Abstract
Leptospirosis and scrub typhus are important causes of acute fever in Southeast Asia. Options for empirical therapy include doxycycline and azithromycin, but it is unclear whether their efficacies are equivalent. We conducted a multicenter, open, randomized controlled trial with adult patients presenting with acute fever (<15 days), without an obvious focus of infection, at four hospitals in Thailand between July 2003 and January 2005. Patients were randomly allocated to receive either a 7-day course of doxycycline or a 3-day course of azithromycin. The cure rate, fever clearance time, and adverse drug events were compared between the two study groups. A total of 296 patients were enrolled in the study. The cause of acute fever was determined for 151 patients (51%): 69 patients (23.3%) had leptospirosis; 57 patients (19.3%) had scrub typhus; 14 patients (4.7%) had murine typhus; and 11 patients (3.7%) had evidence of both leptospirosis and a rickettsial infection. The efficacy of azithromycin was not inferior to that of doxycycline for the treatment of both leptospirosis and scrub typhus, with comparable fever clearance times in the two treatment arms. Adverse events occurred more frequently in the doxycycline group than in the azithromycin group (27.6% and 10.6%, respectively; P = 0.02). In conclusion, doxycycline is an affordable and effective choice for the treatment of both leptospirosis and scrub typhus. Azithromycin was better tolerated than doxycycline but is more expensive and less readily available.
Leptospirosis is a zoonosis with a worldwide distribution (6). It is caused by pathogenic spirochetes of the genus Leptospira which are excreted in the urine of a variety of wild and domestic animals. Human infection occurs through direct contact with infected animals or via exposure to freshwater or soil contaminated by infected animal urine. Leptospirosis is an emerging infection in many countries, including Thailand, where the annual number of reported cases has been increasing since 1997 (12). Scrub typhus is also a zoonotic disease, caused by Orientia tsutsugamushi, an obligate intracellular bacterium transmitted to humans by the bite of a larval leptotrombidium mite (14). Scrub typhus is an important cause of acute fever in the Western Pacific region (9).
Although classical presentations of leptospirosis and scrub typhus are well described, most patients present to hospitals with nonspecific signs and symptoms. Acute undifferentiated fever, i.e., acute fever without an obvious focus of infection, is the most common clinical presentation of both leptospirosis and scrub typhus (11). Early diagnosis of leptospirosis and scrub typhus is essential since antibiotic therapy provides the greatest benefit when initiated early in the course of illness (2, 9). Diagnosis of the early phase of either leptospirosis or scrub typhus is hampered by its nonspecific presentation. The lack of widely available, sensitive, and rapid methods for laboratory diagnosis of both diseases is an important clinical problem when managing patients presenting with acute undifferentiated fever, making it difficult to select appropriate empirical antimicrobial therapy. Doxycycline is potentially an excellent choice of initial antimicrobial treatment for such individuals, though there has been a report of doxycycline-resistant scrub typhus in northern Thailand (13). Although expensive at present azithromycin may be an excellent alternative, particularly when resistance is suspected. We report here the results of a multicenter open randomized controlled trial comparing the efficacies and tolerabilities of doxycycline and azithromycin for the treatment of acute undifferentiated fever with suspicion of either leptospirosis or scrub typhus in areas of high leptospirosis and scrub typhus endemicity.
MATERIALS AND METHODS
Patients and study sites.
This study was conducted between July 2003 and January 2005 at four hospitals in Thailand. Three hospitals are in the northeast part of the country (Udonthani Hospital, Udonthani Province, Maharat Nakhon Rachasima Hospital, Nakhon Rachasima Province, and Chaiyapoom Hospital, Chaiyapoom Province), and one hospital is in the south (Chumphon Hospital, Chumphon Province). Included in the trial were adult patients (age, >14 years) with suspected leptospirosis or scrub typhus—that is, patients who presented with acute fever (oral temperature, ≥38.0°C for <15 days) in the absence of an obvious focus of infection and who in the opinion of the attending physician could receive oral antimicrobial treatment. Patients who were unable to take oral medications, those who were pregnant or breastfeeding, those with a history of allergy to macrolides or tetracyclines, those who had positive malarial blood smear, clinical dengue virus infection consistent with WHO criteria (16), severe leptospirosis- or scrub typhus-related complication, and those who had a definite history of receiving treatment active against leptospirosis or scrub typhus for more than 48 h before enrollment were excluded. The study protocol was approved by the Ethical Review Subcommittee of the Public Health Ministry of Thailand, and written inform consent was obtained from all study volunteers.
Sample size requirements.
The study was designed to test that azithromycin had noninferior efficacy compared with doxycycline for the treatment of both leptospirosis and scrub typhus. Assuming a 90% cure rate for doxycycline in both diseases, a relative difference of ≥20% between the cure rates of the two groups was defined as nonequivalent. On the basis of a one-sided 0.05 significance level and 90% power, respectively, to reject the null hypothesis that the two treatments were not equivalent, testing of noninferior efficacy required that at least 28 patients with confirmed leptospirosis and scrub typhus in each treatment group complete the trial protocol.
Randomization and study protocol.
Independent, computer-generated, simple random allocation sequences were prepared for each study hospital by the investigator team in Bangkok. These were sealed in an opaque envelope and numbered. The investigator in each study hospital assigned study participants to their treatment groups after opening the sealed envelope. Patients were randomly allocated to receive either oral doxycycline (Siam Pharmaceutical) (200 mg in the first dose, followed by 100 mg every 12 h for 7 days) or a 3-day course of azithromycin (Pfizer International) (1 g initially, followed by 500 mg once daily for 2 days). History, physical examination findings, and results of laboratory investigations were recorded on standardized case record forms. During hospitalization, temperature was recorded orally every 4 h. Baseline investigations included a full blood count, plasma glucose and electrolytes, serum urea and creatinine, liver function tests, two aerobic blood cultures, urine analysis, and chest radiography. Five milliliters of blood was placed in a sterile heparinized bottle for leptospire culture using EMJH medium. Leptospira were cultured and identified using standard methodology (17).
Patients were discharged when defervescence had been achieved and maintained for at least 48 h. A follow-up visit was scheduled for 1 to 2 weeks after initial sampling to obtain convalescent-phase serum samples for serological analysis. Sera were stored at −20°C until tested. Data collection was done by the study team, whose members were unaware of the study hypothesis.
Confirmation of leptospirosis and scrub typhus.
Leptospirosis was confirmed on the basis of World Health Organization criteria for leptospirosis (2). Acute and follow-up sera were tested by the microscopic agglutination test (MAT) as previously described (2). Reference leptospira from 24 serogroups, including serovars known to be prevalent in Thailand, were used as the antigen in the MAT. The diagnosis of leptospirosis was made by either the isolation of leptospires from blood or positive serologic tests, which were defined as either a fourfold or greater rise in antibody titer or a titer of at least 1:400 on a single specimen.
Scrub typhus was diagnosed serologically by microimmunofluorescence assays that employed a combination of three O. tsutsugamushi strains (Karp, Gilliam, and Kato) as the antigen. Total antirickettsial immunoglobulins, immunoglobulin G- and immunoglobulin M-specific antibody, were assayed as described previously (4). Criteria for the diagnosis of scrub typhus were either a fourfold or greater rise in immunofluorescence assay titers between paired serum samples or a titer of at least 1:400 or greater on a single specimen.
Analysis of results.
The efficacy of treatment was analyzed on the intention-to-treat and subgroup analysis basis. Intention-to-treat analysis was based on the number of patients who entered the study—145 doxycycline-treated patients and 151 azithromycin-treated patients. Subgroup analysis was based on the number of patients who complete the treatment and had laboratory-confirmed leptospirosis and scrub typhus. The primary efficacy outcome was evaluated according to the following definitions. “Cure” was defined as the resolution of fever within 5 days after initiating the antimicrobial treatment. “Failure” was defined as either persistent fever or the development of any complication after at least 48 h of treatment.
The secondary outcome measure was the time to defervescence, which was defined as the interval between the time at which the first dose of the study drug was administered and the time at which the oral temperature first returned to ≤37.5°C and was maintained for two consecutive measurements without antipyretics.
Patients were assessed for adverse events. “Adverse events” were defined as symptoms or signs that developed after the study drug administration and had not been reported prior to the first dose of the antibiotic. Analyses of baseline characteristics and adverse events were done on the intention-to-treat basis.
Statistical analysis.
All statistical analyses were performed using SPSS, version 13.5 (SPSS). Pearson's or Fisher's exact tests were used to compare rates and proportions, as appropriate. Mann-Whitney U tests were used to analyze continuous variables that were not normally distributed. Independent-sample t tests were used to compare normally distributed variables. Times to fever clearance were compared using the log-rank test. All P values were two-tailed; a P value of ≤0.05 was considered to be statistically significant.
RESULTS
A total of 348 patients were initially evaluated, and 52 patients were excluded prior to randomization (25 patients did not have fever during the baseline examination, 19 patients had severe complications such as hypotension or acute renal failure on admission, and 8 patients did not agree to be admitted to the hospital). Therefore, 296 patients (145 patients in the doxycycline group and 151 patients in the azithromycin group) were randomized. Recruitment by site was as follows: 137 patients at Udonthani (46.3%); 86 patients at Chumphon (29.1%); 39 patients at Nakhon Rachasima (13.2%); and 34 patients at Chaiyapoom (11.5%). Most patients (69.3%) were male, and the median age was 36 (range, 15 to 88) years. Most patients (69.6%) were agricultural workers, mainly rice farmers. The median duration of fever prior to presentation to the hospital was 4.5 days (range, 1 to 15). Forty-three patients received antibiotic treatment within 48 h prior to enrollment in the study (16 [11%] in the doxycycline group and 27 [17.9%] in the azithromycin group; P = 0.095). Most of them received either a single dose of ceftriaxone by injection or oral doxycycline at the district hospital or primary health care center. The distributions of prior antimicrobial treatment were similar in the two treatment groups (data not shown here).
Eighty-nine patients (30.1%; 42 patients in the doxycycline group and 47 patients in the azithromycin group) were lost to follow-up after discharge from the hospital. The median duration of follow-up was 15 days in both groups, with ranges of 6 to 120 days in the doxycycline group and 6 to 150 days in the azithromycin group, respectively. All patients provided blood samples for culture isolation. Among patients who were lost to follow-up, a second serum sample was obtained on day 3 to day 5 of admission for 23 patients. Therefore, 66 patients provided only acute-phase serum.
The causes of acute fever were obtained for 151 out of 296 patients (51%). Of these, the diagnosis was leptospirosis for 69 patients (23.3%), scrub typhus for 57 patients (19.3%), murine typhus for 14 patients (4.7%), and evidence of leptospirosis and scrub or murine typhus coinfection for 11 patients (3.7%). The diagnosis of leptospirosis was made by the isolation of leptospires from blood for 10 patients, a fourfold or greater rise in the MAT titer for 45 patients, and a single titer of 1:400 or greater for 14 patients. The diagnosis of scrub typhus was confirm by a fourfold or greater rise in immunofluorescence assay titers for 34 patients and a single titer of 1:400 or greater for 23 patients. The distribution of the causes of fever was not significantly different between the two study groups.
In the intention-to-treat analysis, treatment failure was observed for three patients in the doxycycline group and four patients in the azithromycin group (P = 0.12) (Table 1). In addition, a severe adverse event occurred for two patients in the doxycycline group. Definite diagnosis was not obtained for these patients. Overall the cure rate of azithromycin was noninferior to that of doxycycline: 96.5% in the doxycycline group and 97.4% in azithromycin group, with a difference of 0.9% (90% confidence interval, −4.6% and 2.8%).
TABLE 1.
Demographic data, final diagnosis, and outcome for all 296 patients included in the study (intention-to-treat analysis)
| Parameter | Value for group treated witha:
|
P value | |
|---|---|---|---|
| Doxycycline | Azithromycin | ||
| No. male/no. female | 101/44 | 104/47 | 0.88 |
| Median age, yr (range) | 38 (15-79) | 38 (15-88) | 0.78 |
| Median no. of days of illness (range) | 4 (1-14) | 5 (1-15) | 0.09 |
| No. (%) with final diagnosis of: | |||
| Leptospirosis | 34 (23.4) | 35 (23.2) | 0.96 |
| Scrub typhus | 27 (18.6) | 30 (19.9) | 0.79 |
| Murine typhus | 6 (4.1) | 8 (5.3) | 0.64 |
| Leptospirosis and rickettsioses | 6 (4.1) | 5 (3.3) | 0.71 |
| Unknown | 72 (49.7) | 73 (48.3) | 0.82 |
| Outcome | |||
| Median (range) time to fever clearance (h) | 48 (8-336) | 48 (8-188) | 0.57 |
| No. (%) for whom outcome was: | |||
| Successful | 140 (96.5) | 147 (97.4) | 0.15 |
| Treatment failure | 3 (2) | 4 (2.6) | |
| No. for whom treatment was stopped due to adverse events | 2 | 0 | |
For the doxycycline group, n = 145; for the azithromycin group, n = 151.
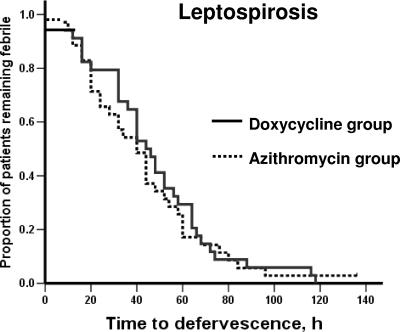
Among patients with laboratory-confirmed leptospirosis, only one patient in the azithromycin group did not show defervescence within 5 days after treatment initiation. Within 48 h after initiation of treatment, 19 patients (55.9%) in the doxycycline group and 23 patients (65.7%) in the azithromycin group became afebrile (P = 0.33). The median time to fever clearance was 45 h (range, 8 to 118 h) in the doxycycline group and 40 h (range, 8 to 136 h) in the azithromycin group, respectively (P = 0.45).
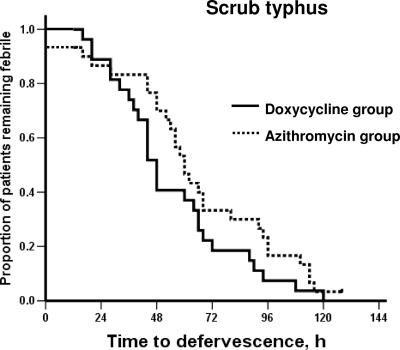
In patients with laboratory-confirmed scrub typhus, treatment failure occurred in one patient in azithromycin group. The median time to fever clearance was 48 h (range, 16 to 120 h) in the doxycycline group and 60 h (range, 12 to 128 h) in the azithromycin group, respectively (P = 0.13). However, within 48 h after initiation of treatment, a significantly higher proportion of the doxycycline-treated group (16 patients [59.3%]) became afebrile than with azithromycin-treated group (9 patients [30%]) (P = 0.03). The analysis of patients who did not receive prior antimicrobial treatment revealed results similar to those of the analysis of all laboratory-confirmed cases.
No relapse was observed in either group over the follow-up period. Kaplan-Meier curves for the time to defervescence, compared between the doxycycline group and the azithromycin group for patients with laboratory-confirmed leptospirosis and for patients with laboratory-confirmed scrub typhus, are shown in Fig. 1 and 2, respectively.
FIG. 1.
Time to defervescence after treatment for patients with confirmed leptospirosis.
FIG. 2.
Time to defervescence after treatment for patients with confirmed scrub typhus.
There were no deaths or serious adverse events in either group. Adverse drug events occurred significantly more frequently in the doxycycline-treated group than in the azithromycin-treated group (Table 2). Doxycycline was discontinued for two patients with unknown diagnosis, due to an adverse reaction, either rash or severe vomiting, in each. One was switched to ceftriaxone injection and the other to orally administered azithromycin. Most patients who developed nausea and/or vomiting after treatment had a history of nausea or vomiting prior to admission. In patients without this history, only six patients in the doxycycline group and two patients in the azithromycin group developed nausea and/or vomiting after treatment.
TABLE 2.
Adverse events in the two treatment groups (intention-to-treat analysis)
| Adverse event | No. (%) of occurrences ina:
|
P value | |
|---|---|---|---|
| Doxycycline group | Azithromycin group | ||
| All | 40 (27.6) | 16 (10.6) | <0.001 |
| Nausea | 3 (2.1) | 1 (0.7) | |
| Vomiting | 22 (15.2) | 10 (5.9) | |
| Nausea and vomiting | 10 (6.9) | 1 (0.7) | |
| Diarrhea | 1 (0.6) | 1 (0.7) | |
| Abdominal pain | 1 (0.6) | 0 | |
| Rash | 1 (0.6) | 3 (0.2) | |
| Dizziness | 2 (1.2) | 0 | |
For the doxycycline group, n = 145; for the azithromycin group, n = 151.
DISCUSSION
Leptospirosis and scrub typhus recently have been recognized as common causes of acute undifferentiated febrile illness in rural southeast Asia (1, 5, 11). Clinical presentations of these infections vary widely, from acute flu-like syndrome, with or without signs of organ dysfunction, such as jaundice or renal insufficiency, to multiorgan dysfunction mimicking severe sepsis syndrome (10). Leptospirosis and scrub typhus were ascertained for approximately half of the patients in this study and were consistent with those found in previous studies in this region (1, 5, 11). Most cases occurred in men, in the working-age group, and in an occupational setting. Approximately 3% of people traveling internationally for a short period report fever that requires prompt attention (8). Leptospirosis and scrub typhus are increasingly recognized as important causes of fever and illness in returning travelers (7).
Diagnosing the causative infections in these fever cases is difficult during the acute phase, and yet appropriate treatment is essential for rapid recovery and the prevention of complications. Presumptive antimicrobial therapy is recommended whenever a case of either leptospirosis or scrub typhus is suspected. Oral doxycycline is the standard treatment for mild cases of leptospirosis and scrub typhus (6, 14). Clinical studies comparing the efficacies of different antimicrobial treatments for mild leptospirosis and scrub typhus are limited. Leptospires are sensitive to most antimicrobials in vitro, including macrolides (6). Results of this study confirmed that oral azithromycin treatment was not inferior to doxycycline treatment for patients with confirmed leptospirosis.
A single oral 500-mg dose of azithromycin was shown to be an effective alternative antimicrobial treatment for mild cases of scrub typhus in a recent study in Korea (3). A 3-day course of azithromycin and a 7-day course of doxycycline were selected for study in Thailand because of awareness of the emergence of doxycycline-resistant O. tsutsugamushi in the north of the country (13, 15). However, all doxycycline-treated patients became afebrile within 5 days after initiation of treatment. Azithromycin was found to be as effective as doxycycline for patients with confirmed scrub typhus, although the proportion of patients who became afebrile within 48 h after azithromycin treatment was significantly lower than that for doxycycline-treated patients. Clinical responses of scrub typhus depend on both the antimicrobial susceptibilities of various O. tsusugamshi strains and the severity of the disease. Studies with a larger number of patients are needed to confirm this finding.
This study was not a randomized double-blinded study. To reduce information bias, baseline data and outcome measurements used in this study were based on well-defined criteria, evaluated by an independent investigator, and the diagnosis was blinded to the statistician until all other data were cleaned and the database was locked. The cure rate in patients with unknown diagnosis in this study was similar to that for those with confirmed leptospirosis or scrub typhus. It was not possible to state that a proportion of these patients could truly have leptospirosis or scrub typhus or that their illnesses were due to other diseases, because we did not obtain paired sera from some of them, and early treatment has been reported to abrogate the rise in antibody titers between paired samples which is required to identify serologically confirmed cases. This is the limitation for the generalizability of results of this study.
In summary, doxycycline and azithromycin were found to be highly effective against both leptospirosis and scrub typhus. Both drugs were also effective as an initial empirical treatment for patients who presented with acute fever without an obvious focus of infection, and no bacterial infection was evident after admission. Azithromycin is an appropriate alternative antimicrobial treatment in areas where doxycycline-resistant scrub typhus is prevalent and also for children under 8 years old or during pregnancy, where doxycycline is contraindicated. Azithromycin was better tolerated than doxycycline but it is more expensive (approximately 10$ versus 2$ per treatment course) and less readily available.
Acknowledgments
We thank the doctors, nurses, and medical technologists of Udonthani Hospital, Maharaj Nakhon Ratchasima Hospital, Chaiyapoom Hospital, Ban Mai Chaiyapod Hospital, and Chumphon Hospital for their cooperation and help during the study period.
The Thailand Research Fund, the Ministry of Public Health, Thailand, and the Wellcome Trust of Great Britain funded this study.
Footnotes
Published ahead of print on 16 July 2007.
REFERENCES
- 1.Brown, G. W., A. Shirai, M. Jegathesan, D. S. Burke, J. G. Twartz, J. P. Saunders, and D. L. Huxsoll. 1984. Febrile illness in Malaysia—an analysis of 1,629 hospitalized patients. Am. J. Trop. Med. Hyg. 33:311-315. [DOI] [PubMed] [Google Scholar]
- 2.Faine, S. B., B. Adler, C. Bolin, and P. Perolat (ed.). 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melburne, Australia.
- 3.Kim, Y. S., H. J. Yun, S. K. Shim, S. H. Koo, S. Y. Kim, and S. Kim. 2004. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin. Infect. Dis. 39:1329-1335. [DOI] [PubMed] [Google Scholar]
- 4.La Scola, B., and D. Raoult. 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leelarasamee, A., C. Chupaprawan, M. Chenchittikul, and S. Udompanthurat. 2004. Etiologies of acute undifferentiated febrile illness in Thailand. J. Med. Assoc. Thail. 87:464-472. [PubMed] [Google Scholar]
- 6.McBride, A. J., D. A. Athanazio, M. G. Reis, and A. Ko. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376-386. [DOI] [PubMed] [Google Scholar]
- 7.Rolain, J. M., M. Jensenius, and D. Raoult. 2004. Rickettsial infections—a threat to travelers? Curr. Opin. Infect. Dis. 17:433-437. [DOI] [PubMed] [Google Scholar]
- 8.Ryan, E. T., M. E. Wilson, and K. C. Kain. 2002. Illness after international travel. N. Engl. J. Med. 347:505-516. [DOI] [PubMed] [Google Scholar]
- 9.Silpapojakul, K. 1997. Scrub typhus in the Western Pacific region. Ann. Acad. Med. Singapore 26:794-800. [PubMed] [Google Scholar]
- 10.Suputtamongkol, Y., K. Niwattayakul, C. Suttinont, K. Losuwanaluk, R. Limpaiboon, W. Chierakul, V. Wuthiekanun, S. Triengrim, M. Chenchittikul, and N. J. White. 2004. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clin. Infect. Dis. 39:1417-1424. [DOI] [PubMed] [Google Scholar]
- 11.Suttinont, C., K. Losuwanaluk, K. Niwatayakul, S. Hoontrakul, W. Intaranongpai, S. Silpasakorn, D. Suwancharoen, P. Panlar, M. Chenchittikul, J. M. Rolain, D. Raoult, and Y. Suputtamongkol. 2006. Causes of acute undifferentiated febrile illness in rural Thailand: a prospective observational study. Ann. Trop. Med. Hyg. 100:363-370. [DOI] [PubMed] [Google Scholar]
- 12.Tangkanakul, W., H. L. Smits, S. Jatanasen, and D. A. Ashford. 2005. Leptospirosis: an emerging health problem in Thailand. Southeast Asian J. Trop. Med. Public Health 36:281-288. [PubMed] [Google Scholar]
- 13.Watt, G., C. Chouriyagune, R. Ruangweerayud, P. Watcharapichat, D. Phulsuksombat, K. Jongsakul, P. Teja-Isavadharm, D. Bhodhidatta, K. D. Corcoran, G. A. Dasch, and D. Strickman. 1996. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348:86-89. [DOI] [PubMed] [Google Scholar]
- 14.Watt, G., and P. Parola. 2003. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 16:429-436. [DOI] [PubMed] [Google Scholar]
- 15.Watt, G., P. Kantipong, K. Jongsakul, P. Watcharapichat, and D. Phulsuksombati. 1999. Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in northern Thailand. Antimicrob. Agents Chemother. 43:2817-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 17.World Heath Organization. 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. http://www.who.int/csr/don/en/WHO_CDS_CSR_EPH_2002.23.pdf.