Abstract
Using an in vitro pharmacodynamic model, a multidrug-resistant strain of Acinetobacter baumannii was exposed to colistin methanesulfonate alone and in combination with ceftazidime. Pre- and postexposure colistin sulfate MICs were determined. A single daily dose of colistin methanesulfonate combined with continuous-infusion ceftazidime prevented regrowth and postexposure MIC increases.
Acinetobacter baumannii is an opportunistic nosocomial pathogen associated with significant morbidity and mortality (3, 9). High rates of resistance to commonly used broad-spectrum antibiotics are frequently reported (2, 5). Current approaches to therapy include carbapenems and aminoglycosides, but resistance to these antibiotics is increasing, as well (4). Should these agents fail, colistin (polymyxin E) may represent the last line of defense.
The purpose of this study was to examine time-kill profiles of colistin methanesulfonate (CMS), the parenteral salt form of colistin, alone and combined with ceftazidime.
CMS (Fluka, Buchs, Switzerland) was prepared 1 day prior to use and stored at 4°C (6). A ceftazidime (GlaxoSmithKline, Research Triangle Park, NC) stock solution was prepared and stored at −80°C. Colistin sulfate (Sigma-Aldrich, St. Louis, MO) stock solutions were prepared for use in MIC trays and stored at 4°C.
A clinical isolate of A. baumannii (06-74; source unknown) was obtained from Regions Hospital (Saint Paul, MN), where identification and sensitivity testing were performed using the Vitek 2 (bioMérieux, Balmes-les-Grottes, France). The time-kill experiments used CMS alone or in combination with continuous-infusion ceftazidime. The previously described in vitro phamacodynamic model (10) contained cation-adjusted Mueller-Hinton broth (Becton Dickinson Microbiology Systems, Sparks, MD). The pump rate was set to create the desired 3-hour half-life of colistin sulfate (8).
Two sets of duplicate 24-hour experiments were performed. In set 1, four experiments simulating a range of peak CMS concentrations were performed to evaluate the time-kill profiles of A. baumannii. At time zero, a bolus dose was injected into the vessel. The desired CMS peak concentrations were 3, 6, 12, and 24 μg/ml. The peak concentrations and half-lives were not verified via assay, so the desired and actual values might have differed. Additionally, one experiment was performed to evaluate the effect of a second 24-μg/ml dose of CMS at 12 h.
Set 2 included three experiments utilizing a CMS bolus of 24 μg/ml administered along with continuous infusions of ceftazidime at 50 μg/ml. To simulate continuous infusions, ceftazidime was added to the media reservoir, and a loading dose was injected into the vessel. In the first experiment, CMS was injected into the vessel at time zero, and the continuous infusion of ceftazidime was initiated at 2 h. In the second experiment, the order was reversed; continuous-infusion ceftazidime was initiated at time zero, followed by a CMS bolus injection at 2 h. Finally, a third experiment was conducted with a continuous infusion of ceftazidime initiated at time zero.
An aliquot of A. baumannii was added to each vessel, resulting in an initial inoculum between 105 and 106 CFU/ml. The drug was introduced, and nine samples were collected between 0.5 and 24 h. Three additional samples were collected when a second dose was administered. Fifty microliters of each sample was deposited onto trypticase soy agar plates supplemented with 5% sheep blood (Becton Dickinson and Company, Sparks, MD) using the WASP 2 spiral plater. The lower limit of accuracy was 400 CFU/ml (20 colonies divided by 0.05 ml deposited on the plate) and was determined using the guidelines outlined in the WASP 2 manual (1). Following incubation, bacteria were enumerated using the aCOLyte automated colony counter (Synbiosis, Cambridge, United Kingdom). Concentration-time-kill curves were compared graphically by plotting log10 CFU/ml versus time.
Microdilution MIC tests for both colistin sulfate and CMS were performed on postexposure isolates present at 24 h using the Bio-Tek Precision 2000 pipetting system (Bio-Tek Instruments, Inc. Winooski, VT) following CLSI guidelines (7). Pseudomonas aeruginosa ATCC 27853 was used as the quality control strain. Ceftazidime MIC testing was performed on A. baumannii isolates from experiments combining ceftazidime and CMS. The resistance stabilities of postexposure isolates from experiments simulating CMS concentrations of 3, 6, 12, and 24 μg/ml were evaluated by subculturing them daily for 10 days and repeating the MIC tests.
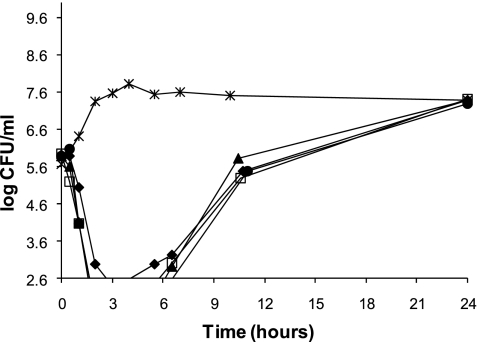
CMS peak concentrations of 3, 6, 12, and 24 μg/ml all reduced the bacterial load by 3 log10 CFU/ml within 2 to 3 h. Regrowth was observed beginning at 3 h for all single-dose CMS experiments and continued through 24 h, reaching approximately 7 log10 CFU/ml (Fig. 1). The addition of a second dose of CMS (24 μg/ml) at 12 h was ineffective in further reducing the bacterial burden or preventing regrowth of A. baumannii.
FIG. 1.
Time-kill curves of CMS against A. baumannii at maximum serum drug concentrations of 3 μg/ml (⧫), 6 μg/ml (•), 12 μg/ml (▴), and 24 μg/ml (□). The growth control (×) is also depicted.
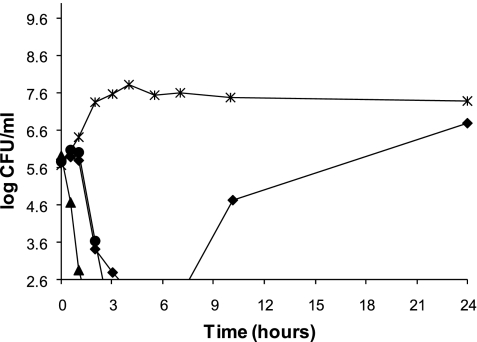
Continuous-infusion ceftazidime administered alone produced a 3 log10 CFU/ml reduction in the bacterial burden within 3 to 4 h, but at 24 h, regrowth to approximately 6.5 log10 CFU/ml had occurred. When a bolus CMS dose was combined with continuous-infusion ceftazidime, a 3 log10 CFU/ml reduction was quickly achieved—within 2 to 3 h—and regrowth was subsequently suppressed (Fig. 2).
FIG. 2.
Time-kill curves of CMS given at time zero plus the addition of continuous-infusion ceftazidime started at 2 h (▴) and continuous-infusion ceftazidime started at time zero plus a CMS bolus given at 2 h (•) against A. baumannii. Continuous-infusion ceftazidime given alone (⧫) and the growth control (×) are also depicted.
The preexposure MICs were 0.5 and 2 μg/ml for colistin sulfate and CMS, respectively. All postexposure isolates from time-kill experiments using CMS alone had CMS MICs of >2,048 μg/ml. In order to obtain definitive endpoints, further MIC testing was performed using colistin sulfate only. Colistin sulfate MICs increased from 0.5 μg/ml preexposure to 32 to >128 μg/ml postexposure for experiments using CMS alone. The addition of continuous-infusion ceftazidime along with CMS prevented resistance to colistin sulfate, and ceftazidime MICs remained unchanged (Table 1). Colistin sulfate MICs were stable at 128 to 256 μg/ml after a 10-day serial passage.
TABLE 1.
Colistin sulfate and ceftazidime MIC results
| Expt | MICa (μg/ml)
|
|
|---|---|---|
| Colistin sulfate | Ceftazidime | |
| A. baumannii preexposure | 0.5 | ≥64 |
| A. baumannii postexposure | ||
| 3 μg/ml CMS | 64-128 | |
| 6 μg/ml CMS | 64->128 | |
| 12 μg/ml CMS | 128 | |
| 24 μg/ml CMS | 32->128 | |
| 24 μg/ml CMS (bolus dose at 0 and 12 h) | ≥128 | |
| 24 μg/ml CMS and 50 μg/ml ceftazidimeb | 0.5 | 32-64 |
| 50 μg/ml ceftazidime and 24 μg/ml CMSc | 0.06 | 32-64 |
Vitek 2 MIC data (μg/ml) for A. baumannii 06-74 are as follows: ampicillin, ≥32; ceftazidime, ≥64; ciprofloxacin, ≥4; levofloxacin, ≥8; gentamicin, ≥16; aztreonam, ≥64; nitrofurantion, ≥512; ampicillin-sulbactam, ≤2/4; amikacin, ≤2; imipenem, ≤1; meropenem, 1; and piperacillin-tazobactam, 32/4.
CMS given as a bolus dose at time zero, followed by ceftazidime administered as a continuous infusion at 2 h.
Ceftazidime administered as a continuous infusion at time zero, followed by CMS given as a bolus dose at 2 h.
The ability to prevent the MIC increases when CMS was combined with ceftazidime and the apparent benefit when the drugs were used in combination were unexpected findings. Likewise, the antibacterial effect of continuous-infusion ceftazidime given alone proved to be an interesting observation, considering that A. baumannii was intrinsically resistant to ceftazidime (MIC ≥ 64 μg/ml).
The order and frequency of antibiotic administration may factor into an optimal dosing strategy for the treatment of A. baumannii infections. The use of CMS first results in a rapid and significant reduction of the bacterial burden. Furthermore, the small numbers of survivors, if now resistant to CMS, may succumb to a second antibiotic, e.g., ceftazidime given as a continuous infusion.
Acknowledgments
We are thankful for support from a Samuel W. Melendy research scholarship and the Antibiotic Pharmacodynamic Research Institute.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Don Whitley Scientific Ltd. 2000. WASP 2 user manual. Don Whitley Scientific Ltd., Shipley, West Yorkshire, England.
- 2.Gales, A. C., R. N. Jones, K. R. Forward, J. Linares, H. S. Sader, and J. Verhoef. 2001. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY antimicrobial surveillance program (1997-1999). Clin. Infect. Dis. 32:S104-S113. [DOI] [PubMed] [Google Scholar]
- 3.Garnacho-Montero, J., C. Ortiz-Leyba, E. Fernández-Hinojosa, T. Aldabó-Pallás, A. Cayuela, J. A. Marquez-Vácaro, A. Garcia-Curiel, and F. J. Jiménez-Jiménez. 2005. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med. 31:649-655. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes, R., J. R. Edwards, and the National Nosocomial Infections Surveillance System. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 5.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, and K. Coulthard. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob. Agents Chemother. 47:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—5th ed. NCCLS document M7-A5. NCCLS, Wayne, PA.
- 8.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 9.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 10.Zabinski, R. A., K. Vance-Bryan, A. J. Krinke, K. J. Walker, J. A. Moody, and J. C. Rotschafer. 1993. Evaluation of activity of temafloxacin against Bacteroides fragalis by an in vitro pharmacodynamic system. Antimicrob. Agents Chemother. 37:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]