0.42 | 4.9 (−12.4-19.5) | 0.56 |
| Death | 23 | 675.9 | 0.03 | 22 | 669.8 | 0.03 | −0.7 (−81.9-44.2) | 0.98 |
PYAR, person-years at risk.
Admission by study physician or respective history.
Considering the complete follow-up period (3 to 24 months), the aforementioned effects waned (Table 3). When the data were stratified into the first and second years of life, protection due to IPTi was found to be most pronounced during infancy whereas no significant effects were seen afterwards, with the exception of asymptomatic parasitemia (Table 3). Basically the same was observed in terms of the risk of the first or only event (data not shown). PE against the first or only episode of malaria was 30.5% (95% CI, 19.6 to 40.0; P < 0.0001) in the first year of life but fell to 3.4% (95% CI, −5.1 to 11.2; P = 0.42) in children aged 13 to 24 months. For severe anemia, the respective figures were 29.1% (95% CI, 6.0 to 46.6; P = 0.02) and 14.3% (95% CI, −0.6 to 26.9; P = 0.06).
TABLE 3.
Frequency of main outcomes during the first and second year of life
| Outcome | Complete follow-up period (3-24 mo)
|
First yr of life (3-12 mo)
|
Second yr of life (13-24 mo)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo group
|
SP group
|
% PE (95% CI) | Placebo group
|
SP group
|
% PE (95% CI) | Placebo group
|
SP group
|
% PE (95% CI) | |||||||
| No. of events | IDe | No. of events | ID | No. of events | ID | No. of events | ID | No. of events | ID | No. of events | ID | ||||
| Malaria | |||||||||||||||
| All episodes | 1,122 | 1.23 | 943 | 1.03 | 16.5 (6.2-25.6)c | 360 | 0.93 | 245 | 0.62 | 33.3 (20.7-43.9)d | 762 | 1.55 | 699 | 1.43 | 8.0 (−5.0-19.3) |
| Episodes with ≥5,000 parasites/μl | 613 | 0.65 | 554 | 0.59 | 9.4 (−4.9-21.8) | 156 | 0.39 | 114 | 0.28 | 27.9 (5.9-44.7)b | 457 | 0.90 | 440 | 0.87 | 2.9 (−14.1-17.4) |
| Severe malaria | 26 | 0.03 | 31 | 0.03 | −20.3 (−106-29.9) | 3 | 0.01 | 4 | 0.01 | −33.6 (−497-70.1) | 23 | 0.04 | 27 | 0.05 | −18.7 (−110-33.4) |
| Anemia | |||||||||||||||
| Hb of <7 g/dl | 439 | 0.46 | 369 | 0.39 | 15.2 (−2.0-29.5) | 125 | 0.32 | 98 | 0.25 | 21.0 (−10.1-43.4) | 315 | 0.61 | 271 | 0.53 | 13.3 (−6.5-29.4) |
| Hb of <7 g/dl plus asymptomatic parasitemia | 267 | 0.28 | 215 | 0.22 | 19.1 (−1.1-35.3) | 59 | 0.15 | 40 | 0.10 | 32.6 (−4.6-56.5) | 208 | 0.40 | 175 | 0.34 | 15.6 (−8.0-34.0) |
| Other | |||||||||||||||
| Hospitalizationa | 339 | 0.35 | 285 | 0.30 | 15.6 (−15.5-38.3) | 87 | 0.21 | 55 | 0.14 | 38.0 (7.0-58.7)b | 250 | 0.48 | 230 | 0.45 | 9.0 (−26.8-34.7) |
| Outpatient attendance | 3,017 | 3.74 | 2,879 | 3.57 | 4.2 (−3.6-11.4) | 1,433 | 4.38 | 1,345 | 4.06 | 7.7 (−0.8-15.4) | 1,586 | 3.34 | 1,538 | 3.22 | 1.5 (−8.9-10.9) |
| Asymptomatic parasitemia | 621 | 0.66 | 444 | 0.47 | 29.0 (17.5-38.9)d | 243 | 0.61 | 145 | 0.36 | 41.5 (27.2-53.0)d | 378 | 0.74 | 299 | 0.58 | 21.1 (5.6-34.1)b |
| Nonmalarial fever | 337 | 0.35 | 317 | 0.33 | 5.3 (−10.5-18.7) | 220 | 0.55 | 216 | 0.54 | 1.7 (−18.6-18.5) | 117 | 0.22 | 101 | 0.19 | 12.8 (−13.8-33.2) |
| Death | 30 | 0.03 | 29 | 0.03 | 0.5 (−79.8-45.0) | 11 | 0.03 | 15 | 0.04 | −37.6 (−199.6-36.8) | 19 | 0.05 | 14 | 0.04 | 17.1 (−2.1-81.2) |
Admission by study physician or respective history.
P < 0.05.
P < 0.005.
P < 0.0001.
ID, incidence density (number of events/person-year at risk).
Next, we analyzed the effects of IPTi during periods of 6 months following each treatment (Table 4). Protection against malaria was most pronounced after dose 2 including a reduction in severe malaria, at borderline statistical significance. IPTi doses 1 and 2 to similar extents prevented severe anemia, but protection against asymptomatic parasitemia and hospitalizations was strongest following IPTi at 3 months of age. No protective effect with respect to any of the outcome parameters was observed after the last IPTi dose. Rather, severe malaria tended to occur more frequently.
TABLE 4.
PEs during 6 months following each dose of SP
| Outcome | Dose 1
|
Dose 2
|
Dose 3
|
|||
|---|---|---|---|---|---|---|
| % PE (95% CI) | P | % PE (95% CI) | P | % PE (95% CI) | P | |
| Malaria | ||||||
| All episodes | 19.9 (0.3-35.7) | 0.047 | 34.0 (18.4-46.6) | <0.0001 | 9.9 (−5.8-23.3) | 0.20 |
| First or only episode | 20.7 (3.7-34.6) | 0.02 | 30.8 (16.5-42.6) | <0.0001 | 3.7 (−7.1-13.4) | 0.49 |
| Malaria with >5,000 parasites/μl | ||||||
| All episodes | 11.3 (−22.0-35.5) | 0.46 | 25.1 (0.2-43.7) | 0.049 | 6.1 (−14.2-22.8) | 0.53 |
| First or only episode | 15.0 (−14.8-37.1) | 0.29 | 24.5 (1.2-42.4) | 0.04 | 3.1 (−12.2-16.3) | 0.67 |
| Severe malaria | ||||||
| All episodes | −104.3 (−2,153-81.5) | 0.56 | 77.2 (−5.6-95.1) | 0.06 | −93.5 (−317.0-10.3) | 0.09 |
| First or only episode | −102.5 (−2,129-81.6) | 0.56 | 77.6 (−3.4-95.1) | 0.06 | −101.1 (−325.8-5.0) | 0.07 |
| Asymptomatic parasitemia | ||||||
| All episodes | 46.5 (28.5-59.9) | <0.0001 | 39.4 (17.7-55.3) | 0.001 | 9.0 (−13.3-26.9) | 0.40 |
| First or only episode | 44.6 (27.4-57.7) | <0.0001 | 33.3 (12.4-49.2) | 0.004 | 10.3 (−8.1-25.5) | 0.25 |
| Anemia (Hb of <7 g/dl) | ||||||
| All episodes | 29.5 (−22.0-59.2) | 0.21 | 32.8 (3.2-53.3) | 0.03 | 14.3 (−9.6-33.1) | 0.22 |
| First or only episode | 39.3 (0.6-62.9) | 0.047 | 39.0 (15.7-55.8) | 0.003 | 15.6 (−2.5-30.5) | 0.09 |
| Hospitalizationa | ||||||
| All episodes | 54.2 (14.3-75.5) | 0.02 | 34.8 (−4.8-59.4) | 0.08 | 13.3 (−26.9-40.8) | 0.46 |
| First or only episode | 55.0 (19.8-74.7) | 0.007 | 21.5 (−15.4-46.6) | 0.22 | 9.3 (−17.1-29.8) | 0.45 |
| Death | −27.5 (−205.3-46.8) | 0.59 | −52.0 (−198.5-22.6) | 0.22 | 2.8 (−51.0-37.4) | 0.90 |
Admission by study physician or respective history. No significant effects were observed with respect to nonmalarial fever or outpatient attendance.
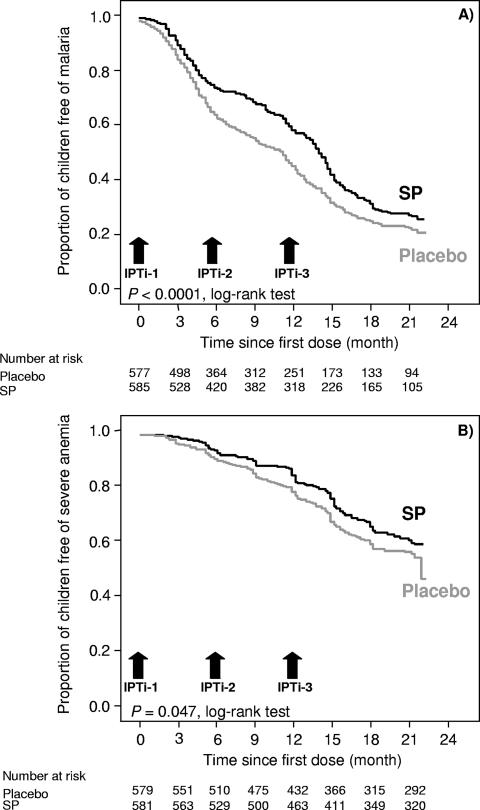
Further stratification revealed that protection against all episodes of malaria was largely confined to 1 month following SP treatment. PE was lowest in the month after dose 1 (83.0% [95% CI, 12.8 to 96.7]; P = 0.03), highest following dose 2 (96.2% [95% CI, 84.7 to 99.1]; P < 0.0001), and also high after dose 3 (91.7% [95% CI, 80.5 to 96.5]; P < 0.0001). Kaplan-Meier analysis of time to the first or only episode of malaria produced corresponding results (Fig. 2). With respect to all episodes of severe anemia, PE tended to increase with the number of dose, i.e., 39.2% (95% CI, −1,726 to 97.8; P = 0.78), 56.7% (95% CI, −341 to 56.7; P = 0.48), and 88.0% (95% CI, 45.1 to 97.4; P = 0.006). In the periods between two treatments, i.e., 5 to <9 and 10 to <15 months of age, protection against all episodes of malaria was substantially lower and at borderline statistical significance (19.6% [95% CI, −0.2 to 35.5; P = 0.05] and 19.6% [95% CI, −2.2 to 36.7; P = 0.08]). For severe anemia, these figures were 21.6% (95% CI, −21.4 to 49.3; P = 0.28) and 33.9% (95% CI, 8.4 to 52.3; P = 0.01), respectively. Not only were episodes of asymptomatic parasitemia prevented for a month after each dose (data not shown), but the effect persisted between dose 1 and dose 2 (41.7% [95% CI, 24.5 to 54.9]; P < 0.0001) and between dose 2 and dose 3 (35.3% [95% CI, 11.8 to 52.5]; P = 0.006).
FIG. 2.
Kaplan-Meier survival estimates of the proportions of children free of malaria (A) and severe anemia (B) by treatment group.
The malaria transmission season had a nonsignificant effect on PE. The vast majority of children (n = 872) received IPTi doses 1 and 3 in the rainy season and dose 2 in the dry season. In these, PE against all episodes of malaria during the intervention period was 20.7% (95% CI, 8.1 to 31.6; P = 0.002) and against severe anemia it was 33.5% (95% CI, 13.8 to 48.7; P = 0.002). The respective figures were higher in the 77 children who received all three doses during the rainy season, i.e., 51.3% (95% CI, 15.9 to 71.8; P = 0.01) and 34.3% (95% CI, −59.9 to 73.0; P = 0.36), and were lower in 82 children who received IPTi doses 1 and 3 in the dry season and dose 2 in the rainy season (11.4% [95% CI, −34.9 to 41.8; P = 0.57] and −8.4% [95% CI, −141.4 to 51.3; P = 0.84]).
Potential rebound effects were examined for a period from 1 month after the last IPTi dose until the end of follow-up. Neither a rebound nor persisting protection was observed with respect to malaria, severe anemia, and mortality (Table 5) or as regards high-parasite-density malaria, hospitalization, outpatient attendance, and nonmalarial fevers (data not shown). The risk of asymptomatic parasitemia remained reduced until the end of the follow-up period in children who had received SP. However, risk and incidence of severe malaria and (virtually overlapping) life-threatening severe malarial anemia (Hb of <5 g/dl) were found to be increased approximately twofold in children who previously had received SP (Table 5). In the SP and placebo groups, 23 and 10 children, respectively, developed severe malarial anemia in the rebound period. This occurred at a median age of 19.8 months (range, 16.5 to 23.3 months), after a median time span of 20 weeks (range, 8 to 35 weeks) following dose 3, and at a median parasite density of 10,118/μl (range, 32 to 43,704/μl), without significant differences between SP and placebo groups. Glucose-6-phosphate dehydrogenase typing (21) of the patients with a rebound effect revealed that three boys in the SP group were hemizygously deficient; however, severe malarial anemia in these children occurred more than 20 weeks after SP administration, rendering drug-induced hemolysis unlikely. Three of the 33 children with severe malarial anemia died (placebo group, one child; SP group, two children) after 1.5, 3, and 9 months. Death was due to new episodes of severe malaria (placebo group, one child; SP group, one child) and to an unknown, febrile disease (SP group, one child).
TABLE 5.
Frequency and risks of outcomes during the potential rebound period (16 to 24 months of age)
| Outcome | Placebo group (n = 529)
|
SP group (n = 525)
|
% PE (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|---|
| No. of events or cases | PYARa | Rateb | No. of events or cases | PYAR | Rate | |||
| Malaria | ||||||||
| All episodes | 553 | 313.1 | 1.77 | 548 | 308.2 | 1.78 | −1.0 (−17.6-13.2) | 0.89 |
| First or only episode | 319 | 0.60 | 311 | 0.59 | 1.8 (−8.5-11.0) | 0.73 | ||
| Anemia (Hb of <7 g/dl) | ||||||||
| All episodes | 220 | 331.4 | 0.66 | 208 | 327.0 | 0.64 | 3.8 (−21.1-23.6) | 0.74 |
| First or only episode | 164 | 0.31 | 151 | 0.29 | 7.2 (−11.7-22.9) | 0.43 | ||
| Severe malaria | ||||||||
| All episodes | 13 | 344.3 | 0.04 | 25 | 338.4 | 0.07 | −97.2 (−296.6-2.0) | 0.06 |
| First or only episode | 12 | 0.02 | 24 | 0.05 | −101.5 (−298.9 to −1.8) | 0.04 | ||
| Severe malarial anemia | ||||||||
| All episodes | 11 | 344.4 | 0.03 | 24 | 338.4 | 0.07 | −124.2 (−373.0 to −6.2) | 0.03 |
| First or only episode | 10 | 0.02 | 23 | 0.04 | −131.8 (−382.3 to −11.4) | 0.03 | ||
| Asymptomatic parasitemia | ||||||||
| All episodes | 282 | 328.8 | 0.86 | 238 | 326.1 | 0.73 | 14.8 (−4.4-30.4) | 0.12 |
| First or only episode | 208 | 0.39 | 172 | 0.33 | 16.7 (2.0-29.1) | 0.03 | ||
| Death | 13 | 0.02 | 6 | 0.01 | 53.1 (−22.4-82.1) | 0.12 | ||
PYAR, person-years at risk. For this calculation person-years at risk starts at 16 months of age.
Rate designates either incidence density (number of events/person-year at risk) or risk (number of cases/children at risk).
Lastly, AEs were analyzed. Vomiting after drug administration occurred in 2.2 to 5% of children and was not related to SP treatment. In total, 9,022 AEs were registered, most of which affected the gastrointestinal and respiratory tracts (Table 6). Overall, AEs occurred at similar rates in children receiving placebo and in children receiving SP. Scabies, however, was less frequently seen in SP-treated children than in placebo-treated children. Analysis of AEs occurring within a month following drug application basically revealed the same pattern. One child developed a moderately severe, pruritic, and exfoliative skin rash which started 4 days after the first dose and which was regarded as possibly related to treatment. Emergency unblinding revealed that SP had been administered; this child was excluded from the study and under symptomatic treatment recovered within 3 days. During the complete follow-up period, 30 deaths occurred in the placebo group, and 29 occurred in the SP group (Table 3); none of these was considered related to treatment.
TABLE 6.
AEs during complete follow-up period and for 1 month after treatments
| AE | Complete follow-up period (3-24 mo)
|
1 mo after treatment
|
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo group
|
SP group
|
Placebo group
|
SP group
|
|||||
| % | No. of events | % | No. of events | % | No. of events | % | No. of events | |
| Total | 100.0 | 4,506 | 100.0 | 4,516 | 100.0 | 522 | 100.0 | 490 |
| Skin | 11.5 | 519 | 9.8 | 442 | 7.7 | 40 | 4.9 | 24 |
| Infection | 4.2 | 189 | 4.3 | 193 | 1.5 | 8 | 1.6 | 8 |
| Rash | 1.9 | 85 | 1.7 | 75 | 2.3 | 12 | 1.0 | 5 |
| Scabies | 4.0 | 182 | 2.8b | 128 | 2.7 | 14 | 1.0 | 5 |
| Gastrointestinal tract | 40.3 | 1,815 | 40.9 | 1,846 | 42.3 | 221 | 42.4 | 208 |
| Diarrhea | 11.7 | 525 | 12.0 | 544 | 18.6 | 97 | 16.9 | 83 |
| Gastroenteritis | 23.8 | 1,071 | 23.8 | 1,074 | 19.5 | 102 | 21.4 | 105 |
| Respiratory tract | 29.0 | 1,306 | 30.4 | 1,371 | 32.0 | 167 | 35.7 | 175 |
| URTIa | 23.4 | 1,054 | 24.6 | 1,110 | 26.1 | 136 | 30.8 | 151 |
| Pneumonia | 2.2 | 98 | 2.5 | 112 | 2.5 | 13 | 2.9 | 14 |
| Otitis | 3.4 | 152 | 3.8 | 172 | 4.8 | 25 | 3.5 | 17 |
| Conjunctivitis | 3.5 | 157 | 3.8 | 172 | 2.1 | 11 | 2.2 | 11 |
| Other | 12.4 | 557 | 11.4 | 513 | 11.1 | 58 | 11.2 | 55 |
URTI, upper respiratory tract infection.
P < 0.0001.
DISCUSSION
IPTi is a simple intervention of minimal infrastructural requirements if given alongside EPI. In this study among 1,200 children from a region of hyperendemicity, overall well-tolerated IPTi prevented 22% of malaria episodes, 34% of cases of asymptomatic parasitemia, 24% of episodes of severe anemia, and 31% of hospital admissions but had no influence on severe malaria and mortality. Overall, these results are in accordance with the 20 to 25% reduction in morbidity observed in the studies following the initial IPTi trial in Tanzania (6, 17, 18, 29). However, following the intervention, 12 additional cases of severe malaria occurred in the SP group. Does this rebound of severe malaria call into question the net benefit of IPTi? If we estimate that some 2% of malaria attacks develop into severe disease (14), the number of uncomplicated episodes prevented translates into three or four averted cases of severe malaria. This would not abolish the excess number of severe malaria cases in the rebound phase but would equalize the total number of such events in the SP and placebo groups. None of the episodes of severe malarial anemia directly contributed to death, which accords with the previously observed absence of fatality among pediatric patients in the Tamale hospital exhibiting this symptom exclusively (22). Altogether, IPTi in this trial significantly reduced malaria-associated morbidity.
There was no evidence for an effect on mortality. However, this study did not have the necessary statistical power to assess this. A meta-analysis of available data is currently being performed by the IPTi Consortium (unpublished data) and should overcome this weakness. A further limitation of the present study might have been caused by the civil conflict during which it was conducted. As a result, parents could have been impeded from bringing their sick children to the health center, resulting in a detection bias towards a lesser effect of IPTi. We cannot exclude this potential bias; however, when the state of emergency was terminated, all children had completed their first year of life, during which the effects of IPTi were most pronounced.
A rebound effect was not observed in Tanzania (29, 30) or in Mozambique (18) but in two studies in Ghana: an increase of high-parasite-density malaria in the very northern part of the country and of anemia (Hb of <7.5 g/dl) in the south (6, 17). Rebound effects are considered to reflect impaired development of protective immunity due to an antimalarial intervention (15, 20). Similarly, persisting asymptomatic and polyclonal Plasmodium falciparum infections have been shown to reduce the prospective risk of clinical malaria and reduced premunition is held responsible for the rebound in morbidity observed after chemoprophylaxis (2, 3).
If so, how can sustained protection against asymptomatic parasitemia following IPTi, no rebound of uncomplicated malaria, and increased risk of severe malaria be explained? This question directly relates to the mode of action of IPTi, which is not fully understood. SP has a half-life of some 3 to 5 days and a chemoprophylactic effect of about a month (34). In the present study, the prevention of uncomplicated malaria was largely confined to 1 month after IPTi applications, suggesting a primarily prophylactic effect. However, additional mechanisms might also be involved. Persisting protection by IPTi into the second year of life in Tanzania has been attributed to an attenuation or containment of parasites exposed to SP, consequently preventing clinical disease but providing an enhanced opportunity to develop protective immunity (13, 30). IPTi in Tamale had the strongest and most sustained effect on asymptomatic parasitemia including the intervals between treatments and the phase following intervention. In regions of high endemicity, immune protection against severe malaria appears to develop fast, requiring only a few episodes of parasite exposure and indicating a component of strain-transcending immunity (16). Preventing asymptomatic infections might interfere with this and consequently increase the risk of severe malaria. On the other hand, decreasing the number of parasitemic episodes or reducing them to submicroscopic levels might still provide sufficient exposure to develop persistent antiparasite immunity. Although this hypothesis is speculative and—in the absence of reliable biomarkers—not demonstrable, it could partially explain the apparent discrepancy between increased risk of severe malaria and continuing prevention of parasitemia following IPTi.
The challenges of IPTi have been reviewed before (26). The success of EPI immunizations appears not to be affected (18, 29), and community acceptance of IPTi appears to be high (27). The timing of IPTi, so far, follows the EPI schedule, which might not be optimal everywhere, e.g., in areas of pronounced seasonal transmission or where the bulk of malaria morbidity occurs at a later age than in early childhood. Also, shorter intervals of the intervention might be an option to increase efficacy, but such would be a measure outside the EPI framework and thus increase operational difficulties. In the present study, we decided to administer one dose in the second year of life to extend protection. However, apart from the 1-month prophylactic effect following the IPTi dose at 15 months of age and the sustained suppression of asymptomatic parasitemia, no significant effect on malaria, anemia, or hospital admission was discernible in the second year of life. This is apparently plausible because only one dose was given in the second year of life compared to two doses in the first. However, comparing the 6-month periods following each IPTi administration, the PE of IPTi dose 3 was found to be substantially lower per se than that of the two preceding ones. Very similar results have recently been reported from southern Ghana (17). We applied SP at a dose of half a tablet irrespective of age as determined a priori. At 15 months of age, children thus received a comparatively lower dose per kg of body weight, which seems to be suboptimal (33). Still, the PEs during 1 month following IPTi dose 3 did not differ significantly from those of the two preceding treatments, indicating that half a tablet of SP was sufficient to at least achieve a prophylactic effect. In southern Ghana, SP-resistant parasites tended to occur more frequently with increasing age in both children receiving IPTi and children receiving placebo (17). While the reason for this is unknown, this could partially explain the lack of substantial benefit of IPTi administered at 15 months of age.
PE of IPTi was highest when all three doses were given in the rainy season, confirming previous results (6). Respective adaptation of dosing alongside EPI seems impossible in areas of perennial malaria transmission, but this finding rationalizes the comparatively high efficacy of “seasonal” IPT, i.e., administration during the transmission season only (8). The choice of drug for IPTi is another critical issue. SP has the benefits of single-dose administration, a relatively long half-life, low cost, extensive previous experience, and a favorable safety profile. In fact, in the present trial, no severe AE occurred and rates of vomiting after treatment and AEs were similar between SP and placebo groups. The reduced incidence of scabies in the IPTi group is unexpected; no reports on the efficacy of SP in this condition are available, but a functionally related substance, co-trimoxazole, has been used occasionally (31). Drug resistance to SP may endanger the efficacy of IPTi, and IPTi may contribute to its spread. In Tamale, SP resistance still is low (23). Following the idea that partial suppression of parasitemia by IPTi is one possible mode of action (13, 30), higher resistance rates might be tolerable, but the critical threshold is unknown. Does IPTi drive SP resistance? Results from Ghana point in this direction (19), but mathematical modeling suggests that the impact on a community base is rather small and less likely to occur in areas where partial resistance is already established (1, 25). Studies of drug resistance, alternative drugs, timing, potential synergisms with insecticide-treated nets, and further issues relevant to the implementation of IPTi are currently being conducted by the IPTi Consortium (28).
In conclusion, IPTi given at 3, 9, and 15 months of age reduced the incidences of malaria, severe anemia, asymptomatic parasitemia, and hospital admissions in northern Ghana, an area of hyperendemicity. The rebound of severe malarial anemia dilutes these beneficial effects and warrants further investigation before IPTi can unconditionally be recommended for implementation.
Acknowledgments
This work was supported by the German Ministry of Education and Research (grant 01KA0202), the German Academic Exchange Service (DAAD), and Charité—University Medicine Berlin (grant 2005-543).
We thank all children and their families for participation in this study as well as all members of the Northern Region Malaria Project (NORMAP). We thank Andrew Seidu-Korkor and Elias Sory, Regional Health Administration, Tamale, for general and infrastructural support; Susanne Röwer and Bärbel Jakob, Institute of Tropical Medicine Berlin, for excellent technical assistance; Cornelia Bevilacqua for the external audit; John Aponte and Andrea Egan, IPTi Consortium, for advice on statistical analysis and general support; and Mark A. James for critically reading the manuscript. F. Hoffmann-La Roche (Basel, Switzerland) provided study drugs free of charge, and Mepha (Basel, Switzerland) donated artesunate.
The authors do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Alexander, N., C. Sutherland, C. Roper, B. Cisse, and D. Schellenberg. 2007. Modelling the impact of intermittent preventive treatment for malaria on selection pressure for drug resistance. Malar. J. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, H. P., I. Felger, P. Vounatsou, R. Hirt, M. Tanner, P. Alonso, and C. Menendez. 1999. Effect of iron supplementation and malaria prophylaxis in infants on Plasmodium falciparum genotypes and multiplicity of infection. Trans. R. Soc. Trop. Med. Hyg. 93(Suppl. 1):41-45. [DOI] [PubMed] [Google Scholar]
- 3.Bereczky, S., A. Liljander, I. Rooth, L. Faraja, F. Granath, S. M. Montgomery, and A. Färnert. 2007. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 9:103-110. [DOI] [PubMed] [Google Scholar]
- 4.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):1-11. [DOI] [PubMed] [Google Scholar]
- 5.Breman, J. G., M. S. Alilio, and A. Mills. 2004. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 71(Suppl. 2):1-15. [PubMed] [Google Scholar]
- 6.Chandramohan, D., S. Owusu-Agyei, I. Carneiro, T. Awine, K. Amponsa-Achiano, N. Mensah, S. Jaffar, R. Baiden, A. Hodgson, F. Binka, and B. Greenwood. 2005. Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ 331:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Onis, M., and M. Blossner. 2003. The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. Int. J. Epidemiol. 32:518-526. [DOI] [PubMed] [Google Scholar]
- 8.Dicko, A., S. Sagara, M. S. Sissoko, O. Guindo, A. B. I. Diallo, M. Kone, M. A. Thera, M. Sacko, and O. K. Doumbo. 2004. Impact of intermittent preventive treatment with sulfadoxine pyrimethamine targetting the transmission season on the incidence of clinical malaria in children of 6 months to 10 years in Kambila, Mali, abstr. 18. Abstr. 53rd Annu. Meet. Am. Soc. Trop. Med. Hyg.
- 9.Dzisi, S. Y. 2002. Childhood malaria in the Bulpeila subdistrict of Tamale, northern Ghana: a study on knowledge, perceptions and attitudes, and socio-demographic factors among care givers. M.Sc. thesis. Charité—University Medicine Berlin, Berlin, Germany.
- 10.Ehrhardt, S., G. D. Burchard, C. Mantel, J. P. Cramer, S. Kaiser, M. Kubo, R. Otchwemah, U. Bienzle, and F. P. Mockenhaupt. 2006. Malaria, anemia, and malnutrition in African children—defining intervention priorities. J. Infect. Dis. 194:108-114. [DOI] [PubMed] [Google Scholar]
- 11.Geerligs, P. D., B. J. Brabin, and T. A. Eggelte. 2003. Analysis of the effects of malaria chemoprophylaxis in children on haematological responses, morbidity and mortality. Bull. W. H. O. 81:205-216. [PMC free article] [PubMed] [Google Scholar]
- 12.Ghana Health Service. 2004. Standard treatment guidelines, 5th ed. Ghana National Drugs Programme, Accra, Ghana.
- 13.Greenwood, B. 2007. Intermittent preventive antimalarial treatment in infants. Clin. Infect. Dis. 45:26-28. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood, B., K. Marsh, and R. Snow. 1991. Why do some African children develop severe malaria? Parasitol. Today 7:277-281. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood, B. M., P. H. David, L. N. Otoo-Forbes, S. J. Allen, P. L. Alonso, J. R. Armstrong Schellenberg, P. Byass, M. Hurwitz, A. Menon, and R. W. Snow. 1995. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans. R. Soc. Trop. Med. Hyg. 89:629-633. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 17.Kobbe, R., C. Kreuzberg, S. Adjei, B. Thompson, I. Langefeld, P. A. Thompson, H. H. Abruquah, B. Kreuels, M. Ayim, W. Busch, F. Marks, K. Amoah, E. Opoku, C. G. Meyer, O. Adjei, and J. May. 2007. A randomized controlled trial of intermittent preventive antimalarial treatment in infants. Clin. Infect. Dis. 45:16-25. [DOI] [PubMed] [Google Scholar]
- 18.Macete, E., P. Aide, J. J. Aponte, S. Sanz, I. Mandomando, M. Espasa, B. Sigauque, C. Dobano, S. Mabunda, M. Dgedge, P. Alonso, and C. Menendez. 2006. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J. Infect. Dis. 194:276-285. [DOI] [PubMed] [Google Scholar]
- 19.Marks, F., V. von Kalckreuth, R. Kobbe, S. Adjei, O. Adjei, R. D. Horstmann, C. G. Meyer, and J. May. 2005. Parasitological rebound effect and emergence of pyrimethamine resistance in Plasmodium falciparum after single-dose sulfadoxine-pyrimethamine. J. Infect. Dis. 192:1962-1965. [DOI] [PubMed] [Google Scholar]
- 20.Menendez, C., E. Kahigwa, R. Hirt, P. Vounatsou, J. J. Aponte, F. Font, C. J. Acosta, D. M. Schellenberg, C. M. Galindo, J. Kimario, H. Urassa, B. Brabin, T. A. Smith, A. Y. Kitua, M. Tanner, and P. L. Alonso. 1997. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet 350:844-850. [DOI] [PubMed] [Google Scholar]
- 21.Mockenhaupt, F. P., J. Mandelkow, H. Till, S. Ehrhardt, T. A. Eggelte, and U. Bienzle. 2003. Reduced prevalence of Plasmodium falciparum infection and of concomitant anaemia in pregnant women with heterozygous G6PD deficiency. Trop. Med. Int. Health 8:118-124. [DOI] [PubMed] [Google Scholar]
- 22.Mockenhaupt, F. P., S. Ehrhardt, J. Burkhardt, S. Y. Bosomtwe, S. Laryea, S. D. Anemana, R. N. Otchwemah, J. P. Cramer, E. Dietz, S. Gellert, and U. Bienzle. 2004. Manifestation and outcome of severe malaria in children in northern Ghana. Am. J. Trop. Med. Hyg. 71:167-172. [PubMed] [Google Scholar]
- 23.Mockenhaupt, F. P., S. Ehrhardt, S. Y. Dzisi, T. J. Bousema, N. Wassilew, J. Schreiber, S. D. Anemana, J. P. Cramer, R. N. Otchwemah, R. W. Sauerwein, T. A. Eggelte, and U. Bienzle. 2005. A randomized, placebo-controlled, double-blind trial on sulfadoxine-pyrimethamine alone or combined with artesunate or amodiaquine in uncomplicated malaria. Trop. Med. Int. Health 10:512-520. [DOI] [PubMed] [Google Scholar]
- 24.Mockenhaupt, F. P., S. Ehrhardt, T. A. Eggelte, P. Agana-Nsiire, K. Stollberg, A. Mathieu, M. Markert, R. N. Otchwemah, and U. Bienzle. 2005. Chloroquine-treatment failure in northern Ghana: roles of pfcrt T76 and pfmdr1 Y86. Ann. Trop. Med. Parasitol. 99:723-732. [DOI] [PubMed] [Google Scholar]
- 25.O'Meara, W. P., D. L. Smith, and F. E. McKenzie. 2006. Potential impact of intermittent preventive treatment (IPT) on spread of drug-resistant malaria. PLoS Med. 3:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Meara, W. P., J. G. Breman, and F. E. McKenzie. 2005. The promise and potential challenges of intermittent preventive treatment for malaria in infants (IPTi). Malar. J. 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pool, R., K. Munguambe, E. Macete, P. Aide, G. Juma, P. Alonso, and C. Menendez. 2006. Community response to intermittent preventive treatment delivered to infants (IPTi) through the EPI system in Manhica, Mozambique. Trop. Med. Int. Health 11:1670-1678. [DOI] [PubMed] [Google Scholar]
- 28.Schellenberg, D., B. Cisse, and C. Menendez. 2006. The IPTi Consortium: research for policy and action. Trends Parasitol. 22:296-300. [DOI] [PubMed] [Google Scholar]
- 29.Schellenberg, D., C. Menendez, E. Kahigwa, J. Aponte, J. Vidal, M. Tanner, M. Mshinda, and P. Alonso. 2001. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: a randomised, placebo-controlled trial. Lancet 357:1471-1477. [DOI] [PubMed] [Google Scholar]
- 30.Schellenberg, D., C. Menendez, J. J. Aponte, E. Kahigwa, M. Tanner, H. Mshinda, and P. Alonso. 2005. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 365:1481-1483. [DOI] [PubMed] [Google Scholar]
- 31.Shashindran, C. H., I. S. Gandhi, and S. Lal. 1979. A trial of cotrimoxazole in scabies. Br. J. Dermatol. 100:483. [DOI] [PubMed] [Google Scholar]
- 32.Stoltzfus, R. 1997. Rethinking anaemia surveillance. Lancet 349:1764-1766. [DOI] [PubMed] [Google Scholar]
- 33.Terlouw, D. J., B. L. Nahlen, J. M. Courval, S. K. Kariuki, O. S. Rosenberg, A. J. Oloo, M. S. Kolczak, W. A. Hawley, A. A. Lal, and F. O. Kuile. 2003. Sulfadoxine-pyrimethamine in treatment of malaria in Western Kenya: increasing resistance and underdosing. Antimicrob. Agents Chemother. 47:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winstanley, P. A., W. M. Watkins, C. R. Newton, C. Nevill, E. Mberu, P. A. Warn, C. M. Waruiru, I. N. Mwangi, D. A. Warrell, and K. Marsh. 1992. The disposition of oral and intramuscular pyrimethamine/sulphadoxine in Kenyan children with high parasitaemia but clinically non-severe falciparum malaria. Br. J. Clin. Pharmacol. 33:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. 1986. WHO Expert Committee on Malaria. Eighteenth report. WHO Tech. Rep. Ser. 735:65-66. [PubMed] [Google Scholar]
- 36.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):1-90. [PubMed] [Google Scholar]