Abstract
The in vitro and in vivo antipneumococcal activities of the main pneumococcal autolysin (LytA) and Cpl-1, a lysozyme encoded by phage Cp-1, were studied. Intraperitoneal therapy with LytA or high-dose Cpl-1 remarkably reduced peritoneal bacterial counts (>5 log10 CFU/ml) compared with those for the controls. After intravenous injection, LytA was the most effective treatment.
New therapeutic strategies for combating infections caused by antibiotic-resistant Streptococcus pneumoniae are being sought. Lytic enzymes produced by bacteria and phages may be useful alternatives for the treatment of pneumococcal infections. The LytA amidase is the main pneumococcal autolysin (3, 6) and is responsible for the lytic effects of penicillin and other cell wall inhibitors (5, 10, 11). Cpl-1 is a lysozyme encoded by pneumococcal phage Cp-1 (1, 2, 4).
The aim of this study was to compare the in vitro and in vivo activities of purified LytA and Cpl-1 with those of cefotaxime (CTX) against a β-lactam-resistant (penicillin MIC, 2 μg/ml) meningeal pneumococcal isolate (strain MJD3693).
The MICs of LytA, Cpl-1, and CTX for strain MJD3693 were 16, 32, and 4 μg/ml, respectively, as determined by the broth microdilution methodology (7). LytA and Cpl-1 were overproduced and purified by affinity chromatography in DEAE-cellulose (8). One unit of enzymatic activity (5) was defined as the amount of enzyme that catalyzed the hydrolysis of 1 μg (∼715 net cpm) of [methyl-3H]choline-labeled pneumococcal cell walls (6) in 10 min at 37°C. Time-kill experiments were performed in three separate assays by exposing early-log-phase cultures of MJD3693 to 50 μg/ml of LytA, Cpl-1, or CTX in tubes with either cation-adjusted Mueller-Hinton broth with 4% lysed horse blood (CA-MHB-LHB) or phosphate-buffered saline (PBS; pH 7.0). Such a concentration was over 12-fold greater than the CTX MIC but only 1.6- and 3-fold higher than the MICs of Cpl-1 and LytA, respectively. After incubation at 35°C, the bacterial titers were compared at 1, 3, and 5 h (Table 1). The killing effects of the enzymes were very rapid and were similar in both media, and their antipneumococcal activities was remarkably higher than that of CTX, particularly in PBS. In CA-MHB-LHB at 1 and 3 h, LytA and Cpl-1 were significantly more effective than CTX (P ≤ 0.018), although after 5 h incubation, only LytA demonstrated a significantly higher activity than CTX (P = 0.004). In PBS, LytA and Cpl-1 exerted a profound bactericidal effect at all times, whereas, as expected, the effect of CTX in PBS was quite poor (Table 1).
TABLE 1.
Time-kill experiments with pneumococcal strain MJD3693 in either CA-MHB-LHB or PBS after exposure to LytA, Cpl-1, or cefotaxime
| Time (h) | Difference in log10 CFU/mla
|
|||||
|---|---|---|---|---|---|---|
| CA-MHB-LHB
|
PBS
|
|||||
| LytA | Cpl-1 | Cefotaxime | LytA | Cpl-1 | Cefotaxime | |
| 1 | −3.5 ± 0.0 | −3.4 ± 0.9 | −0.7 ± 0.1 | −4.1 ± 0.6 | −3.7 ± 0.8 | −0.1 ± 0.2 |
| 3 | −5.4 ± 0.1 | −5.0 ± 0.8 | −3.0 ± 0.3 | −4.8 ± 0.3 | −4.1 ± 0.4 | −0.5 ± 0.2 |
| 5 | −5.3 ± 0.2 | −5.3 ± 0.7 | −4.2 ± 0.3 | −5.4 ± 0.8 | −5.3 ± 0.6 | −0.7 ± 0.2 |
Differences in bacterial colony counts (expressed as log10 CFU/ml) between those found in enzyme- or antibiotic-containing tubes and those shown in control tubes at different time points. Values are expressed as the means ± standard deviations from three experiments where the baseline (time zero) inoculum was 7.14 ± 0.03 log10 CFU/ml. LytA (specific activity, 9.3 × 105 U/mg), Cpl-1 (specific activity, 9.8 × 105 U/mg), and CTX were each used at a final concentration of 50 μg/ml.
The plasma pharmacokinetics of purified LytA and the in vivo efficacies of the compounds were studied by using adult Swiss mice, with the approval of the Fundación Jiménez Díaz Ethics Committee. The plasma pharmacokinetics of LytA were determined by using a solution of 3.1 mg/ml (specific activity, 5.9 × 105 U/mg). After the administration of single intravenous (i.v.) doses (via the tail vein) of LytA (25 mg/kg) to uninfected mice, blood samples were obtained from three animals per group, which were killed at 5, 15, 60, and 120 min; and the plasma enzyme activity was measured. The mean plasma area under the enzymatic activity-time curve was 3,440 U·min·μl−1, with an intercept value of 101 U/μl. The plasma half-life of LytA was 22.5 min, which was similar to that of Cpl-1 (20.5 min) (4) and longer than that of CTX (ca. 12 min) (9).
To induce the peritonitis-sepsis model, the mice were intraperitoneally (i.p.) inoculated with 0.25 ml of the pneumococcal suspension (7.3 ± 0.3 log10 CFU per mouse). This inoculum was 100% lethal within 1 to 3 days and provided a detectable number of bacteria in the peritoneal cavity and blood. Treatments were started 1 h after bacterial challenge as single 0.25-ml doses by i.p. or i.v. administration. Ten groups of infected mice (five to six animals per group) were used; half of them were treated by each route. The mice were treated with LytA (1.7 mg; 57 mg/kg of body weight; specific activity, 8.9 × 105 U/mg), Cpl-1 (1.4 mg; 47 mg/kg; specific activity, 9.8 × 105 U/mg [“low-dose” regimen]), Cpl-1 (3.3 mg; 110 mg/kg; specific activity, 6 × 105 U/mg [“high-dose” regimen]), or CTX (12 mg; 400 mg/kg); control mice received sterile saline. Four hours after injection the animals were killed and blood and peritoneal lavage fluid specimens were processed for determination of bacterial counts. The lower limit of organism quantification in these studies was 10 CFU/ml. For analytical purposes, specimens with <10 CFU/ml were arbitrarily assigned a value of 1 CFU/ml.
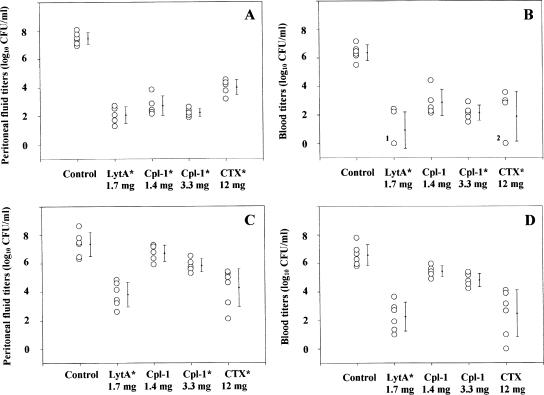
Figure 1 and Table 2 summarize the efficacies of the agents in reducing the bacterial loads in the peritoneal lavage fluid and blood after i.p. and i.v. administration of the different agents. At 5 h postchallenge, the bacterial titers in the peritoneal fluid and the blood of the control animals were 7.4 ± 0.6 and 6.5 ± 0.7 log10 CFU/ml, respectively.
FIG. 1.
Antipneumococcal activities in the peritoneal fluid (A and C) and in the blood (B and D) of lytic enzymes or CTX 4 h after administration by the i.p. (A and B) or i.v. (C and D) route in mice with β-lactam-resistant S. pneumoniae peritonitis-sepsis. Each point represents one mouse; five to six animals were included in each group; the mean ± standard deviation for each group is also shown. In panel B, the label 1 indicates that three animals treated with LytA i.p. showed bacterial clearance from the blood and the label 2 indicates that two animals treated with CTX i.p. also showed bacterial clearance from the blood. *, statistically significant difference compared to the results for the control.
TABLE 2.
Decline in bacterial counts in peritoneal fluid and blood samples of animals with pneumococcal peritonitis-sepsis after 4 h posttherapy compared to those of controls
| Test group (dose [mg] per mouse) | Injection route | Mean ± SD differences in bacterial counts (log10 CFU/ml)a
|
|
|---|---|---|---|
| Peritoneal fluid | Blood | ||
| LytA (1.7) | i.p. | −5.4 ± 0.6b | −5.4 ± 1.3b |
| i.v. | −3.5 ± 0.9b | −4.3 ± 1.0b | |
| Cpl-1 (1.4) | i.p. | −4.8 ± 0.7b | −3.5 ± 0.9 |
| i.v. | −0.7 ± 0.6 | −1.1 ± 0.3 | |
| Cpl-1 (3.3) | i.p. | −5.2 ± 0.3b | −4.2 ± 0.5b |
| i.v. | −1.5 ± 0.5b | −1.8 ± 0.3 | |
| CTX (12) | i.p. | −3.5 ± 0.6b | −4.5 ± 1.7b |
| i.v. | −3.1 ± 1.3b | −4.1 ± 1.6 | |
Differences in bacterial titers between treated animals and untreated controls.
Statistically significant differences compared with the results for the controls (P < 0.05).
The bacterial counts diminished significantly (compared with the counts for the controls) following the i.p. administration of all agents. The most important effect was observed with LytA, although a profound killing effect was also seen after the i.p. administration of Cpl-1 at either the high or the low dose. The mice treated i.p. with CTX experienced the smallest antibacterial effect in the peritoneum. After the i.v. administration of LytA, CTX, or high-dose Cpl-1, the bacterial titers in the peritoneal fluid were significantly diminished compared with those in the controls. LytA administered i.v. had a more potent effect in the peritoneum than either of the two Cpl-1 i.v. regimens.
The i.p. administration of LytA, high-dose Cpl-1, or CTX significantly reduced the bacterial load in the blood; indeed, three of five mice treated with LytA and two of five mice treated with CTX had no detectable bacteremia (Fig. 1). LytA administered i.v. had the greatest bactericidal effect in blood. The antipneumococcal activities of all the agents tested were superior when they were administered i.p. than when they were injected i.v., although the differences were significant only for both doses of Cpl-1 and the bacterial load in peritoneal fluid and blood (P < 0.001) and for LytA and the bacterial load in peritoneal fluid (P < 0.01).
The reduction in the peritoneal and blood colony counts achieved by i.p. LytA treatment reflects the intrinsic activity and bactericidal capacity of LytA. The two Cpl-1 regimens tested were also more effective than CTX when the i.p. route was used. Comparison of the results of the bacterial killing achieved with the i.v. and i.p. routes suggests that LytA is acceptably distributed throughout the circulatory system and enters the peritoneal cavity, which is a highly vascularized site.
To our knowledge, the present study is the first to treat mice with β-lactam-resistant pneumococcal peritonitis-sepsis with LytA. These results may therefore represent a step forward toward the potential use of enzybiotic therapy.
Acknowledgments
We thank A. Burton for correcting the English version of the manuscript.
This research was supported by grants from the Red Temática de Investigación Cooperativa Spanish Pneumococcal Infection Study Network (grant G03/103) (Ministerio de Sanidad y Consumo, Spain) and the program COMBACT Nuevas Dianas para Combatir a las Bacterias Patógenas (grant S-BIO-026-2006) (Consejería de Educación, Comunidad de Madrid, Madrid, Spain). L.H. and G.D.P. received scholarships from the Fundación Conchita Rábago; M.G. received a scholarship from the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Spain.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Fischetti, V. A. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491-496. [DOI] [PubMed] [Google Scholar]
- 2.García, J. L., E. García, A. Arrarás, P. García, C. Ronda, and R. López. 1987. Cloning, purification, and biochemical characterization of the pneumococcal bacteriophage Cp-1 lysin. J. Virol. 61:2573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard, L. V., and H. Gooder. 1974. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J. Bacteriol. 117:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López, R., C. Ronda, and E. García. 1990. Autolysins are direct involved in the bactericidal effect caused by penicillin in wild type and in tolerant pneumococci. FEMS Microbiol. Lett. 66:317-322. [DOI] [PubMed] [Google Scholar]
- 6.Mosser, J. L., and A. Tomasz. 1970. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity of lysis by an autolytic enzyme. J. Biol. Chem. 245:287-298. [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard. NCCLS document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Sánchez-Puelles, J. M., J. M. Sanz, J. L. García, and E. García. 1992. Immobilization and single-step purification of fusion proteins using DEAE-cellulose. Eur. J. Biochem. 203:153-159. [DOI] [PubMed] [Google Scholar]
- 9.Soriano, F., P. García-Corbeira, C. Ponte, R. Fernández-Roblas, and I. Gadea. 1996. Correlation of pharmacodynamic parameters of five β-lactam antibiotics with therapeutic efficacies in an animal model. Antimicrob. Agents Chemother. 40:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasz, A. 1979. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu. Rev. Microbiol. 33:113-137. [DOI] [PubMed] [Google Scholar]
- 11.Tomasz, A., and S. Waks. 1975. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of the cell wall synthesis. Proc. Natl. Acad. Sci. USA 72:4162-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]