Abstract
Using the rabbit endocarditis model, we compared the activity of a new broad-spectrum cephalosporin, ceftaroline, with those of linezolid and vancomycin against methicillin-resistant Staphylococcus aureus. After a 4-day treatment, ceftaroline exhibited superior bactericidal in vivo activity against resistant S. aureus strains and appeared to be the most effective drug against a heterogeneous glycopeptide-intermediate S. aureus strain.
Ceftaroline is a novel broad-spectrum cephalosporin with potent activity against methicillin-resistant Staphylococcus aureus (MRSA) strains due to its strong affinity for S. aureus penicillin-binding proteins (PBPs), including PBP 2A, the additional protein responsible for the methicillin resistance mechanism (6, 15). Ceftaroline acetate (PPI-0903) is an N-phosphono water-soluble prodrug rapidly metabolized in vivo into the bioactive metabolite ceftaroline (PPI-0903 M). No study has been performed by using a challenging rabbit infection model of experimental endocarditis, which has proved to be highly valuable in evaluating the in vivo effectiveness of antibiotics. The aim of the present study was to evaluate the in vivo activity of ceftaroline compared with those of other antistaphylococcal drugs by using a rabbit model of aortic valve endocarditis with doses projected to be therapeutic for humans.
We studied two MRSA strains isolated from blood cultures. The MRSA strain (originally designated SA-2) was a strain with heterogeneous high-level methicillin resistance (methicillin MIC = 128 mg/liter) (7), and the heterogeneous glycopeptide-intermediate S. aureus strain (hGISA) exhibited homogeneous resistance to methicillin (methicillin MIC > 1,024 mg/liter) and heterogeneous resistance to glycopeptides (8). The MICs were determined in cation-supplemented Mueller-Hinton broth by the microdilution technique (1, 11). Bactericidal activity was assessed on the basis of the determination of minimal bactericidal concentrations (MBCs) by the microdilution method and on the basis of the results of time-kill experiments with an inoculum of 5 × 106 CFU/ml (12).
High-performance liquid chromatography was used to determine the concentrations of linezolid (13) (lower detection limit, 0.1 mg/liter; coefficient of variation, <10%). Assays with vancomycin were performed by an immunoenzymatic method with a COBAS MIRA unit and EMIT reagents (Behring Diagnostics Inc., Cupertino, CA) (detection threshold, 2.5 mg/liter; coefficient of variation, 4.1 to 6.9%). Active ceftaroline concentrations were determined by a microbiologic assay with Bacillus subtilis as the test organism and antibiotic medium 2 (Difco Laboratories, Detroit, MI) as the diffusion medium (lower detection limit, 0.25 mg/liter; intraday and interday variations, <10%). Simulation of the pharmacokinetics of linezolid was performed as validated previously (7). For ceftaroline, blood samples were taken from six healthy rabbits after administration of a ceftaroline acetate bolus of 10 and 30 mg/kg of body weight in order to determine the spontaneous drug kinetics. The simulation was intended to provide apparent values of pharmacokinetic parameters close to those observed in healthy volunteers after a 1-h infusion of a 600-mg dose (ca. 10 mg/kg) of ceftaroline acetate: mean half-life (t1/2), 1.57 to 2.63 h; peak concentration (Cmax), 18.96 to 21.02 mg/liter; and area under the curve (AUC), 56.08 mg·h/liter (Cerexa, Inc., unpublished data). A total dose of 58 mg/kg needed to be infused into the rabbit over a 12-h period in order to simulate the kinetics in human serum after the administration of a 10-mg/kg dose (i.e., 600 mg twice daily).
For each MRSA strain, the animals were randomly assigned to either no treatment (controls), ceftaroline regimen mimicking the human dose of 10 mg/kg every 12 h (q12h) (600 mg q12h), a linezolid regimen mimicking the human dose of 10 mg/kg q12h (600 mg q12h), and vancomycin administered by a constant intravenous infusion in order to reach a steady-state 20× MIC in serum (18).
Experimental endocarditis was induced with an inoculum of 108 CFU of S. aureus (5, 14) and was approved by The Committee of Animal Ethics of the University of Nantes. Treatment was started 24 h after inoculation for a 4-day regimen. Aortic valve vegetations were excised, weighed, and then homogenized in 0.5 ml of saline buffer and used for quantitative cultures on agar for 24 h at 37°C. Dilutions at 10−1, 10−2, and 10−4 were prepared to eliminate potential carryover effects. To evaluate whether ceftaroline treatment could induce the selection of variants resistant in vivo, undiluted vegetation homogenates were spread on agar plates containing the active form of ceftaroline at a concentration corresponding to fourfold the MIC. Bacterial counts were determined after 48 h of incubation at 37°C.
Statistical analyses were performed with GraphPad Prism software (version 4.0; GraphPad Software, San Diego, CA). For each strain studied, analysis of variance was used to compare the effects between the different groups, followed by a Bonferroni's test to compare the treated groups two by two. A P value of ≤0.05 was considered significant.
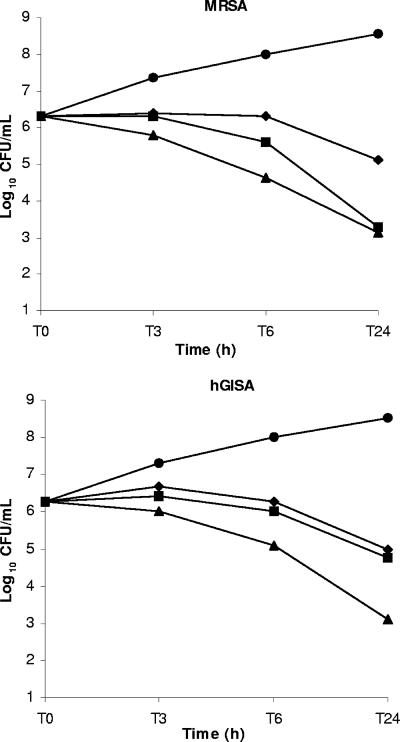
The MICs and MBCs for the MRSA and hGISA strains were 1 and 1 mg/liter and 2 and 2 mg/liter, respectively, for ceftaroline; 1 and 2 mg/liter and 4 and 8 mg/liter, respectively, for vancomycin; and 2 and >64 mg/liter and 1 and 16 mg/liter, respectively, for linezolid. The MICs of vancomycin and teicoplanin were increased for the hGISA strain (4 and 12 mg/liter, respectively). The results of the time-kill curve studies are shown in Fig. 1.
FIG. 1.
Killing curves for ceftaroline, linezolid, and vancomycin at 8× MIC against MRSA and hGISA strains. The results obtained with the control (circles), ceftaroline (triangles), linezolid (diamonds), and vancomycin (squares) are shown.
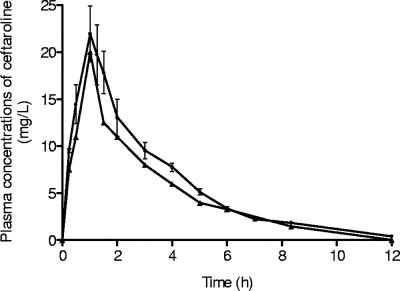
The corresponding Cmax, AUC, and t1/2 values were 21.9 ± 3.0 mg/liter, 71.2 mg·h/liter, and 2.4 h, respectively (Fig. 2). For linezolid, the corresponding mean Cmax, AUC, and t1/2 values were 11.9 ± 1.1 mg/liter, 76.3 mg·h/liter, and 2.7 h, respectively, at the first dose and 19.6 ± 1.1 mg/liter, 136.9 mg·h/liter, and 3.3 h, at day 4. The steady-state concentration of vancomycin confirmed that the target concentration (23.1 ± 8.3 mg/liter) was achieved.
FIG. 2.
Experimental pharmacokinetics of ceftaroline in animal plasma after administration of a dose simulating a 600-mg dose in humans (•) and the corresponding human pharmacokinetics (▴). Error bars represent standard deviations.
The in vivo outcome after a 4-day treatment regimen and the rate of sterilization of the vegetations produced by both strains are shown in Table 1. Linezolid showed only moderate activity and failed to exhibit bactericidal activity against both MRSA strains. Vancomycin displayed bactericidal activity against the MRSA strain but only bacteriostatic activity against the hGISA strain, as was observed previously in the same experimental model (3). Ceftaroline demonstrated excellent bactericidal activity against both S. aureus strains, with at least a 6-log10 CFU/g decrease in growth compared with the growth of the controls. Ceftaroline demonstrated superior efficacy compared with those of linezolid and vancomycin against the hGISA strain tested. Agar plates containing ceftaroline at four times the MIC showed no S. aureus colony after 48 h of incubation at 37°C.
TABLE 1.
Bacterial titers in vegetations after 4 days of treatment
| Regimen | Mean ± SD log10 CFU/g of vegetation (no. of sterile vegetationsa/total no. of vegetations) (%)
|
|
|---|---|---|
| MRSA | hGISA | |
| Control | 8.9 ± 0.5 (0/6) (0) | 9.4 ± 0.3 (0/6) (0) |
| Ceftaroline (10 mg/kg q12h)e | 2.5 ± 0.3 (9/10) (90)b,c | 3.0 ± 0.9 (6/10) (60)b,d |
| Linezolid (10 mg/kg q12h)e | 7.1 ± 0.6 (0/7) (0)b | 6.9 ± 0.4 (0/8) (0)b |
| Vancomycin | 2.7 ± 0.8 (4/6) (67)b,c | 6.7 ± 0.4 (0/5) (0)b |
We considered a vegetation to be sterile if the cultures yielded no growth after 48 h of incubation at 37°C.
P < 0.001 versus the results for the controls.
P < 0.001 versus the results for the linezolid.
P < 0.001 versus the results for the linezolid and vancomycin; Bonferroni's test after analysis of variance.
Simulated dose for humans.
Staphylococcus aureus is able to accumulate mechanisms of resistance to all clinically available compounds, including β-lactams, glycopeptides, and, more recently, oxazolidinones. In the present circumstances, the search for new agents targeting the cell wall and, more specifically, the PBPs is a promising approach (4, 10, 16, 17).
The in vitro study of ceftaroline and comparison of the results with those obtained with the comparators confirmed the excellent activity of this new drug against MRSA strains. Bactericidal activity and time-dependent killing were observed at 24 h against both MRSA strains by using clinical achievable concentrations. Regarding the hGISA strain, ceftaroline was the only drug that showed a bactericidal effect, while vancomycin and linezolid displayed bacteriostatic activity. These in vitro data emphasize the potential of ceftaroline for the therapy of infections caused by difficult-to-treat bacteria. Moreover, ceftaroline demonstrated activity against gram-negative bacteria similar to those of the expanded-spectrum cephalosporins against members of the family Enterobacteriaceae (e.g., Citrobacter freundii, Escherichia coli, Klebsiella pneumoniae, and Morganella morganii), Haemophilus influenzae, Moraxella catarrhalis, and Neisseria meningiditis; but expanded-spectrum β-lactamase-producing strains and Pseudomonas aeruginosa showed decreased susceptibilities to ceftaroline (MIC90, >32 mg/liter) (15).
By using the rabbit model of infective endocarditis, ceftaroline acetate exhibited a highly bactericidal effect and showed homogeneous activity against both MRSA strains. While the comparators displayed only bacteriostatic activity against the hGISA strain, the use of ceftaroline resulted in a 6-log10-CFU/g decrease in growth compared to the growth of the controls and confirmed the superiority of this drug observed in vitro. Moreover, ceftaroline achieved sterilization of 90% and 60% of the vegetations infected by the MRSA or hGISA strain, respectively, whereas vancomycin sterilized 67% and 0% of the vegetations, respectively (Table 1). This new cephalosporin is not influenced by reduced susceptibility to glycopeptides, as would be expected from the mechanism of action of this antibiotic. These results are concordant with those obtained with other animal models of infection, such as the systemic infection model and the pneumonia model in mice (6). The in vivo efficacy of ceftobiprole was demonstrated by using the same experimental model against MRSA strains (4). Both studies confirm that anti-MRSA cephalosporins are highly efficacious in the rabbit endocarditis model and demonstrate the potential of these drugs for use for the treatment of severe MRSA infections.
Andes and Craig have intensively studied the pharmacodynamic characteristics of ceftaroline using murine thigh and lung infection models (2). The parameter consisting of the percentage of time that the concentration remains above the MIC (% T > MIC) was the pharmacokinetic-pharmacodynamic index that best correlated with efficacy. Moreover, a T > MIC of 33% to 45% achieved a reduction in the burden of organisms from the start of therapy of 1 to 2 log10 CFU/thigh for S. aureus strains. In our study, the T > MICs were about 62% and 75% for the hGISA strain and the MRSA strain, respectively. These values were associated with highly bactericidal in vivo activity after a 4-day treatment.
A simulation was performed with ceftaroline in order to mimic in animals as closely as possible the human pharmacokinetics of a dose of 600 mg q12h. A pharmacokinetic equivalent between human and animals was necessary due to the very short spontaneous t1/2 of ceftaroline in the rabbit (<20 min). The target parameters were achieved (Fig. 2), and the current study—with the excellent in vivo activity observed in this MRSA severe infection model—validates the choice of a ceftaroline regimen of 600 mg q12h for therapy for serious complicated skin and skin structure infections in humans. Moreover, ceftaroline exhibited a low level of plasma protein binding in humans (<20%), as in rabbits (<20%), and these data improve the ability to extrapolate experimental in vivo results to human therapy (9).
By using the rabbit endocarditis model, ceftaroline showed highly bactericidal in vivo activity against MRSA and hGISA strains after a 4-day treatment regimen with a simulated dosage equivalent to that used in humans. In comparison with linezolid and vancomycin, ceftaroline was the most effective treatment against the hGISA strain. This new cephalosporin appears to be a promising and effective option for the treatment of severe MRSA infections.
Acknowledgments
This work was supported by a grant from Cerexa, Inc.
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Amsterdam, D. 1996. Susceptibility testing of antibiotics in liquid media, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 2.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asseray, N., C. Jacqueline, V. Le Mabecque, E. Batard, D. Bugnon, G. Potel, and J. Caillon. 2005. Activity of glycopeptides against Staphylococcus aureus infection in a rabbit endocarditis model: MICs do not predict in vivo efficacy. Antimicrob. Agents Chemother. 49:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers, H. F. 2005. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. II. Survival of bacteria in endocardial vegetations. Br. J. Exp. Pathol. 53:50-53. [PMC free article] [PubMed] [Google Scholar]
- 6.Iizawa, Y., J. Nagai, T. Ishikawa, S. Hashiguchi, M. Nakao, and K. Okonogi. 2004. In vitro antimicrobial activity of T-91825, a novel anti-MRSA cephalosporin, and in vivo anti-MRSA activity of its prodrug, TAK-599. J. Infect. Chemother. 10:146-156. [DOI] [PubMed] [Google Scholar]
- 7.Jacqueline, C., E. Batard, L. Perez, D. Boutoille, A. Hamel, J. Caillon, M. F. Kergueris, G. Potel, and D. Bugnon. 2002. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob. Agents Chemother. 46:3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacqueline, C., D. Navas, E. Batard, A. F. Miegeville, V. Le Mabecque, M. F. Kergueris, D. Bugnon, G. Potel, and J. Caillon. 2005. In vitro and in vivo synergistic activities of linezolid combined with subinhibitory concentrations of imipenem against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge, Y., and A. Hubbel. 2006. In vitro evaluation of plasma protein binding and metabolic stability of ceftaroline (PPI-0903), abstr. A-1935. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Malouin, F., J. Blais, S. Chamberland, M. Hoang, C. Park, C. Chan, K. Mathias, et al. 2003. RWJ-54428 (MC-02,479), a new cephalosporin with high affinity for penicillin-binding proteins, including PBP 2a, and stability to staphylococcal beta-lactamases. Antimicrob. Agents Chemother. 47:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 12.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapmann. 1980. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, T. Zenki, and N. Ozawa. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 14.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 15.Sader, H. S., T. R. Fritsche, K. Kaniga, Y. Ge, and R. N. Jones. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji, M., M. Takema, H. Miwa, J. Shimada, and S. kuwahara. 2003. In vivo antibacterial activity of S-3578, a new broad-spectrum cephalosporin: methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa experimental infection models. Antimicrob. Agents Chemother. 47:2507-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vouillamoz, J., J. M. Entenza, P. Hohl, and P. Moreillon. 2004. LB11058, a new cephalosporin with high penicillin-binding protein 2a affinity and activity in experimental endocarditis due to homogeneously methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 48:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wysocki, M., F. Delatour, F. Faurisson, A. Rauss, Y. Pean, B. Misset, F. Thomas, et al. 2001. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob. Agents Chemother. 45:2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]