Abstract
A novel oxazolidinone, AM 7359, was evaluated in two mouse models of Staphylococcus aureus infection. AM 7359 and linezolid were equally efficacious in a methicillin-susceptible S. aureus organ burden model and a methicillin-resistant S. aureus localized infection model. However, AM 7359 was eightfold more efficacious than linezolid against a linezolid- and methicillin-resistant S. aureus strain in this localized (thigh) infection model.
The emerging resistance of Staphylococcus aureus and Enterococcus spp. to presently available antibiotics has reduced the options for antimicrobial therapy (3, 5, 7).
The oxazolidinones are a new therapeutic class of drugs that inhibit the initiation of protein synthesis (8, 16). The widely used oxazolidinone linezolid (Zyvox) is active against most gram-positive pathogens, including methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus spp., and penicillin-resistant Streptococcus pneumoniae (14, 15). However, linezolid is limited by its inhibition of monoamine oxidase and the potential for myelosuppression (2, 11; linezolid package insert [Pharmacia]).
AM 7359 is a novel oxazolidinone which exhibits improved efficacy and activity against a broader spectrum of organisms, including Haemophilus influenzae and Moraxella catarrhalis (for which the MIC90s are 2 and 0.5 μg/ml, respectively) (10). We report here the preliminary evaluation of AM 7359 against methicillin-susceptible S. aureus (MSSA) strain Smith, MRSA strain COL, and linezolid-resistant MRSA (LMRSA) isolates in two mouse models of infection. In vitro MICs of AM 7359 and linezolid for these isolates were determined by procedures recommended by the Clinical and Laboratory Standards Institute (4).
(Results of this work were presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006.)
Efficacy in the organ burden model was assessed by intraperitoneally challenging C3H mice (Harlan Laboratories, Indianapolis, IN) with 2 × 103 CFU of MSSA Smith suspended in 0.5 ml of 5% hog gastric mucin. Within 15 min of challenge, AM 7359 and linezolid were given as single doses either orally (p.o.) by gavage or intravenously (i.v.). One day after challenge, paired kidneys from euthanized mice were removed aseptically, weighed, and placed in sterile Whirl-Pak bags (Fisher Scientific, Springfield, NJ) containing 5 ml of sterile saline. Kidneys were homogenized in the bags and serially diluted in saline, and aliquots were plated onto mannitol salt agar plates (BBL, Cockeysville, MD). Plates were incubated at 35°C for 48 h, and the numbers of CFU of the challenge organism were determined.
In the localized infection model, 0.2 ml of Trypticase soy broth containing 3.6 × 108 CFU of MRSA COL was injected intramuscularly into the right thighs of DBA/2 mice (Taconic Laboratories, Germantown, NY). These mice are deficient in complement component 5, which renders them more susceptible to challenge with MRSA. For p.o. dosing, the first antibiotic treatment was administered 2 h after challenge. Mice were treated again at 6, 10, 24, 48, 72, 96, and 120 h postinfection (eight doses in 5 days). Due to difficulty encountered in multiple i.v. dosing in the lateral tail veins, the MRSA-challenged mice treated i.v. received only the first four antibiotic doses (0.2 ml at 2, 6, 10, and 24 h post challenge). C3H mice were challenged with 109 CFU of LMRSA/thigh, and compounds were administered only p.o. as described above. Two days after the final antibiotic dose, mice were euthanized and denuded thighs were surgically removed, placed into tubes containing 4 ml of phosphate-buffered saline, and ground with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY). Serial dilutions in saline were plated onto mannitol salt agar, the plates were incubated at 35°C for 48 h, and the numbers of CFU of the organisms per thigh were determined.
The mean log10 numbers of CFU of the organisms per gram of kidneys or per thigh from the drug-treated groups were compared to those from the sham-treated control by using Student's t test (two-tailed and unpaired) on Microsoft Excel. Comparisons were deemed significant at a level of α of 0.05. Inverse regression was subsequently used for both tests to estimate 99% effective doses (ED99) defined as the doses (in milligrams per kilogram of body weight) that reduced the number of CFU per organ by 99% compared to the number for the sham-treated control (9, 12).
In the organ burden model, p.o. or i.v. treatment with AM 7359 or linezolid at 25 or 12.5 mg/kg significantly reduced kidney burdens relative to those of sham-treated mice (Table 1). While both compounds were effective in lowering the bacterial tissue burden, renal sterilization was seen only at the 25-mg/kg p.o. dose of linezolid. Based on the ED99, AM 7359 was equivalent to linezolid in efficacy against the MSSA strain in this model when administered p.o. (ED99, 4.9 versus 7.0 mg/kg, respectively) or i.v. (ED99, 6.3 versus 12 mg/kg) (Table 2).
TABLE 1.
Comparative efficacies of AM 7359 and linezolid against MSSA Smith in an organ burden assay
| Compound (mode of administration) and MIC (μg/ml) | Dose (mg/kg/day) | % Mortalitya | Log CFU/g of kidneyb (% eradication) | Reduction (SD)c |
|---|---|---|---|---|
| AM 7359 (p.o.), 0.125 | 25 | 0 | 3.11* (0) | 5.23 (0.58) |
| 12.5 | 0 | 5.41* (0) | 2.93 (0.59) | |
| 6.25 | 0 | 5.71* (0) | 2.63 (0.80) | |
| 3.12 | 0 | 8.60 (0) | −0.26 (0.37) | |
| Linezolid (p.o.), 2.0 | 25 | 0 | 2.27* (100) | 6.07 (0.04) |
| 12.5 | 0 | 5.63* (0) | 2.71 (0.37) | |
| 6.25 | 0 | 6.90* (0) | 1.44 (0.59) | |
| 3.12 | 0 | 8.87 (0) | −0.53 (0.10) | |
| AM 7359 (i.v.), 0.125 | 25 | 0 | 3.81* (0) | 4.59 (0.15) |
| 12.5 | 0 | 3.94* (0) | 4.46 (0.16) | |
| 6.25 | 0 | 6.37* (0) | 2.03 (0.61) | |
| 3.12 | 0 | 8.34 (0) | 0.06 (0.15) | |
| Linezolid (i.v.), 2.0 | 25 | 0 | 4.13* (0) | 4.27 (0.70) |
| 12.5 | 0 | 6.96* (0) | 1.44 (1.62) | |
| 6.25 | 0 | 8.52 (0) | −0.12 (0.20) | |
| 3.12 | 0 | 8.67 (0) | −0.27 (0.11) | |
| Control (distilled H2O; p.o.) | 20 | 8.34 (0) | ||
| Control (distilled H2O; i.v.) | 0 | 8.40 (0) |
Calculated as the number dead divided by the total number in the group.
*, significant reduction compared to sham-treated control as determined by Student's t test.
Reduction in log number of CFU compared to sham-treated control. Significant reductions are shown in bold. SD, standard deviation of results for the four mice per group.
TABLE 2.
ED99 of AM 7359 and linezolid in mouse efficacy models
| Model (organism) | ED99 (mg/kg) (95% CI) of:
|
|||
|---|---|---|---|---|
| AM 7359
|
Linezolid
|
|||
| p.o. | i.v. | p.o. | i.v. | |
| Kidney (MSSA)a | 4.9 (3.1-7.6) | 6.3 (4.2-9.5) | 7.0 (5.3-9.2) | 12 (7.8-18.5) |
| Thigh (MRSA)b | 4.2 (2.8-6.1) | 5.6 (2.3-∞) | 5.2 (4.2-6.6) | 6.9 (4.5-∞) |
| Thigh (LMRSA) | 10.2 (4.3-24) | NTa | 85 (50.2-∞) | NT |
Values are based on mice receiving a single dose 15 min postchallenge; therefore, ED99 values are equal to the total mg/kg/day given each mouse.
Orally dosed mice received a total of eight doses in 5 days and i.v. dosed mice a total of four doses in 24 h; therefore, the ED99 values represent the value of each individual dose given in each model.
c NT, not tested.
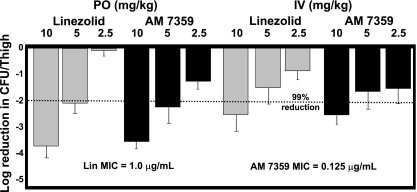
In the localized MRSA infection model, AM 7359 and linezolid administered p.o. were equally efficacious at 10 mg/kg (decrease, log10 ∼3.7 CFU) and 5 mg/kg (decrease, log10 ∼2.0 CFU) (Fig. 1). When given i.v., AM 7359 and linezolid reduced the burden by approximately log10 2.4 and 1.5 CFU at 10 and 5 mg/kg, respectively, compared to the burden in the sham-treated control. The ED99 of AM 7359 and linezolid in these localized assays are listed in Table 2. The better efficacy of approximately log10 1 CFU achieved when these compounds were administered p.o. rather than i.v. may be explained by the difference in dosing regimens or perhaps the greater time of exposure to the antibiotics, with only four doses given in the i.v. assay compared to eight doses (four in the first 24 h) in the p.o. assay. As others have shown previously, the best pharmacokinetics or pharmacodynamics parameter to correlate with the efficacy of this class of compounds against S. aureus is the ratio of the area under the concentration-time curve to the MIC (1). Interestingly, the greater in vitro activity of AM 7359 did not result in better efficacy than that of linezolid in these models. While the normalized areas under the concentration-time curves obtained at a 25 mg/kg dose were similar, 1.16 and 0.78 μg·h/ml for AM 7359 and linezolid, respectively, the levels of plasma protein binding of these two compounds were significantly different, with 19 and 68% free compound for AM 7359 and linezolid, respectively. This difference in protein binding was likely enough to bridge the difference in MICs and thereby equalize the ED99 data. Other factors such as lower bioavailability, 30% for AM 7359 versus 100% for linezolid, and a lower plasma clearance rate may also play a role in the discrepancy between these in vitro and in vivo results.
FIG. 1.
Efficacy of AM 7359 and linezolid (Lin) against an MRSA COL strain in a localized infection model. DBA/2 mice (five per group) were challenged intramuscularly with 3.6 × 108 CFU of MRSA in a 0.2-ml inoculum and treated p.o. three times a day for 1 day and once a day for 5 days or i.v. three times a day for 1 day and once a day for 1 day.
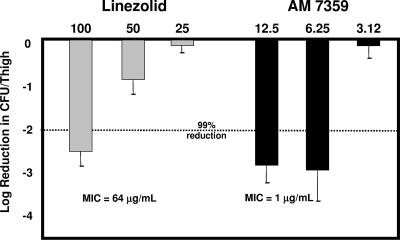
In the LMRSA thigh infection model, the in vitro activity seen with AM 7359 did translate into good in vivo efficacy (Fig. 2). While a reduction of log10 2.5 CFU by linezolid at 100 mg/kg compared to the sham-treated control was observed, the same or slightly better efficacy was seen for AM 7359 at 12.5 and 6.25 mg/kg. AM 7359 was eightfold (ED99 of 10.2 versus 85 mg/kg) more efficacious than linezolid against this LMRSA strain. To date, the complete mode of action of this series of compounds has not been elucidated. The activity against this LMRSA strain may indicate a different or supplemental biological target for AM 7359. This difference would allow AM 7359 to be used to treat infections caused by linezolid-resistant organisms.
FIG. 2.
Efficacy of AM 7359 and linezolid against an LMRSA strain in a localized infection model. C3H mice (five per group) were challenged intramuscularly with 109 CFU of LMRSA in a 0.2-ml inoculum and treated p.o. three times a day for 1 day and once a day for 5 days.
MRSA and vancomycin-resistant Enterococcus infections in hospitals and, more recently, community-acquired MRSA infections have resulted in increased mortality and costs (6, 13, 17). Even with the approval of three new antibacterials, quinupristin-dalfopristin, linezolid, and daptomycin, there is still a medical need for agents to treat the multidrug-resistant pathogens. AM 7359 is an oxazolidinone compound which exhibits a broad in vitro spectrum of activity against gram-positive organisms, including MRSA, vancomycin-resistant Enterococcus spp., and penicillin-resistant Streptococcus pneumoniae, and is also active against some gram-negative respiratory tract pathogens. These properties, along with the efficacy exhibited by AM 7359 against three different S. aureus strains, including a linezolid-resistant strain, in two mouse models of bacterial infection, warrant further evaluation of this compound series.
Acknowledgments
We thank Karen Dorso and Debbie Suber of the Clinical Microbiology Department for determining the MICs of the compounds and T. C. Wang of the Biometrics Research Department for performing the statistical analyses. We thank Cameron Douglas for critical reading and helpful discussion of the manuscript.
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 3.Auckland, C., L. Teare, F. Cooke, M. E. Kaufman, M. Warner, G. Jones, K. Bamford, H. Ayles, and A. P. Johnson. 2002. Linezolid-resistant enterococci: report of the first isolates in the United Kingdom. J. Antimicrob. Chemother. 50:743-746. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-45(20). Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Craig, W. A. 1996. Antimicrobial resistance issues of the future. Diagn. Microbiol. Infect. Dis. 25:213-217. [DOI] [PubMed] [Google Scholar]
- 6.Dibo, I., S. K. Pillai, H. S. Gold, M. R. Baer, M. Wetzler, J. L. Snack, P. A. Hazamy, D. Ball, C. B. Hsiao, P. L. McCarthy, Jr., and B. H. Segal. 2004. Linezolid-resistant Enterococcus faecalis isolated from a cord blood transplant recipient. J. Clin. Microbiol. 42:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the Sentry Participants Group. 2001. Survey of infection due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe and the Western Pacific region for the Sentry Antimicrobial Surveillance program, 1997-1999. Clin. Infect. Dis. 15:S114-S132. [DOI] [PubMed] [Google Scholar]
- 8.Diekema, D. J., and R. N. Jones. 2000. Oxazolidinones, a review. Drugs 59:7-16. [DOI] [PubMed] [Google Scholar]
- 9.Draper, N. R., and H. Smith. 1981. Applied regression analysis. John Wiley and Sons, Inc., New York, NY.
- 10.Ebisu, H., R. Abuki, M. Takei, and H. Fukuda. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F1-966.
- 11.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291-1293. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen, L. F., and J. M. Curtis. 1947. The use of angular transformation in biological assays. J. Am. Stat. Soc. 42:282-296. [DOI] [PubMed] [Google Scholar]
- 13.Michel, M., and L. Gutmann. 1997. Methicillin resistant Staphylococcus aureus and vancomycin resistant enterococci: therapeutic realities and possibilities. Lancet 349:1901-1906. [DOI] [PubMed] [Google Scholar]
- 14.Norrby, R. 2001. Linezolid: a review of the first oxazolidinone. Expert Opin. Pharmacother. 2:203-302. [DOI] [PubMed] [Google Scholar]
- 15.Noskin, G. A., F. Siddiqui, V. Storsor, D. Hacek, and L. R. Peterson. 1999. In vitro activity of linezolid against important gram-positive bacterial pathogens including vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 43:2059-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits the initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]