Abstract
Three clinically relevant intermittent regimens, and a continuous infusion, of colistin were simulated in an in vitro pharmacokinetic/pharmacodynamic model against two colistin-heteroresistant strains of Acinetobacter baumannii. Extensive initial killing was followed by regrowth as early as 6 h later; bacterial density in the 24- to 72-h period was within 1 log10 CFU/ml of growth control. Population analysis profiles revealed extensive emergence of resistant subpopulations regardless of the colistin regimen.
Multidrug-resistant (MDR) Acinetobacter baumannii has emerged in recent times as a major cause of nosocomial infections worldwide (4, 6, 20, 24, 25), particularly in patients who are critically ill or immunocompromised. Of clinical concern are reports from global antimicrobial resistance surveillance programs that indicate a stepwise trend towards multidrug resistance (25, 26). A. baumannii clinical strains have become resistant to almost all antibiotics that are currently available, except polymyxins (e.g., colistin) (3, 7, 20, 24). As a result, clinicians have been forced to reappraise the clinical value of the “old” drug colistin (2, 14, 15). Two forms of “colistin” are available commercially: colistin sulfate and sodium colistin methanesulfonate (CMS), the latter being the form that is administered parenterally. Recent clinical reports suggest that CMS therapy against MDR A. baumannii infections has been successful (8, 9, 19, 21, 23). However, the majority of reports evaluating the clinical use of CMS have been retrospective (2, 7) and must therefore be interpreted with some caution (15). Unfortunately, resistance to colistin has emerged with increasing use of CMS (13), and the recent observation of heteroresistance to colistin among clinical strains of MDR A. baumannii is also a significant cause for concern (17). There is an urgent need to investigate the effects of various dosage regimens with a view to optimizing therapy. Our aim was to evaluate the antibacterial activity of clinically relevant CMS dosage regimens against heteroresistant A. baumannii. In addition, the effect of the dosage regimens on the emergence of resistance to colistin was examined.
It is important to note that CMS is an inactive prodrug of colistin (1), and therefore the pharmacokinetic (PK) profiles employed in the present study were from the administration of colistin sulfate to mimic the PKs of colistin, generated from CMS, in humans (10, 16). The in vitro PK/pharmacodynamic (PD) model resembled the one-compartment PK model described previously (5). The temperature of the central compartment was maintained at 37°C, and a peristaltic pump (Masterflex L/S; Cole-Parmer) was used to deliver sterile cation-adjusted Mueller-Hinton broth (Oxoid) from a reservoir into the central compartment. An aliquot (1 ml) of early-log-phase bacterial suspension was inoculated into the central compartment to achieve approximately 106 CFU/ml at the start of the experiments. Furthermore, the simulations were based upon the fact that the unbound fraction of colistin in human plasma is approximately 0.5 (unpublished data). Four colistin regimens were simulated in the present study (Table 1). For the three intermittent regimens (regimens 1 to 3), the appropriate loading dose of colistin (sulfate) (lot 123K1382; Sigma-Aldrich) was introduced into the central compartment at the start of the experimental period followed by intermittent maintenance doses of colistin at 8-, 12-, or 24-h intervals. The volume of the central compartment (100 ml) and flow conditions (0.3 ml/min) produced a target colistin half-life of 4 h. The fourth regimen, simulating a continuous infusion producing a steady-state concentration of colistin of 4.5 μg/ml, was achieved by spiking this concentration of colistin into the cation-adjusted Mueller-Hinton broth that was placed in the reservoir. Colistin is stable in solution at 37°C for up to 120 h (11). Controls were included to define growth dynamics in the absence of colistin. All experiments were conducted in three replicates. Sampling times are shown in Table 1. Samples were measured by high-performance liquid chromatography (12) to verify the simulated colistin PKs. Counting of viable bacteria was conducted by spiral plating (WASP2; Don Whitley Scientific Ltd.) and using a ProtoCOL colony counter (Don Whitley Scientific Ltd.); the limit of detection was 20 CFU/ml. Real-time population analysis profiles (PAPs) were conducted on samples collected at 24, 48, and 72 h to detect the presence of colistin-heteroresistant bacterial subpopulations (17).
TABLE 1.
Colistin dosage regimens and sampling times in the in vitro PK/PD model, and the resultant inoculum-normalized AUBC0-72 values
| Parameter | Result for:
|
|||
|---|---|---|---|---|
| Regimen 1 | Regimen 2 | Regimen 3 | Regimen 4 | |
| Loading dose (mg) | 0.30 | 0.45 | 0.90 | NAb |
| Maintenance dose (mg) | 0.23 | 0.39 | 0.89 | NAb |
| Dosage interval (h) | 8 | 12 | 24 | NAb |
| Target Cmax (μg/ml) | 3.0 | 4.5 | 9.0 | NAb |
| Target Cmin (μg/ml) | 0.75 | 0.56 | 0.14 | NAb |
| Target Css (μg/ml) | NAa | NAa | NAa | 4.5 |
| Sampling times (h) for microbiological measurements | 0, 0.5, 1, 2, 4, 6, 8, 8.5, 16, 16.5, 24, 24.5, 25, 26, 32, 32.5, 40, 40.5, 48, 48.5, 49, 50, 56, 56.5, 64, 64.5, and 72 | 0, 0.5, 1, 2, 4, 6, 8, 12, 12.5, 24, 24.5, 25, 26, 36, 36.5, 48, 48.5, 49, 50, 60, 60.5, and 72 | 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 24.5, 25, 26, 36, 48, 48.5, 49, 50, 60, and 72 | 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 24.5, 25, 26, 36, 48, 48.5, 49, 50, 60, and 72 |
| AUBC0-72/(log10 CFU/mlt= 0) (n = 3) | ||||
| Clinical strain 6 | 68.0 ± 1.21 | 64.9 ± 3.55 | 58.5 ± 3.56 | 65.6 ± 0.92 |
| ATCC 19606 | 68.5 ± 0.31 | 68.5 ± 1.80 | 67.0 ± 0.10 | 72.7 ± 2.29 |
Not applicable (NA), as the regimens involved the intermittent administration of colistin.
Not applicable (NA), as a constant steady-state concentration (Css) was generated.
Regimen 1 (Table 1) simulated the unbound plasma concentration-time profile of colistin achieved with the recommended daily dosage regimen (5 mg/kg of body weight/day of colistin base activity) (Coly-Mycin M parenteral product information; Monarch Pharmaceuticals, Bristol, TN) of CMS administered every 8 hours in humans with normal renal function. Regimens 2 and 3 simulated larger intermittent doses of CMS administered in humans at longer dosage intervals (12 h and 24 h, respectively). The product information indicates that for patients with normal renal function, a 12-h dosage interval may be used, and although not recommended in the product information, the administration of larger doses at 24-h intervals has recently been reported (22). Again, although not recommended in the product information, CMS has been administered by continuous intravenous infusion in humans (8, 18); this situation was simulated by regimen 4. A reference strain (A. baumannii ATCC 19606 [American Type Culture Collection, Manassas, VA]) and a clinical strain (clinical strain number 6) (17) were examined. Both strains belonged to distinct clonotypes (17). By use of the VITEK card (GNS-424; bioMérieux), ATCC 19606 was resistant to gentamicin and ciprofloxacin, clinical strain 6 was resistant to ceftazidime, and both strains were resistant to ceftriaxone (17). The preexposure MICs for both strains were 1 μg/ml for colistin (sulfate). The time-averaged microbiological response was quantified by calculation of the area under the bacterial kill-time curves from 0 to 72 h (AUBC0-72), using the linear trapezoidal rule, and was normalized by the respective log10 CFU/ml value before the introduction of colistin (Fig. 1; Table 1).
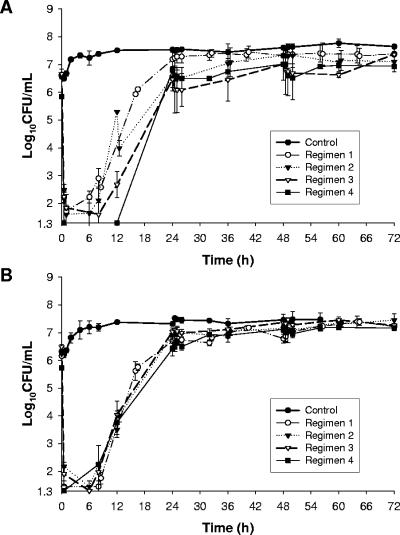
FIG. 1.
Microbiological responses observed in the in vitro PK/PD model simulating the colistin PKs of different dosing regimens against clinical strain 6 (A) and ATCC 19606 (B) for regimen 1 (maximum concentration of drug in plasma [Cmax], 3 μg/ml), regimen 2 (Cmax, 4.5 μg/ml), regimen 3 (Cmax, 9 μg/ml), and regimen 4 (continuous maintenance of 4.5 μg/ml). Data are presented as means and standard deviations.
The microbiological responses for all regimens and each strain are shown in Fig. 1. The features common to all dosing regimens for both strains were as follows: (i) extensive bacterial killing (>4 log10 reduction in CFU/ml) within 30 min of the initiation of colistin administration; (ii) regrowth observed as early as 6 h from the commencement of the colistin regimens; (iii) minor or no bacterial killing evident after the second and subsequent doses (with the intermittent regimens); and (iv) very extensive regrowth across the 6- to 24-h period, such that the bacterial density in the 24- to 72-h period was within approximately 1 log10 CFU/ml of that of the corresponding growth control (Fig. 1). The inoculum-normalized AUBC0-72 values were very similar for all regimens and strains (Table 1). The regrowth observed in the first several hours after initiation of colistin administration, despite the existence of colistin concentrations well above the MIC, is consistent with the amplification of resistant subpopulations. Indeed, real-time PAPs revealed a very substantial increase in the proportion of resistant populations at 24, 48, and 72 h (Fig. 2); these were substantially different from the PAP profiles at the baseline (i.e., prior to colistin exposure) and also those for the growth controls at 24 and 48 h (data not shown) and at 72 h (Fig. 2). The data presented for regimen 1 in Fig. 2 are highly representative of the PAPs for all other regimens.
FIG. 2.
PAPs of clinical strain 6 (A) and ATCC 19606 (B) from the in vitro PK/PD model for regimen 1. The profile for baseline is from reference 17.
In conclusion, colistin is believed to be very active against MDR A. baumannii based on MICs, and its use has been substantially increased worldwide in recent years. However, we have shown that the exposure to colistin generated from current recommended dosage regimens for CMS is inadequate to prevent the emergence of resistance in vitro. This study suggests very strongly that great care is required with monotherapy with intravenous CMS, especially in immunocompromised patients, for infections caused by colistin-heteroresistant A. baumannii, due to the potential risk of emergence of resistant bacteria. It is imperative that we preserve the utility of this important antibiotic by promoting its judicious use and developing new therapeutic strategies to combat the emergence of resistance.
Footnotes
Published ahead of print on 9 July 2007.
REFERENCES
- 1.Bergen, P. J., J. Li, C. R. Rayner, and R. L. Nation. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas, M., and S. Kasiakou. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333-1341. [DOI] [PubMed] [Google Scholar]
- 3.Gales, A., R. Jones, and H. Sader. 2006. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001-2004). Clin. Microbiol. Infect. 12:315-321. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes, R., and J. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 5.Grasso, S., G. Meinardi, I. De Carneri, and V. Tamassia. 1978. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob. Agents Chemother. 13:570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Infectious Diseases Society of America. 2004. Bad bugs, no drugs. Infectious Diseases Society of America. http://www.idsociety.org/pa/IDSA_Paper4_final_web.pdf. [DOI] [PubMed]
- 7.Jain, R., and L. H. Danziger. 2004. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann. Pharmacother. 38:1449-1459. [DOI] [PubMed] [Google Scholar]
- 8.Kasiakou, S. K., K. Fragoulis, G. Tzagarakis, P. Mistidis, A. Kapaskelis, and M. E. Falagas. 2005. Cure of multidrug-resistant Acinetobacter baumannii fixation device-related orthopedic infections in two patients with intravenous colistin. Microb. Drug Resist. 11:287-289. [DOI] [PubMed] [Google Scholar]
- 9.Katragkou, A., and E. Roilides. 2005. Successful treatment of multidrug-resistant Acinetobacter baumannii central nervous system infections with colistin. J. Clin. Microbiol. 43:4916-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, J., K. Coulthard, R. Milne, R. L. Nation, S. Conway, D. Peckham, C. Etherington, and J. Turnidge. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 52:987-992. [DOI] [PubMed] [Google Scholar]
- 11.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, and K. Coulthard. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma studied by high-performance liquid chromatography. Antimicrob. Agents Chemother. 47:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and D. W. Johnson. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B 761:167-175. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., and R. L. Nation. 2006. Old polymyxins are back: is resistance close? Clin. Infect. Dis. 43:663-664. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., R. L. Nation, R. W. Milne, J. D. Turnidge, and K. Coulthard. 2005. Evaluation of colistin as an agent against multi-resistant gram-negative bacteria. Int. J. Antimicrob. Agents 25:11-25. [DOI] [PubMed] [Google Scholar]
- 15.Li, J., R. L. Nation, J. D. Turnidge, R. W. Milne, K. Coulthard, C. R. Rayner, and D. L. Paterson. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 6:589-601. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., C. R. Rayner, R. L. Nation, R. Deans, R. Boots, N. Widdecombe, A. Douglas, and J. Lipman. 2005. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob. Agents Chemother. 49:4814-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, J., C. R. Rayner, R. L. Nation, R. J. Owen, D. Spelman, K. E. Tan, and L. Liolios. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalopoulos, A., S. Kasiakou, E. Rosmarakis, and M. Falagas. 2005. Cure of multidrug-resistant Acinetobacter baumannii bacteraemia with continuous intravenous infusion of colistin. Scand. J. Infect. Dis. 37:142-145. [DOI] [PubMed] [Google Scholar]
- 19.Mubareka, S., and E. Rubinstein. 2005. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Crit. Care 9:29-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peleg, A. Y., and D. L. Paterson. 2006. Multidrug-resistant Acinetobacter: a threat to the antibiotic era. Intern. Med. J. 36:479-482. [DOI] [PubMed] [Google Scholar]
- 21.Reina, R., E. Estenssoro, G. Saenz, H. S. Canales, R. Gonzalvo, G. Vidal, G. Martins, A. Das Neves, O. Santander, and C. Ramos. 2005. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med. 31:1058-1065. [DOI] [PubMed] [Google Scholar]
- 22.Rosenvinge, A., T. Pressler, and N. Hoiby. 2005. Colistin intravenously is a safe and effective treatment of multidrug-resistant microorganisms in cystic fibrosis. J. Cyst. Fibros. 4:S32. [Google Scholar]
- 23.Sueke, H., H. Marsh, and A. Dhital. 2005. Using intrathecal colistin for multidrug resistant shunt infection. Br. J. Neurosurg. 19:51-52. [DOI] [PubMed] [Google Scholar]
- 24.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 25.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10:684-704. [DOI] [PubMed] [Google Scholar]
- 26.Villegas, M., and A. Hartstein. 2003. Acinetobacter outbreaks, 1977-2000. Infect. Control Hosp. Epidemiol. 24:284-295. [DOI] [PubMed] [Google Scholar]