Abstract
Increasing numbers of Neisseria gonorrhoeae strains with decreased susceptibilities to ceftriaxone and other oral cephalosporins widely used for the treatment of gonorrhea have been isolated in Sydney, Australia, over several years. In this study, we examined the complete penicillin-binding protein 2 (PBP 2) amino acid sequences of 109 gonococci, selected on the basis of their diverse temporal and geographic origins and because they exhibited a range of ceftriaxone MICs: ≤0.03 μg/ml (n = 59), 0.06 μg/ml (n = 43), and 0.125 μg/ml (n = 7). Auxotyping, serotyping, and genotyping by N. gonorrhoeae multiantigen sequence typing sequence-based analysis was also performed. In total, 20 different amino acid sequence patterns were identified, indicating considerable variation in the PBP 2 sequences in this study sample. Only some of the N. gonorrhoeae isolates with significantly higher ceftriaxone MICs contained a mosaic PBP 2 pattern, while more isolates exhibited a nonmosaic PBP 2 pattern containing an A501V substitution. Although particular N. gonorrhoeae genotypes in our sample were shown to be less susceptible to ceftriaxone, the reduced susceptibility to ceftriaxone was not specific to any particular genotype and was observed in a broad range of auxotypes, serotypes, and genotypes. Overall, the results of our study show that N. gonorrhoeae strains exhibiting reduced sensitivity to ceftriaxone are not of a particular subtype and that a number of different mutations in PBP 2 may contribute to this phenomenon.
The options for the effective treatment of Neisseria gonorrhoeae infections have been significantly reduced over time by the emergence and spread of gonococci resistant to the penicillins, tetracyclines, and the macrolide and quinolone groups of antibiotics (22). Cephalosporin antibiotics are now the mainstay of treatment in many settings, and the injectable agent ceftriaxone is used extensively in Australia and elsewhere. However, over recent years, reports, most notably from Japan (1, 8, 21), but also elsewhere (7, 27), of N. gonorrhoeae strains with decreased susceptibility to cephalosporin agents have appeared with increasing frequency.
The mechanism of gonococcal resistance to ceftriaxone is still unclear. N. gonorrhoeae has two penicillin-binding proteins (PBPs 1 and 2) that are the major targets of beta-lactam antibiotics. PBP 2, which is encoded by the penA gene, has a 10-fold greater affinity for penicillin than PBP 1 (6). Thus, mutations in PBP 2 are the major contributor to the emergence of chromosomally mediated resistance to beta-lactam antibiotics in N. gonorrhoeae, and particular attention has been paid to the penA gene. Alterations in penA that affect the activities of beta-lactam antibiotics have long been recognized. Insertion of a single aspartic acid residue preceding Asp-345 (Asp-345A) in PBP 2 resulted in the expression of an altered PBP 2 and decreased susceptibility to penicillins (3). The acquisition by gonococci of other mutations and sequential alterations to other genes was shown to result in cumulative high-level chromosomal resistance to the penicillins (18), but these changes were said to have but little effect on the clinical efficacies of cephalosporin antibiotics (8).
Ameyama et al. (1) described a mosaic penA gene that was associated with reduced susceptibility to a number of beta-lactam agents, including cephalosporins, in gonococci from Tokyo, Japan. The mosaic penA gene was thought to be acquired by gonococci from commensal Neisseria strains by in vivo transformation (19). Reductions in the susceptibilities of in vitro transformants with this mosaic sequence were much greater for oral cephalosporins than for ceftriaxone. Ito et al. (8) examined the penA sequences of 70 clinical isolates of N. gonorrhoeae from Gifu, Japan, with a range of susceptibilities to ceftriaxone and other beta-lactam agents and described 10 PBP 2 patterns of sequence alteration numbered patterns I to X. The distribution of MICs for penicillin, the oral agents cefixime and cefdinir, and the injectable agent ceftriaxone was correlated with the penA patterns determined. Isolates with patterns I to IX contained the Asp-345A insertion, but isolates with pattern X did not; rather, they possessed the mosaic PBP 2 sequence described by Ameyama et al. (1). The MICs of ceftriaxone for gonococci with pattern X that contained the mosaic penA gene ranged from 0.015 to 0.25 μg/ml but were said to be “significantly higher” than the MICs of isolates with patterns I to IX.
Tanaka et al. (21) examined the alterations present in a multiresistant gonococcus isolated in Fukuoka City, Japan, that was also less susceptible to ceftriaxone (MIC, 0.5 μg/ml). That group found mutations present in penA, mtrR, penB, and ponA and postulated that mutations that confer high-level resistance to penicillin also confer resistance to cephalosporins, including ceftriaxone. Although their isolate possessed the mosaic gene described by Ameyama et al. (1) and Ito et al. (8), it also lacked the Asp-345A substitution in PBP 2. Lindberg et al. (12) also noted that polymorphisms were present in penA, mtrR, penB, and ponA in 18 gonococci less susceptible to ceftriaxone and concluded that an “unambiguous” association existed between the increased ceftriaxone MICs, these polymorphisms, and penA mosaic alleles. Takahata et al. (20) more recently demonstrated that particular additional amino acid substitutions in the mosaic PBP 2 sequence were associated with decreased susceptibility to cefixime but had little effect on susceptibility to ceftriaxone. That study also found only minor reductions in ceftriaxone susceptibility in transformation experiments that introduced the full-length mosaic into a susceptible strain. We (26) described a second mosaic PBP 2 sequence with an Asp-345A change, but without the three key substitutions described by Takahata et al. (20), in a gonococcus fully susceptible to ceftriaxone and suggested that the extent of the contribution of mosaic PBP 2 sequences to reduced ceftriaxone susceptibility was yet to be fully elucidated.
In this study, selected gonococci that were isolated between 1997 and 2005 in Sydney, Australia, and that had altered susceptibilities to ceftriaxone were examined together with isolates susceptible to ceftriaxone. We explored further the relationship between penA alteration and ceftriaxone susceptibility by sequencing the penA genes of all isolates. Ito et al. (8) and a Danish group (7) have also suggested that the spread of these gonococci with decreased susceptibilities to ceftriaxone may be the result of the wider dissemination of a limited number of gonococcal subtypes. We therefore typed and compared all isolates by phenotypic and genotypic procedures and included in the selection of the strains studied a number of gonococci that were examined in Australia but that were isolated from patients who acquired their infection in a number of different countries.
MATERIALS AND METHODS
N. gonorrhoeae strains.
The South Eastern Area Laboratory Services laboratory in Sydney is the reference laboratory for N. gonorrhoeae for the state of New South Wales, Australia. It undertakes the primary isolation of N. gonorrhoeae from clinical samples from a number of sexual health clinics and hospitals and also receives isolates from public- and private-sector laboratories throughout the state. Approximately 1,200 gonococcal isolates are examined and stored annually, as described previously (11). A total of 109 gonococcal clinical isolates were retrospectively selected on the basis of their ceftriaxone MICs and their geographical and temporal diversities. The ceftriaxone MICs for the isolates were in the range of ≤0.008 to 0.03 μg/ml (n = 59), 0.06 μg/ml (n = 43), and 0.125 μg/ml (n = 7). The penicillin G MICs were in the range of <0.125 μg/ml (n = 6), ≥0.125 μg/ml to <0.5 μg/ml (n = 18), and ≥1.0 μg/ml (n = 75); and another 10 isolates were penicillinase-producing gonococci (see Table 3). The isolates examined included local and international strains; 13 isolates were obtained from various parts of Asia (China, n = 1; Indonesia, n = 1; Japan, n = 3; Korea, n = 5 [28]; Mongolia, n = 1 [13]; not specified, n = 2), and the remaining 96 strains were collected from various regions of the Australian state of New South Wales. The years of isolation included 1988 (n = 3), 1997 (n = 1), 1999 (n = 1), 2000 (n = 3), 2001 (n = 12), 2002 (n = 11), 2003 (n = 7), 2004 (n = 12), and 2005 (n = 59). Included among the isolates examined were gonococci used as reference cultures in World Health Organization and Australian quality assurance programs (2, 24, 27) and gonococci less susceptible to ceftriaxone, which were kindly supplied by T. Muratani (16).
TABLE 3.
Profiles for the 50 N. gonorrhoeae isolates with reduced susceptibilities to ceftriaxone (MIC ≥ 0.06 μg/ml), indicating the PBP 2 amino acid pattern, ceftriaxone MIC, auxotype, serotype, and genotype dataa
| PBP 2 pattern | Ceftriaxone MIC (μg/ml) | NG MAST ST | Auxotype | Serotypeb | No. of isolates |
|---|---|---|---|---|---|
| IV | 0.06 | 69 | NR | Brpyut | 1 |
| IV | 0.12 | 327 | Pro | Brpust | 1 |
| V | 0.06 | 1,410 | Pro | Bropyst | 2 |
| V | 0.06 | 1,688 | Pro | Bropyst | 1 |
| VII | 0.06 | 1,619 | Pro | Brpyust | 1 |
| VII | 0.06 | 1,622 | Pro | Byust | 1 |
| VII | 0.12 | 1,422 | Pro | Brpyust | 1 |
| IX | 0.06 | 5 | NR | Bropyst | 6 |
| IX | 0.06 | 21 | NR | Bopyst | 1 |
| X | 0.06 | 1,414 | Pro | Bpyust | 3 |
| X | 0.06 | 1,424 | Pro | Bpyust | 3 |
| X | 0.06 | 1,677 | Pro | Byust | 1 |
| X | 0.06 | 326 | Pro | Bropyst | 1 |
| X | 0.06 | 835 | Pro | Bpyust | 1 |
| X | 0.06 | 925 | Pro | Boys | 1 |
| XI | 0.06 | 1,420 | Pro | Bro | 1 |
| XI | 0.06 | 5 | NR | Bropyst | 1 |
| XII | 0.06 | 1,426 | NR | Brpyst | 1 |
| XII | 0.06 | 1,703 | NR | Bopt | 1 |
| XII | 0.06 | 604 | NR | Bropyst | 1 |
| XII | 0.06 | 604 | NR | Broypt | 1 |
| XIII | 0.06 | 1,409 | NR | Bropyst | 2 |
| XIII | 0.06 | 1,623 | NR | Bropyst | 4 |
| XIII | 0.06 | 1,624 | NR | Byvut | 1 |
| XIII | 0.06 | 1,676 | NR | Byvut | 1 |
| XIII | 0.12 | 1,148 | Pro | Bropyst | 1 |
| XIII | 0.12 | 1,413 | NR | Bropyst | 1 |
| XIII | 0.12 | 1,425 | NR | Bropyst | 1 |
| XVII | 0.06 | 1,342 | Pro | Bropyst | 2 |
| XVII | 0.12 | 1,620 | Pro | Bropyst | 1 |
| XVIII | 0.06 | 864 | Pro | Bopt | 2 |
| XVIII | 0.12 | 1,621 | Pro | Bropyst | 1 |
| XX | 0.06 | 211 | NR | Arst | 2 |
Nine profiles were identified for more than one isolate. ST, sequence type; NR, nonrequiring; Pro, proline requiring.
Serotype by use of the nomenclature of Bygdeman (4).
Nucleotide sequencing of penA genes.
The full-length penA gene sequences of the isolates were amplified and sequenced as described previously (8). Briefly, three separate PCRs were used to amplify three fragments of the penA gene. The PCR products were purified and then sequenced by using an ABI PRISM BigDye sequencing kit (Applied Biosystems).
Phenotypic and genotypic characterization and MIC determinations.
Phenotypic and genotypic characterization and MIC determinations were performed for all 109 isolates as described previously (11, 24). Briefly, the extended phenotype of each isolate was derived by determination of the antibiotic susceptibility by the agar plate dilution methods of the Australian Gonococcal Surveillance Programme (24), which uses Sensitest agar (Oxoid, Basingstoke, United Kingdom) with 8% saponin-lysed horse blood as the test medium and an inoculum of 104 CFU per spot, and by auxotype/serovar classification. The ceftriaxone MIC was determined in triplicate examinations on separate occasions for those gonococci for which the MIC was raised. Auxotyping was done by the method of La Scolea and Young (10), and serovar determinations were done with a panel of 14 monoclonal reagents (Boule, Huddinge, Sweden) and by use of the nomenclature of Bygdeman (4) Genotyping was performed by N. gonorrhoeae multiantigen sequence typing (NG MAST), as described previously (15).
Statistical analysis.
Data analysis was performed by using the Mann-Whitney U test. Significance was set at a P value of <0.05.
RESULTS
Mutations of PBP 2 in clinical isolates.
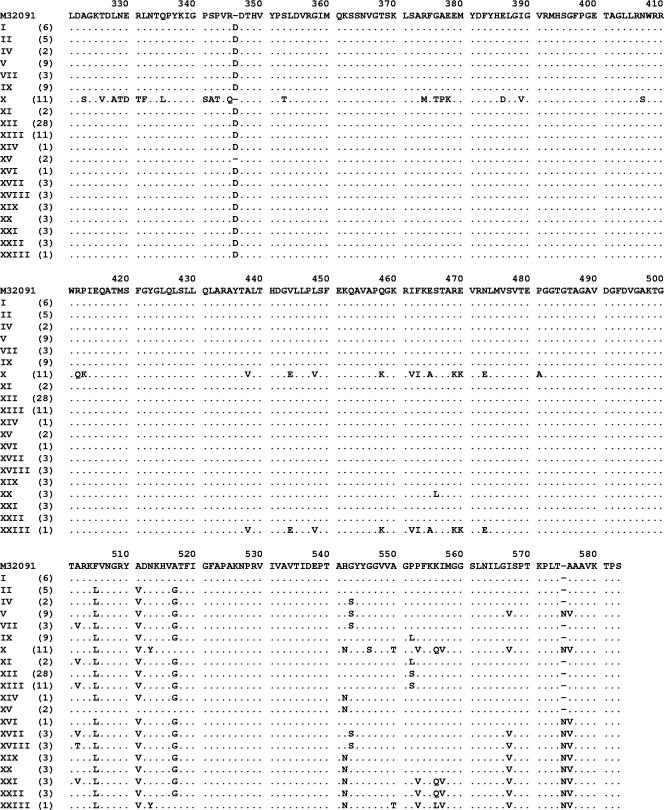
The complete nucleotide sequences of the penA genes of 109 clinical isolates of N. gonorrhoeae were determined. In total, 20 different amino acid sequence patterns were identified (patterns I to XXIII; Fig. 1), indicating the presence of considerable variation among the PBP 2 proteins in this study sample. Briefly, the first 340 amino acids of all 20 patterns except pattern X were identical to those in the GenBank reference sequence (accession number M32091). Various substitutions in the remaining sequence, particularly amino acids 500 to 580, accounted for most of the variation in the sequence patterns observed. Sequence patterns I to X were previously described by Ito et al. (8), whereas patterns XI to XXIII were identified in this study. Overall, sequence pattern XII was the most common (28 isolates), followed by patterns X and XIII (11 isolates each). Patterns III, VI, and VIII, previously described by Ito et al. (8), were not identified in this sample.
FIG. 1.
Partial PBP 2 amino acid sequences (positions 321 to 581) from the 109 N. gonorrhoeae isolates used in this study. The sequences are classified into different amino acid patterns (patterns I to XXIII) and are aligned with a GenBank N. gonorrhoeae sequence (accession number M32091). Sequence patterns I to X were previously described by Ito et al. (8), whereas patterns XI to XXIII were identified in this study. The numbers of isolates with each pattern are indicated in parentheses. Patterns III, VI, and VIII, previously described by Ito et al. (8), were not identified in this sample.
Sequence pattern X (n = 11) represented the previously described mosaic PBP 2 sequence, confirming the presence of this sequence in Australian gonococcal strains. Sequence pattern XXIII was a variation of the previously described pattern X mosaic sequence (26) (Fig. 1). Briefly, the first 430 amino acids of this pattern XXIII mosaic sequence were nearly identical to those in the GenBank reference sequence (accession number M32091) except for an additional Asp-345A codon, whereas the remaining amino acids were similar to those of the pattern X mosaic sequence but with the three substitutions described by Takahata et al. (20).
Association of PBP 2 with antimicrobial susceptibility.
Overall, considerable variation in the ceftriaxone and penicillin G MICs was observed for each of the PBP 2 sequence patterns (Table 1). Significant associations with ceftriaxone susceptibility were observed for five PBP 2 patterns. Significantly higher ceftriaxone MICs were observed for the N. gonorrhoeae strains with the mosaic PBP 2 pattern X (P < 0.01), as well as those with pattern XIII (P < 0.001). Significantly lower ceftriaxone MICs were observed for strains with patterns I (P < 0.01), II (P < 0.01), and XII (P < 0.05). Ten of the 11 strains with mosaic pattern X were less susceptible to ceftriaxone (MICs = 0.06 μg/ml), whereas one strain with pattern X and the one strain with variant mosaic pattern XXIII were both sensitive (MICs = 0.03 μg/ml and 0.008 μg/ml, respectively; Table 1).
TABLE 1.
Ceftriaxone MICs for the various PBP 2 amino acid patterns and associated N. gonorrhoeae NG MAST sequence types
| PBP 2 pattern | No. of isolates with the following ceftriaxone MIC (μg/ml):
|
No. of NG MAST STsa | |||||
|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.016 | 0.03 | 0.06 | 0.12 | Total | ||
| I | 6 | 6 | 6 | ||||
| II | 5 | 5 | 3 | ||||
| IV | 1 | 1 | 2 | 2 | |||
| V | 3 | 3 | 3 | 9 | 5 | ||
| VII | 2 | 1 | 3 | 3 | |||
| IX | 1 | 1 | 7 | 9 | 3 | ||
| X | 1 | 10 | 11 | 7 | |||
| XI | 2 | 2 | 2 | ||||
| XII | 9 | 5 | 10 | 4 | 28 | 12 | |
| XIII | 8 | 3 | 11 | 7 | |||
| XIV | 1 | 1 | 1 | ||||
| XV | 2 | 2 | 2 | ||||
| XVI | 1 | 1 | 1 | ||||
| XVII | 2 | 1 | 3 | 2 | |||
| XVIII | 2 | 1 | 3 | 2 | |||
| XIX | 2 | 1 | 3 | 3 | |||
| XX | 1 | 2 | 3 | 1 | |||
| XXI | 3 | 3 | 2 | ||||
| XXII | 2 | 1 | 3 | 3 | |||
| XXIII | 1 | 1 | 1 | ||||
| Total | 36 | 9 | 14 | 43 | 7 | 109 | 64 |
STs, sequence types.
Significant associations with penicillin G susceptibility were observed for five PBP 2 patterns (Table 2). Significantly higher penicillin G MICs were observed for N. gonorrhoeae strains with mosaic PBP 2 pattern X (P < 0.001) as well as those with pattern XX (P < 0.01). Strains with patterns I, XV, and XXII demonstrated significantly lower penicillin G MICs (P < 0.01). Only 4 of 95 N. gonorrhoeae strains with the additional Asp-345A codon were susceptible to penicillin G, including strains with PBP 2 patterns I (1 isolate), XII (1 isolate), and XXII (2 isolates). The two gonococcal strains with PBP 2 pattern XV, which lacks the Asp-345A codon, were both susceptible to penicillin G, with low MICs (MIC = 0.0016 μg/ml).
TABLE 2.
Penicillin G MICs for the various PBP 2 amino acid patterns
| PBP 2 pattern | No. of isolates with the following penicillin G MIC (μg/ml) or characteristic:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ≤0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | PPNGa | Total | |
| I | 1 | 3 | 1 | 1 | 6 | |||
| II | 1 | 3 | 1 | 5 | ||||
| IV | 1 | 1 | 2 | |||||
| V | 5 | 4 | 9 | |||||
| VII | 1 | 2 | 3 | |||||
| IX | 1 | 7 | 1 | 9 | ||||
| X | 2 | 9 | 11 | |||||
| XI | 1 | 1 | 2 | |||||
| XII | 1 | 2 | 19 | 5 | 27 | |||
| XIII | 9 | 1 | 1 | 11 | ||||
| XIV | 1 | 1 | ||||||
| XV | 2 | 2 | ||||||
| XVI | 1 | 1 | ||||||
| XVII | 2 | 1 | 3 | |||||
| XVIII | 3 | 3 | ||||||
| XIX | 1 | 2 | 3 | |||||
| XX | 3 | 3 | ||||||
| XXI | 1 | 1 | 1 | 3 | ||||
| XXII | 2 | 1 | 3 | |||||
| XXIII | 1 | 1 | ||||||
| Total | 6 | 4 | 8 | 5 | 47 | 28 | 10 | |
PPNG, penicillinase-producing N. gonorrhoeae.
Association of N. gonorrhoeae typing with ceftriaxone susceptibility.
There was considerable diversity among the isolates tested, with seven auxotypes, 23 serotypes, and 64 NG MAST sequence types observed. The PBP 2 amino acid patterns, ceftriaxone MICs, auxotypes, serotypes, and sequence types for the 50 N. gonorrhoeae isolates with reduced susceptibilities to ceftriaxone are provided in Table 3. Briefly, the strains with reduced susceptibilities to ceftriaxone did not have any particular type and were found to have a broad range of auxotypes, serotypes, and genotypes.
Also of note was the presence of 14 isolates of sequence type 225, all with PBP 2 pattern XII. The ceftriaxone MICs for this sequence type were ≤0.008 μg/ml (n = 6), 0.016 μg/ml (n = 4), and 0.03 μg/ml (n = 4). There were another 14 isolates also of PBP 2 pattern XII but of seven other sequence types (data not shown) and with ceftriaxone MICs ≤0.008 μg/ml (n = 3), 0.016 μg/ml (n = 1), 0.03 μg/ml (n = 6), and 0.06 μg/ml (n = 4). Sequence type 225 was the “clone” identified in the Danish cluster (7), but no PBP 2 sequencing was performed in that study.
DISCUSSION
This study shows the considerable diversity that exists in PBP 2 proteins in N. gonorrhoeae strains less susceptible to ceftriaxone. The 20 different amino acid sequences detected included the 7 previously described by Ito et al. (8) and 13 additional variations. Many of the individual PBP 2 sequence patterns displayed a range of ceftriaxone MICs and were found in multiple gonococcal subtypes, although some were associated with significantly higher (patterns X and XIII) or lower (patterns I, II, and XII) ceftriaxone MICs. As demonstrated in a previous study (8), we also found a significant association between the presence of the pattern X mosaic sequence and reduced ceftriaxone susceptibility. However, in contrast to the previous report (8), the mosaic sequences in our sample did not account for a large proportion of strains with reduced sensitivities to ceftriaxone, and only 6 of the 50 strains with reduced sensitivities (MICs = 0.06 μg/ml or more) in this study possessed the mosaic PBP 2 sequence (Table 3). Thus, the presence of a mosaic PBP 2 sequence, previously assigned a pivotal role in this phenomenon, could not account for the majority of instances of this reduced sensitivity to ceftriaxone.
We raised further questions regarding the role of mosaic sequences in conferring reduced ceftriaxone susceptibility when we described a strain with variant mosaic pattern XXIII that was fully susceptible to ceftriaxone (26). In this context, the highly significant association between PBP 2 pattern XIII and reduced susceptibility to ceftriaxone was also of particular interest. This PBP 2 pattern contained a substitution at position 501 (A501V), and all 11 strains with pattern XIII were less susceptible to ceftriaxone, with MICs of 0.06 μg/ml or greater. In fact, 22 of the 25 strains in our sample containing a substitution at position 501 (A501V or A501T; patterns VII, XI, XIII, XVII, XVIII, and XXI) were less susceptible to ceftriaxone and comprised 6 of the 7 strains with MICs of 0.125 μg/ml. During transformation experiments with mosaic penA sequences, Takahata et al. (20) found that a spontaneous mutant with a single A501V mutation was selected with cephalexin. Subsequent analysis revealed that this A501V mutation led to two- to fourfold increases in the MICs of cefixime and other cephems (20). Ito et al. (8) also observed that all five strains with PBP 2 pattern VII were less susceptible to ceftriaxone (8). These changes at position 501 were also present in some gonococci less susceptible to ceftriaxone in the studies of Ito et al. (8) and Lindberg et al. (12) but were not specifically remarked upon by either group. Overall, this suggests that the mutations at position 501 are also of interest and, based on our data, are more significant as a contribution to ceftriaxone susceptibility than the mosaic sequences. However, questions are again raised over the relevance of this mutation in isolation, given that all three strains in our study with pattern XXI (which contains the A501V mutation) were fully susceptible to ceftriaxone (MICs = 0.008 μg/ml).
It was also shown in this study that while particular N. gonorrhoeae genotypes, e.g., sequence type 5, were associated with decreased susceptibility to ceftriaxone, in general, this reduced susceptibility to ceftriaxone was not restricted to any particular genotype and was observed in strains of a broad range of auxotypes, serotypes, and genotypes. Thus, the mechanisms responsible for the reduced susceptibility to ceftriaxone appear to be present in a broad range of gonococcal types and strains and were not associated with a particular subtype, as has been observed previously (7, 8). Both of those earlier studies were with gonococci isolated in a limited geographic area over a defined period, whereas this study was of gonococci selected for their temporal and geographic diversities. Other broadly based studies of the diagnostic features (11, 14) and the pathogenic features (17) of gonococci have also revealed a wider distribution of gonococcal subtypes with a particular characteristic than that revealed by earlier and more narrowly based studies. The findings of those earlier studies and those of this study are thus consistent with the known population dynamics of gonococci (9, 23), and the earlier data probably reflect the expansion of a particular subtype within a wider gonococcal population at a particular time and place. The presence in both Denmark and Australia of N. gonorrhoeae NG MAST sequence type 225 strains with some increase in the ceftriaxone MIC (MIC = 0.03 μg/ml) also suggests the possibility of the international spread of these strains. The International spread of gonococci with other specific characteristics has also been documented recently (25).
The clinical significance of these findings is difficult to determine at present. The history of antimicrobial resistance in N. gonorrhoeae indicates that problems with any antibiotic that is used extensively for the treatment of N. gonorrhoeae disease will eventually be encountered (5, 22). The emergence of N. gonorrhoeae strains with reduced susceptibilities to ceftriaxone and other expanded-spectrum cephalosporins suggests that the gonococci are now developing mechanisms of resistance to yet another important group of antibiotics. These isolates with reduced susceptibilities to ceftriaxone are often also quinolone resistant (7), and this was also the case with the isolates studied here (data not shown). Although treatment failure with oral expanded-spectrum agents, including cefixime, which is not available in Australia, have been recorded in Japan (16), it should be noted that no documented treatment failures have been associated with the injectable agent ceftriaxone when the higher 250-mg dose recommended for use in Australia is used, even for strains containing the mosaic penA sequence (22). Thus, it would appear that additional resistance mechanisms are required before we will see gonococci that are fully resistant to ceftriaxone.
Overall, the results of our study show that mosaic PBP 2 sequences alone cannot account for the reduced ceftriaxone sensitivity observed in our N. gonorrhoeae strains and that strains exhibiting this phenotype are not necessarily of a particular subtype. Furthermore, the results suggest that gradual mutation in the N. gonorrhoeae penA gene sequence, in addition to genetic recombination, which occurs frequently in gonococci (23), contributes not only to reduced susceptibility to ceftriaxone but also to the diversity of changes observed here. The separate phenomenon of strain diversity seen also, but separately, reflects the high rate of transformation that occurs in N. gonorrhoeae. Other mechanisms that may confer reduced susceptibility to newer cephalosporins, including mutations in the ponA (PBP 1) gene, have been proposed (21). Thus, further investigation is needed to fully understand the impacts of these genetic mechanisms both alone and in combination. We emphasize that N. gonorrhoeae strains with reduced susceptibilities to ceftriaxone represent a potential threat to current treatment regimens. Hence, the continued spread of these strains needs to be closely monitored.
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Ameyama, S., S. Onodera, M. Takahata, S. Minami, N. Maki, K. Endo, H. Goto, H. Suzuki, and Y. Oishi. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Gonococcal Surveillance Programme. 2006. Annual report of the Australian Gonococcal Surveillance Programme, 2005. Commun. Dis. Intell. 30:205-210. [PubMed] [Google Scholar]
- 3.Brannigan, J. A., I. A. Tirodimos, Q. Y. Zhang, C. G. Dowson, and B. G. Spratt. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913-919. [DOI] [PubMed] [Google Scholar]
- 4.Bygdeman, S. M. 1988. Polyclonal and monoclonal antibodies applied to the epidemiology of gonococcal infection, p. 117-166. In H. Young and A. McMillan (ed.), Immunological diagnosis of sexually transmitted diseases. Marcel Dekker, New York, NY.
- 5.Centers for Disease Control and Prevention. 2007. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. Morb. Mortal. Wkly. Rep. 56:332-336. [PubMed] [Google Scholar]
- 6.Dougherty, T. J., A. E. Koller, and A. Tomasz. 1980. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 18:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann, S. H., L. Lambertsen, L. Berthelsen, and S. Cowan. 2005. Neisseria gonorrhoeae with increased ceftriaxone MIC in Denmark 2004: bi-locus sequence typing, serotyping, and sexual orientation, abstr. WP-035. ISSTDR 16th Meeting, Amsterdam, The Netherlands.
- 8.Ito, M., T. Deguchi, K. Mizutani, M. Yasuda, S. Yokoi, S. Ito, Y. Takahashi, S. Ishihara, Y. Kawamura, and T. Ezaki. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 49:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapp, J. S., M. R. Tam, R. C. Nowinski, K. K. Holmes, and E. G. Sandstrom. 1984. Serological classification of N. gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J. Infect. Dis. 150:44-48. [DOI] [PubMed] [Google Scholar]
- 10.La Scolea, L. J., Jr., and F. E. Young. 1974. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl. Microbiol. 28:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limnios, E. A., N.-L. Nguyen, S. Ray, C. J. McIver, and J. W. Tapsall. 2006. Dynamics of appearance and expansion of a prolyliminopeptidase-negative subtype among Neisseria gonorrhoeae isolates collected in Sydney, Australia, from 2002 to 2005. J. Clin. Microbiol. 44:1400-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg, R., H. Fredlund, R. Nicholas, and M. Unemo. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porbIb, and ponA. Antimicrob. Agents Chemother. 51:2117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lkhamsuren, E., T. R. Shultz, E. A. Limnios, and J. W. Tapsall. 2001. The antibiotic susceptibility of Neisseria gonorrhoeae isolated in Ulaanbaatar, Mongolia. Sex. Transm. Infect. 77:218-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lum, G., K. Freeman, N. L. Nguyen, E. A. Limnios, S. N. Tabrizi, I. Carter, I. W. Chambers, D. M. Whiley, T. P. Sloots, S. M. Garland, and J. W. Tapsall. 2005. A cluster of culture positive gonococcal infections but with false-negative cppB-gene-based PCR. Sex. Transm. Infect. 81:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, I. M. C., C. A. Ison, D. A. Aanensen, K. A. Fenton, and B. G. Spratt. 2004. Rapid sequence-based identification of gonococcal clusters in a large metropolitan area. J. Infect. Dis. 189:1497-1505. [DOI] [PubMed] [Google Scholar]
- 16.Muratani, T., S. Akasaka, T. Kobayashi, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimcrob. Agents Chemother. 45:3603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power, P. M., S. C. Ku, K. Rutter, M. J. Warren, E. A. Limnios, J. W. Tapsall, and M. P. Jennings. 2007. The phase-variable allele of the pili glycosylation gene pglA is not strongly associated with strains of Neisseria gonorrhoeae isolated from patients with disseminated gonococcal infection. Infect. Immun. 75:3202-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ropp, P. A., M. Hu, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein I, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 20.Takahata, S., N. Senju, Y. Osaki, T. Yoshida, and T. Ida. 2006. Amino acid substitutions of mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 50:3638-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka, M., H. Nakayama, K. Huruya, I. Konomi, S. Irie, A. Kanayama, T. Saika, and I. Kobayashi. 2006. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 27:20-26. [DOI] [PubMed] [Google Scholar]
- 22.Tapsall, J. 2006. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert. Rev. Anti.-Infect. Ther. 4:619-628. [DOI] [PubMed] [Google Scholar]
- 23.Tapsall, J., D. Whiley, and T. Sloots. 2006. Applications of molecular testing in clinical laboratories for the diagnosis and control of gonorrhea. Future Microbiol. 1:317-324. [DOI] [PubMed] [Google Scholar]
- 24.Tapsall, J. W., and Members of the National Neisseria Network of Australia. 2004. Antimicrobial testing and applications in the pathogenic Neisseria, p. 173-186. In J. Merlino (ed.), Antimicrobial susceptibility testing: methods and practices with an Australian perspective. Australian Society for Microbiology, Sydney, Australia.
- 25.Unemo, M., H. H. Palmer, T. Blackmore, G. Herrera, H. Fredlund, E. A. Limnios, N. Nguyen, and J. Tapsall. 2007. Global transmission of prolyliminopeptidase-negative Neisseria gonorrhoeae strains: implications for changes in diagnostic strategies. Sex. Transm. Infect. 83:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiley, D. M., E. A. Limnios, S. Ray, T. P. Sloots, and J. W. Tapsall. 2007. Further questions regarding the role of mosaic penA sequences in conferring reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 51:802-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. 2006. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2005. Commun. Dis. Intell. 30:430-433. [PubMed] [Google Scholar]
- 28.Yong, D., T. S. Kim, J. R. Choi, J. H. Yum, K. Lee, Y. Chong, H.-B. Oh, T. R. Shultz, and J. W. Tapsall. 2004. Epidemiologic characteristics and molecular basis of fluoroquinolone-resistant Neisseria gonorrhoeae strains isolated in Korea and nearby countries. J. Antimicrob. Chemother. 54:451-455. [DOI] [PubMed] [Google Scholar]