Abstract
Intragastric inoculation of Klebsiella pneumoniae can cause invasive diseases, including necrosis of liver tissues and bacteremia. The effect of concanavalin A (ConA) on K. pneumoniae was tested. Pretreatment with ConA was able to protect mice from K. pneumoniae infection in an intragastric model. K. pneumoniae-induced mouse death and liver injury such as liver necrosis, as well as blood levels of aspartate aminotransferase and alanine aminotransferase, were inhibited in a dose-dependent manner by ConA. ConA administered intravenously as late as 24 h after K. pneumoniae inoculation was still protective. In an in vitro assay, ConA was able to bind K. pneumoniae cells directly and further agglutinate them but had no effect on their in vitro growth. Surveys of bacterial counts of ConA-treated mice revealed that the bacteria were eliminated effectively in both blood and liver tissues. Furthermore, the bactericidal activity of macrophages against K. pneumoniae was also enhanced in a dose-dependent manner by ConA in an in vitro culture. These data suggest that ConA is a potentially therapeutic agent for K. pneumoniae-induced liver infection.
Klebsiella pneumoniae is an important nosocomial pathogen that causes severe diseases such as septicemia, pneumonia, and urinary tract infection in immunocompromised individuals (30). In the past two decades, community-acquired infection by invasive K. pneumoniae has emerged in Taiwan that is characterized by primary liver abscess with sepsis and bacteremia (5, 6, 12, 14, 20, 21, 36), and about 10% to 12% of patients develop complications of metastatic meningitis and endophthalmitis (6, 12, 14). Diabetes mellitus is regarded as an important risk factor for patients with K. pneumoniae liver abscess (5, 12, 14, 20, 21, 36), but individuals displaying no apparent underlying diseases are also attacked by the invasive strains (6, 12). Despite intensive care that includes pigtail catheter drainage and antimicrobial therapy, the mortality rate has remained at 10% to 30%, especially for patients with metastatic complications (11, 19). K. pneumoniae-induced primary liver abscess has also been reported in other countries, including the United States (22), Europe (3), and Japan (29).
Although the mechanism of K. pneumoniae-induced primary liver abscess is not clearly understood, epidemiological analysis indicates that the transmission of K. pneumoniae is through the fecal-oral route, and these invasive bacteria may reach the liver through the portal vein and cause liver abscess (5). Several genetic loci such as the cps cluster (2), wb cluster (25), rmpA (1), htrA (8), and magA (7, 12) have been identified as virulence genes of K. pneumoniae. Most clinical K. pneumoniae isolates from liver abscess patients express K1 capsular polysaccharide (7, 14) and MagA outer membrane protein (7, 12), which are the two virulence factors that offer bacterial resistance to serum and phagocyte killing (12).
Concanavalin A (ConA), a lectin derived from the seeds of jack beans (Canavalia ensiformis), exhibits broad biological activity by interaction with its sugar binding site, α-linked mannose (23). The well-known activities of ConA include mitogenic activation of T cells (10), induction of T-cell-mediated hepatitis (35), and inhibition of tumor growth (26). In the present study, the effect of ConA on K. pneumoniae infection was evaluated. Using an intragastric infection model, which mimics the clinical infection route, we found that a nonhepatotoxic dose of ConA was able to inhibit K. pneumoniae-induced liver injury and bacteremia by decreasing the bacterial burden in the liver.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, or from Charles River Japan, Inc. (Atsugi, Japan). They were maintained on standard laboratory chow and water ad libitum in the animal center of National Cheng Kung University. The animals were raised and cared for in accordance with guidelines established by the National Science Council of the Republic of China. Eight- to 10-week-old male mice were used in all experiments.
Bacterium.
K. pneumoniae NK-5, isolated from a patient with primary liver abscess and septicemia at the National Cheng Kung University Hospital, was resistant to serum killing, and its 100% lethal dose (LD100) by intraperitoneal injection in B6 mice was 50 K. pneumoniae cells. K. pneumoniae was cultured in tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) for 18 h at 37°C and then subcultured in fresh broth (1:50 [vol/vol]) for another 3 h. The concentration of bacteria was determined with a spectrophotometer (Beckman Instruments, Somerset, NJ), with an optical density at 600 nm of 1 being equal to 2.8 × 108 CFU/ml.
ConA.
ConA (type V) (Sigma Chemical Co., St. Louis, MO) was dissolved in distilled water (10 mg/ml) and stored at −20°C as a stock. In this study, ConA was diluted with phosphate-buffered saline (PBS) (100 μg/ml) immediately before intravenous injection.
Force-feeding model of infection.
For the induction of liver abscess, groups of 6 to 14 mice were given 0.2 ml of a solution of 0.2 M NaHCO3 orally, through a sterile gastric gavage, to neutralize acidity (24). Through the same route, 2 × 108 K. pneumoniae cells in 0.2 ml of sterile PBS were administered immediately after the bicarbonate treatment. The LD100 by intragastric administration in B6 mice was 2.5 × 108 K. pneumoniae cells. The animals were observed every day for a total of 9 days. In ConA inhibition experiments, the mice were given an intravenous injection of ConA (approximately 0.1 to 2 mg/kg of body weight) 2 h before K. pneumoniae infection followed by intravenous injection of ConA (approximately 0.1 to 2 mg/kg) every 2 or 3 days until day 4 postinfection. In ConA therapeutic experiments, ConA (1 or 2 mg/kg) was given intravenously at day 1 and day 3 post-K. pneumoniae infection. Survival curves were then determined.
In some ConA inhibition experiments, groups of six to seven B6 mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse. ConA (1 mg/kg) was administrated intravenously 2 h before infection followed by ConA treatment at day 3 postinfection. At different times after infection, mouse serum samples were collected, and the livers were removed, fixed in 3.7% formaldehyde, and embedded in paraffin. The 5-μm-thick tissues were sliced and stained with hematoxylin and eosin. The degree of liver inflammation was determined by a blinded histopathology score. Three different sections of the largest liver lobule of each mouse were examined. A score of 1 indicates that the number of microabscesses on each liver section was less than 5 and that no necrosis region was found. A score of 2 indicates that the number of microabscesses on each liver section was more than 5 and less than 15 and that no necrosis region was found. A score of 3 indicates that the number of microabscesses on each liver section was more than 15 and less than 25 and that no necrosis region was found. A score of 4 indicates that the number of microabscesses on each liver section was more than 25 and that no necrosis region was found. A score of 5 indicates that the number of microabscesses on each liver section was more than 25 and that necrosis regions were found. The average score for each group was generated by examination of liver sections from six mice. In another ConA inhibition experiment, heparin-containing blood or liver, which was homogenized in PBS, was aseptically collected and serial diluted, poured (0.1 ml) into LB agar plates, and incubated at 37°C overnight. The number of CFU of K. pneumoniae was then quantitated and expressed as the mean ± standard deviation. The minimum dilution in this assay was 100×; hence, the limit of detection was 1,000 CFU per g or ml.
Determining aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in serum samples.
Mouse serum samples were collected, and the concentrations of AST and ALT were determined using GOT-JS and GPT-JS kits (Denka Seiken Co., Ltd., Niigata, Japan), respectively, and were then detected using an automated biochemical analyzer (model TBA-200FR; Toshiba Co., Tokyo, Japan) (17).
Bacterial growth curves.
K. pneumoniae NK-5 was cultured in TSB at 37°C overnight, and then the bacterial suspension was subcultured (1:50 [vol/vol]) in fresh TSB for another 12 h. At the time of subculture, different concentrations of ConA were added to the bacterial suspension, and the growth of bacteria at different times was determined with a spectrophotometer by measuring the absorbance at 600 nm. For exact quantification of bacteria, bacterial suspensions collected at different times were plated on LB agar and incubated for 24 h at 37°C. The results of one of three experiments are reported.
ConA binding assay.
K. pneumoniae cells (5 × 107/ml) were coincubated with 5, 10, or 20 μg/ml of ConA or fluorescein-conjugated ConA (Vector, Burlingame, CA) for 30 min at 37°C. The capacity of ConA or ConA-fluorescein isothiocyanate binding to cells was determined by flow cytometric analysis (BD Biosciences, San Jose, CA).
Immunohistochemistry.
Groups of three to four mice were killed by perfusion via cardiac puncture with PBS. The livers were removed and embedded in OCT compound (Miles Inc., Elkhart, IN) and were then frozen in liquid nitrogen. Cryosections (4 μm) were made and were fixed with 3.7% formaldehyde-PBS for 3 min. They were then stained with a primary rat anti-mouse F4/80 monoclonal antibody (MCA497GA; Serotec, Toronto, Ontario, Canada) or a rat anti-mouse neutrophil monoclonal antibody, which was collected from the culture supernatant of hybridoma RB6-8C5 (9). The secondary antibody was peroxidase-conjugated donkey anti-rat immunoglobulin G (Jackson ImmunoResearch Laboratories Inc., West Grove, PA). A peroxidase stain with a reddish-brown color was developed with an aminoethyl carbazole substrate kit (Zymed Laboratories, San Francisco, CA).
Assay for serum ConA.
To detect ConA pharmacokinetics, groups of four B6 mice were given an intravenous injection of ConA (1 mg or 2 mg/kg). At different times (0 min, 10 min, 1 h, 6 h, or 24 h) after ConA injection, mouse serum samples were collected and detected by the enzyme-linked immunosorbent assay (ELISA) method as follows. The wells of the ELISA plate were precoated with goat anti-ConA polyclonal antibody (Vector, Burlingame, CA) (2 μg/well) and were then blocked with 3% bovine serum albumin (Sigma). Thereafter, ConA standard solutions (approximately 0.8 to 51.2 μg/ml) and mouse serum samples were added and incubated at room temperature for 2 h. Mannose-biotin (GlycoTech Co., Gaithersburg, MD) (1 μg/well) was then added, and the mixture was incubated for another 2 h at room temperature. Finally, peroxidase-conjugated streptavidin (R&D Systems, Inc., Minneapolis, MN) was added and the mixture was incubated for 30 min. A peroxidase reaction was developed with a tetramethyl benzidine substrate kit (Zymed Laboratories), and absorbance values were read by use of a Multiskan Ex ELISA reader (Thermo Electron Co., Waltham, MA) at 450 nm.
The bactericidal assay of macrophage.
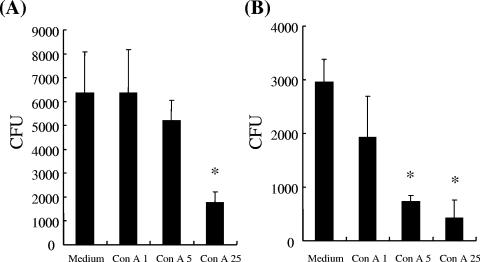
RAW264.7 macrophage cells were grown in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum at 37°C. RAW264.7 cells (4 × 105 cells/well) in 24-well culture plates were pretreated with different concentrations of ConA (1, 5, or 25 μg/ml) at 37°C for 1 h, and the cells were then cocultured with K. pneumoniae cells at a ratio of 15:1 (bacteria to RAW264.7 cells). The plates were centrifuged for 5 min at 500 × g to enhance and synchronize infection. The cells were incubated for 2 h at 37°C to permit phagocytosis, and the free bacteria were removed by three washes with sterile PBS. Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 25 μg/ml of gentamicin was added, and the mixture was further incubated for another 1 h or 3 h at 37°C (34). After several washes with PBS, 0.2 ml of sterile distilled water was added to lyse the cells of each well, and the mixture in each well was then serial diluted, poured in LB agar plates, and incubated at 37°C overnight. The number of CFU of K. pneumoniae was then quantitated and expressed as the mean ± standard deviation.
Statistics.
The statistical analysis was done using Prism 3.0 software (GraphPad Software, San Diego, CA). For the mouse model, survival curves were compared for significance using the Mantel-Haenszel log rank test. Tests for significance of differences between treatment groups were determined using the t test. Statistical significance was set at P < 0.05.
RESULTS
Inhibition of K. pneumoniae-induced death by ConA.
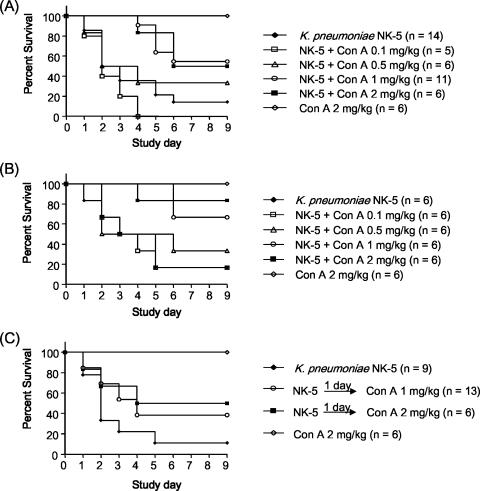
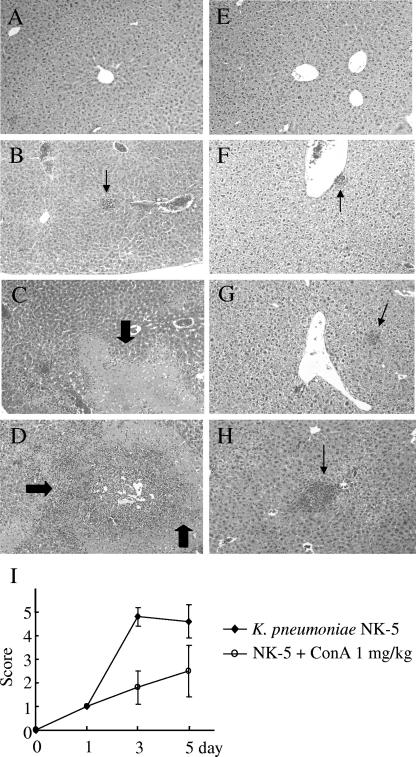
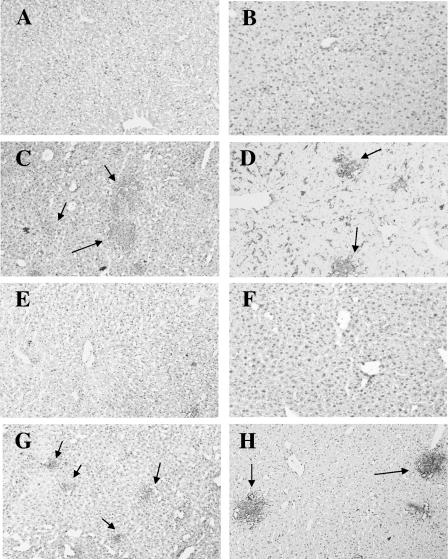
ConA is known to induce acute hepatitis in mice. As the dose has to be higher than 5 mg/kg of body weight, we choose a nonhepatotoxic dose (<3 mg/kg) of ConA (35, 37) to examine its effect on K. pneumoniae-induced mouse death. Without treatment, 90% of the mice would die within 5 days after intragastric inoculation of 2 × 108 K. pneumoniae cells (the LD90 for mice). In contrast, intravenous pretreatment with ConA 2 h before inoculation and a second ConA treatment at day 3 thereafter inhibited the K. pneumoniae induced death in a dose-dependent manner: 0.5 mg of ConA/kg resulted in 33% survival (2 of 6 mice) whereas 1 mg/kg resulted in 55% survival (6 of 11). However, 2 mg of ConA/kg resulted in a protective effect similar to that seen with 1 mg/kg of ConA (Fig. 1A). Although 0.1 mg of ConA/kg seemed to increase the mortality rate, no statistically significant differences were observed compared with the results seen with K. pneumoniae-infected group (Fig. 1A). The histological examination showed that liver tissue had massive necrosis of hepatocytes and microabscess formation at day 3 and day 5 after K. pneumoniae infection (Fig. 2C and D). ConA treatment significantly inhibited necrosis of the liver tissue, although some liver abscesses still existed (Fig. 2G and H). The degree of inflammation in liver tissues was quantitated by histological examination and is shown in Fig. 2I.
FIG. 1.
Inhibition of K. pneumoniae-induced mortality by ConA. (A) ConA inhibits K. pneumoniae NK-5-induced death in B6 mice in a dose-dependent manner. Groups of 6 to 14 B6 mice were inoculated via the intragastric route with 2 × 108 K. pneumoniae NK-5 cells per mouse. Various doses of ConA were administrated intravenously 2 h before infection followed by ConA treatment at day 3 postinfection. ConA alone at 2 mg/kg of body weight had no effect on mice. Survival curves were compared for significance using the Mantel-Haenszel log rank test of NK-5 plus ConA (1 mg/kg) treatment versus the NK-5 group (P = 0.007) and NK-5 plus ConA (2 mg/kg) treatment versus the NK-5 group (P = 0.033). (B) Increasing the frequency of ConA treatment after K. pneumoniae infection inhibits mouse death. Groups of six B6 mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse. Various doses of ConA were administrated intravenously 2 h before infection followed by ConA treatment every 2 days until day 4 postinfection. Survival curves were compared for significance using the Mantel-Haenszel log rank test of NK-5 plus ConA (1 mg/kg) treatment versus the NK-5 group (P = 0.0196) and NK-5 plus ConA (2 mg/kg) treatment versus the NK-5 group (P = 0.028). (C) Therapeutic effect of ConA against K. pneumoniae NK-5 infection in B6 mice. Groups of 6 to 13 B6 mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse. ConA (1 mg or 2 mg/kg) was administrated intravenously at day 1 and day 3 postinfection. Survival curves were compared for significance using the Mantel-Haenszel log rank test of NK-5 plus ConA (2 mg/kg) treatment versus the NK-5 group (P = 0.0451).
FIG. 2.
Inhibition of K. pneumoniae-induced liver damage by ConA. Groups of six to seven B6 mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse. ConA (1 mg/kg of body weight) was administrated as described for Fig. 1A. The mice were sacrificed at different times postinfection, and the liver sections were prepared as described in Materials and Methods. (A) PBS, without K. pneumoniae, day 3; (B) PBS, K. pneumoniae, day 1; (C) PBS, K. pneumoniae, day 3; (D) PBS, K. pneumoniae, day 5; (E) ConA, no K. pneumoniae, day 5; (F) ConA, K. pneumoniae, day 1; (G) ConA, K. pneumoniae, day 3; (H) ConA, K. pneumoniae, day 5. The thick arrows indicate the necrosis region; the thin arrows indicate the liver abscess. Magnification, ×400. Figure 2I indicates the degree of liver inflammation as determined by histological examination as described in Materials and Methods.
In addition to the first treatment with ConA 2 h before inoculation, increasing the total dose of ConA treatment after K. pneumoniae infection also enhanced mouse survival. As shown in Fig. 1B, intravenous injection of 1 mg of ConA/kg every 2 days until day 4 postinfection resulted in 67% survival (four of six mice) whereas 2 mg/kg resulted in 83% survival (five of six). In contrast, intravenous injection of 2 mg of ConA/kg daily for three successive days postinfection worsened mouse survival (data not shown). Furthermore, there was a therapeutic effect of ConA on K. pneumoniae infection. Intravenous administration of 1 mg and 2 mg of ConA/kg at day 1 and day 3 after K. pneumoniae infection still protected 38% (5 of 13) and 50% (3 of 6) of mice from death, respectively (Fig. 1C). These results indicate that ConA could inhibit K. pneumoniae-induced death in this force-feeding infection model.
ConA decreases the bacterial burden and inhibits liver damage.
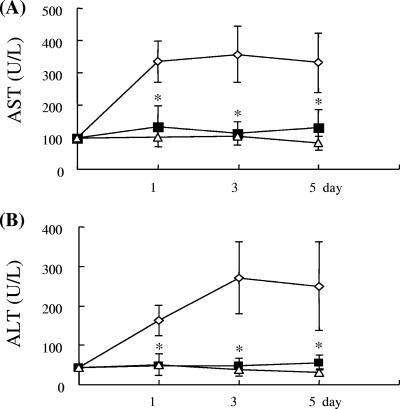
The bacterial counts in the liver and blood were quantitated further. After intragastric inoculation of 2 × 108 K. pneumoniae cells (the LD90 for mice), bacteria were found in livers at day 1, with the amount peaking at day 3 and decreasing by day 5 after infection. The bacteremia began at day 1 and subsequently gradually decreased (Table 1). In contrast, the ConA treatment significantly decreased the bacterial load at day 1 and day 3 in both liver and blood (1,000× difference compared to nontreated control results) (Table 1). K. pneumoniae was gradually cleared after day 5 (data not shown). Furthermore, the liver functions were also impaired, as shown by increased serum AST and ALT levels after K. pneumoniae infection (Fig. 3), but no such elevated AST and ALT levels were found in ConA-treated mice. Intravenous injection of ConA (1 mg/kg) alone resulted in no effects on naive B6 mice (Fig. 3). These results suggest that a subhepatotoxic dose of ConA could decrease the bacterial burden and liver impairment in K. pneumoniae-infected mice.
TABLE 1.
Bacterial counts in liver tissues and blood after ConA treatment of K. pneumoniae infectiona
| Day | Bacterial count in liver (log10 CFU/g) after indicated treatment
|
Bacterial count in blood (log10 CFU/ml) after indicated treatment
|
||
|---|---|---|---|---|
| NK-5 | NK-5 + ConA | NK-5 | NK-5 + ConA | |
| 1 | 6.12 ± 1.05 | 3.42 ± 0.12* | 6.11 ± 0.48 | 2.96 ± 0.24** |
| 3 | 7.05 ± 0.08 | 3.80 ± 0.37* | 5.97 ± 0.09 | 3.15 ± 0.21* |
| 5 | 6.28 ± 0.28 | <3b | 4.97 ± 0.64 | <3b |
Data are presented as means ± standard deviations. *, P < 0.05 after indicated treatment; **, P < 0.01 compared with results for K. pneumoniae-treated mice.
No detectable CFU.
FIG. 3.
Serum AST (A) and ALT (B) levels after ConA treatment of K. pneumoniae infection. Groups of four mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse. ConA (1 mg/kg of body weight) was administrated as described for Fig. 1A. The mice were sacrificed at different times postinfection, and the mouse serum samples were collected and detected as described in Materials and Methods. Treatments: ⋄, K. pneumoniae NK-5 only; ▪, K. pneumoniae NK-5 plus ConA (1 mg/kg); ▵, ConA (1 mg/kg) only. *, P < 0.05 compared with values determined for K. pneumoniae-treated mice.
In vitro effect of ConA on K. pneumoniae.
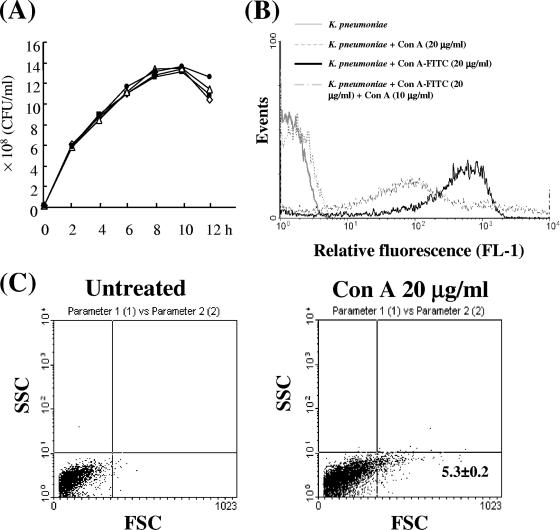
ConA had no direct antimicrobial activity with respect to growth of K. pneumoniae in an in vitro TSB culture (Fig. 4A). However, fluorescein-conjugated ConA (approximately 5 to 20 μg/ml) could bind K. pneumoniae cells in a dose-dependent manner according to flow cytometric analysis (data not shown), and the binding of fluorescein isothiocyanate-ConA (20 μg/ml) to K. pneumoniae cells was inhibited by unlabeled ConA (Fig. 4B). Moreover, ConA (20 μg/ml) was able to agglutinate 5% of K. pneumoniae cells in an in vitro culture (Fig. 4C) based on forward scatter analysis of flow cytometry, indicating that ConA could bind and agglutinate K. pneumoniae cells directly but could not affect their growth.
FIG. 4.
In vitro effect of ConA on K. pneumoniae. (A) Effect of ConA on the in vitro growth of K. pneumoniae. K. pneumoniae NK-5 (1 × 107 cells/ml) was cultured with various concentrations of ConA in TSB, and bacterial growth was determined by counting colonies on LB agar plates, as described in Materials and Methods. One of three experiments is represented. Amount of ConA: ⋄, none; ▪, 1 μg/ml; ▵, 10 μg/ml; •, 50 μg/ml. The binding (B) and agglutinating (C) activity of ConA for K. pneumoniae. K. pneumoniae cells (5 × 107/ml) were coincubated with 20 μg/ml of ConA, 20 μg/ml of fluorescein-conjugated ConA, or 20 μg/ml of fluorescein-conjugated ConA plus 10 μg/ml of ConA in PBS as described in Materials and Methods, and the binding activity was determined by flow cytometric analysis.
Immunomodulatory effect of ConA.
As shown above, the presence of ConA decreased the bacterial burden in the liver and resulted in an increased number of inflammatory cells within liver abscesses by day 3 postinfection (Table 1 and Fig. 2). The infiltrating cells in liver tissues were identified by immunohistochemistry. F4/80 and RB6-8C5 monoclonal antibodies were used to detect pan-macrophages (33) and neutrophils (9), respectively. In addition to F4/80-positive cells, there were few infiltrated neutrophils in the liver at day 1 postinfection irrespective of ConA treatment (data not shown). In contrast, more F4/80-positive cells and infiltrated neutrophils were found in liver abscess foci of the ConA-treated mice than in those of the PBS-treated group at day 3 (data not shown) and day 5 post-K. pneumoniae infection (Fig. 5D and H versus Fig. 5C and G), indicating that ConA likely inhibited K. pneumoniae infection through increasing the interaction of macrophages and neutrophils in the liver.
FIG. 5.
ConA treatment enhanced recruitment of phagocytes induced by K. pneumoniae in B6 mice. Groups of three mice were inoculated intragastrically with 2 × 108 K. pneumoniae NK-5 cells per mouse, and the mice were sacrificed at day 5 postinfection. (B, D, F, and H) ConA (1 mg/kg of body weight) was administered intravenously 2 h before infection followed by ConA treatment at day 3 postinfection. Cryosections (4 μm) of frozen liver tissues were stained with anti-F4/80 (A to D) or anti-neutrophil (RB6-8C5) (E to H) monoclonal antibodies as described in Materials and Methods. (A and E) mock control; (B and F) ConA only, day 5; (C and G) K. pneumoniae only, day 5; (D and H) K. pneumoniae and ConA treatment, day 5. Red coloring indicates positive staining. The thin arrows indicate the liver abscess. Magnification, ×400.
After intravenous administration of 2 mg of ConA/kg into B6 mice, the total ConA concentration in the serum declined from 40 ± 2.4 to 2 ± 0.6 μg per ml within 10 min, as measured by the ELISA detection method, and the serum ConA amount could not be detected thereafter. In contrast, ConA bound rapidly to liver tissues, such as endothelial cells and Kupffer cells (liver macrophages), but not to spleen, kidney, and brain tissues at 10 min after ConA injection, according to the results of immunohistochemistry with anti-ConA antibody, and the ConA signal on liver tissues continued to be present until 1 h post-ConA treatment (data not shown). To further examine the effect of ConA on macrophage killing, a mouse macrophage cell line, RAW264.7, was used for an in vitro assay. As shown in Fig. 6, pretreatment of ConA increased the bactericidal activity of macrophages against K. pneumoniae in a dose-dependent manner, indicating that ConA effectively enhanced macrophage killing activity.
FIG. 6.
In vitro effect of ConA on macrophage bactericidal activity. ConA-stimulated RAW264.7 cells were challenged with K. pneumoniae NK-5 as described in Materials and Methods. The numbers of viable bacteria inside the cells were determined at 3 h (A) and 5 h (B) postinfection. The experiment was repeated three times, and the results are shown for a representative experiment. *, P < 0.05 compared with medium control.
DISCUSSION
Primary K. pneumoniae liver abscess has emerged in Taiwan, where over 900 cases have been reported over the last two decades (5, 6, 12, 14, 20, 21, 36). Its complications are associated with a high mortality rate, especially for metastatic meningitis (11, 19). Currently, the isolated K. pneumoniae strains responsible for liver abscesses are uniformly susceptible to cephalosporins and aminoglycosides (36) but constitutively resistant to amoxicillin and ampicillin (4). However, inappropriate use of antibiotics will lead to the emergence of multiresistant K. pneumoniae, so another anti-K. pneumoniae agent needs to be considered. In this study, we demonstrate that ConA, a nonantibiotic agent, can inhibit K. pneumoniae infection by enhancing the bactericidal activity of phagocytes, which effectively reduces the bacterial burden in the liver.
The most likely infection route of K. pneumoniae-induced liver abscess is through the gastrointestinal tract, and these invasive bacteria approach the liver via the portal vein (5). Kupffer cells, residing within the lumen of the liver sinusoids, constitute the first macrophage population to contact bacteria derived from the gastrointestinal tract in the periportal area. Several reports demonstrate that adherence of microorganisms to Kupffer cell surface receptors such as lectins, macrophage scavenger receptors, and Toll-like receptors plays a significant role in activation of Kupffer cells and further bacterial clearance (16). In this study, we found that ConA decreased the burden of K. pneumoniae in both the liver and blood as early as 24 h after infection (Table 1); however, at this early stage, no significant neutrophil infiltration was found in the liver after ConA treatment (data not shown). This indicates that Kupffer cells may play an important role in the early bacterial clearance by ConA. Based on pharmacokinetic and immunohistochemistry assays (data not shown), we found that most of ConA bound rapidly to liver endothelial cells and Kupffer cells within 10 min after intravenous injection of ConA. Moreover, ConA was able to enhance macrophage killing against K. pneumoniae in a dose-dependent manner (Fig. 6). These results further emphasize the role of liver macrophages in decreasing the bacterial burden by ConA after K. pneumoniae infection.
Intravenous treatment of ConA increased the staining of F4/80 in the liver starting from day 1 postinfection (data not shown), and this staining level was sustained until day 5 (Fig. 5D), suggesting that activated macrophages may be recruited into the liver post-ConA treatment, thereby eliminating bacteria effectively in the liver. Moresco et al. reported that intraperitoneal pretreatment with a high dose of ConA in a Candida albicans infection model increased the number of peritoneal macrophages after infection with C. albicans (27). The phagocytic and candidacidal activities of the macrophages were enhanced by ConA through induction of gamma interferon via Th1 responses (13, 18, 31).
Kupffer cells are regarded as the first macrophage population to take up bacteria in the liver; however, they usually coordinate with infiltrating neutrophils to eliminate bacteria in systemic infection. Bactericidal neutrophils accumulate at the Kupffer cell surface through the activity of complementary adhesion molecules (i.e., CD11b/CD18 and CD54) and then internalize and kill the bacteria bound extracellularly to Kupffer cells. Thereafter, apoptotic neutrophils sequestered in the liver sinusoids are ingested and destroyed quickly by Kupffer cells, suppressing the release of toxic metabolic products and degradative enzymes and further reducing liver injury (16). In ConA-treated mice, additional activated macrophages, but not infiltrating neutrophils, were found at day 1 postinfection (data not shown); however, at day 3 (data not shown) and day 5 (Fig. 5D and H) postinfection, additional activated macrophages and infiltrating neutrophils concentrated together on the abscess foci. These results divide ConA-mediated bacterial clearance into two stages; the early stage is macrophage dependent, and the late stage is mediated by the interaction of activated macrophages and infiltrating neutrophils, leading to decreasing liver injury.
ConA pretreatment reduced the bacterial count in the liver (Table 1). In this study, ConA was found to directly bind and partially agglutinate K. pneumoniae cells in an in vitro culture (Fig. 4B and C). It is also possible that ConA might play the partial role of enhancing the activity of phagocytes through lectinophagocytosis, which is mediated by specific recognition between phagocytes and their targets through the interaction of carbohydrate-binding proteins (28). Several lectins, including ConA, Glycine max lectin, and Arachis hypogea lectin, have been demonstrated to enhance hepatic clearance and killing of C. albicans in isolated perfused mouse livers (32). From both our own observations and the results of the research of another group, we note that following intravenous injection of ConA into mice, the concentration of ConA in mouse livers reaches a peak at 30 min postinjection (15). Our study adopted multi-injection administration of ConA to treat K. pneumoniae-infected mice; given the resulting increased potential for lectinophagocytosis, we suggest that ConA may act as a bridge between the phagocyte and the bacterium itself, partially enhancing bacterial clearance by phagocytes.
Different doses of ConA are found to result in differential effects on liver damage. Intravenous treatment with a high dose of ConA (>15 mg/kg of body weight) induces T-cell-dependent hepatitis (35), while intravenous pretreatment with a low, nonhepatotoxic dose of ConA (3 mg/kg) induces a high level of interleukin-6 to prevent liver damage induced by the high dose of ConA (15 mg/kg) (37). In our study, pretreatment with a nonhepatotoxic dose of ConA inhibited K. pneumoniae-induced production of tumor necrosis factor alpha, interleukin-6, and gamma interferon at the early (day 1) but not the late (day 5) stage, as indicated by immunohistochemical staining (unpublished observation). Moreover, a nonhepatotoxic low dose of ConA has been found to preferentially activate natural killer cells to induce an antitumor effect against metastatic liver tumor (26). Furthermore, the CD4 T cell is regarded as the mediator in the enhancement by ConA of phagocytic and candidacidal activities of macrophages (13, 18, 31). These factors, including the roles of natural killer cells, CD4 T cells, and the differential expression of cytokines involved in ConA-mediated inhibition of K. pneumoniae infection, need further examination.
In this study, we established a K. pneumoniae-induced liver abscess model through an intragastric infection route. In addition to liver abscess formation, the mice developed meningitis beginning on day 3 postinfection (unpublished observation). Using this infection model, ConA has been shown to be a potential agent in decreasing the burden of K. pneumoniae in the liver. In our results, we found that 2 mg of ConA/kg of body weight, administered every 2 days until day 4 postinfection, resulted in 83% survival (Fig. 1B). However, intravenous treatment with ConA (2 mg/kg) daily for 3 days continuously postinfection worsened the mouse survival, a result which may have been due to the liver toxicity caused by the total dose of 8 mg of ConA/kg in each mouse (data not shown). Based on the results shown in Fig. 1, we consider that the first 2 days postinfection are the critical time for ConA treatment. If the bacterial load in the liver is limited efficiently in the first 2 days after ConA treatment, then the bacteremia is inhibited further and the survival of mice is increased. Although the higher dose (>5 mg/kg) of ConA represents a hepatotoxin (35, 37), the low dose of ConA seems to be an activator of innate immunity and could then enhance macrophage killing of K. pneumoniae (Fig. 6) and also activate natural killer cells (26). Thus, a combination of a nonhepatotoxic dose of ConA with other antimicrobial agents may be a more efficient method to inhibit K. pneumoniae infection.
Acknowledgments
This work was supported by grants NSC94-2320-B214-004 and NSC 95-2320-B-214-004 from the National Science Council, Taiwan.
We acknowledge the editorial assistance of Jonathan Courtenay.
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Arakawa, Y., M. Ohta, R. Wacharotayankun, M. Mori, N. Kido, H. Ito, T. Komatsu, T. Sugiyama, and N. Kato. 1991. Biosynthesis of Klebsiella K2 capsular polysaccharide in Escherichia coli HB101 requires the functions of rmpA and the chromosomal cps gene cluster of the virulent strain Klebsiella pneumoniae Chedid (O1:K2). Infect. Immun. 59:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa, Y., R. Wacharotayankun, T. Nagatsuka, H. Ito, N. Kato, and M. Ohta. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J. Bacteriol. 177:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill, M., B. Chang, and A. Murray. 2000. Bilateral endogenous bacterial endophthalmitis associated with pyogenic hepatic abscess. Br. J. Ophthalmol. 84:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, S. C., C. T. Fang, P. R. Hsueh, Y. C. Chen, and K. T. Luh. 2000. Klebsiella pneumoniae isolates causing liver abscess in Taiwan. Diagn. Microbiol. Infect. Dis. 37:279-284. [DOI] [PubMed] [Google Scholar]
- 5.Chiu, C. H., L. H. Su, T. L. Wu, and I. J. Hung. 2001. Liver abscess caused by Klebsiella pneumoniae in siblings. J. Clin. Microbiol. 39:2351-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou, H. C., C. Z. Lee, L. C. Ma, C. T. Fang, S. C. Chang, and J. T. Wang. 2004. Isolation of a chromosomal region of Klebsiella pneumoniae associated with allantoin metabolism and liver infection. Infect. Immun. 72:3783-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang, Y. P., C. T. Fang, S. Y. Lai, S. C. Chang, and J. T. Wang. 2006. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis. 193:645-654. [DOI] [PubMed] [Google Scholar]
- 8.Cortés, G., B. de Astorza, V. J. Benedi, and S. Alberti. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 10.DiSabato, G., J. M. Hall, and L. Thompson. 1987. T cell mitogens and polyclonal B cell activators. Methods Enzymol. 150:3-17. [DOI] [PubMed] [Google Scholar]
- 11.Fang, C. T., Y. C. Chen, S. C. Chang, W. Y. Shau, and K. T. Luh. 2000. Klebsiella pneumoniae meningitis: timing of antimicrobial therapy and prognosis. QJM 93:45-53. [DOI] [PubMed] [Google Scholar]
- 12.Fang, C. T., Y. P. Chuang, C. T. Shun, S. C. Chang, and J. T. Wang. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 199:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felipe, I., S. Bim, and C. C. Somensi. 1995. Increased clearance of Candida albicans from the peritoneal cavity of mice pre-treated with concanavalin-A or jacalin. Braz. J. Med. Biol. Res. 28:477-483. [PubMed] [Google Scholar]
- 14.Fung, C. P., F. Y. Chang, S. C. Lee, B. S. Hu, B. T. Kuo, C. Y. Liu, and L. K. Siu. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gantner, F., M. Leist, A. W. Lohse, P. G. Germann, and G. Tiegs. 1995. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21:190-198. [DOI] [PubMed] [Google Scholar]
- 16.Gregory, S. H., and E. J. Wing. 2002. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J. Leukoc. Biol. 72:239-248. [PubMed] [Google Scholar]
- 17.Huang, L. R., H. L. Wu, P. J. Chen, and D. S. Chen. 2006. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 103:17862-17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Káposzta, R., P. Tree, L. Maródi, and S. Gordon. 1998. Characteristics of invasive candidiasis in gamma interferon and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect. Immun. 66:1708-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko, W. C., D. L. Paterson, A. J. Sagnimeni, D. S. Hansen, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis. 8:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai, Y. C., S. L. Yang, H. L. Peng, and H. Y. Chang. 2000. Identification of genes present specifically in a virulent strain of Klebsiella pneumoniae. Infect. Immun. 68:7149-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau, Y. J., B. S. Hu, W. L. Wu, Y. H. Lin, H. Y. Chang, and Z. Y. Shi. 2000. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. J. Clin. Microbiol. 38:412-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lederman, E. R., and N. F. Crum. 2005. Pyrogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 100:322-331. [DOI] [PubMed] [Google Scholar]
- 23.Leist, M., and A. Wendel. 1996. A novel mechanism of murine hepatocyte death inducible by concanavalin A. J. Hepatol. 25:948-959. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 25.Merino, S., M. Altarriba, L. Izquierdo, M. M. Nogueras, M. Regue, and J. M. Tomas. 2000. Cloning and sequencing of the Klebsiella pneumoniae O5 wb gene cluster and its role in pathogenesis. Infect. Immun. 68:2435-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyagi, T., T. Takehara, T. Tatsumi, T. Suzuki, M. Jinushi, Y. Kanazawa, N. Hiramatsu, T. Kanto, S. Tsuji, M. Hori, and N. Hayashi. 2004. Concanavalin A injection activates intrahepatic innate immune cells to provoke an antitumor effect in murine liver. Hepatology 40:1190-1196. [DOI] [PubMed] [Google Scholar]
- 27.Moresco, T. R., L. C. J. Gaziri, Y. Yasumoto, and I. Felipe. 2002. Phagocytic and candidacidal activities of macrophages from suckling and adult mice pretreated with concanavalin-A. Med. Mycol. 40:393-397. [DOI] [PubMed] [Google Scholar]
- 28.Ofek, I., and N. Sharon. 1988. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect. Immun. 56:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmori, S., K. Shiraki, K. Ito, H. Inoue, T. Ito, T. Sakai, K. Takase, and T. Nakano. 2002. Septic endophthalmitis and meningitis associated with Klebsiella pneumoniae liver abscess. Hepatol. Res. 22:307-312. [DOI] [PubMed] [Google Scholar]
- 30.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romani, L., A. Mencacci, E. Cenci, R. Spaccapelo, P. Mosci, P. Puccetti, and F. Bistoni. 1993. CD4+ subset expression in murine candidiasis: Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J. Immunol. 150:925-931. [PubMed] [Google Scholar]
- 32.Sawyer, R. T., R. E. Garner, and J. A. Hudson. 1992. Effect of lectins on hepatic clearance and killing of Candida albicans by the isolated perfused mouse liver. Infect. Immun. 60:1041-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shultz, L. D., P. A. Schweitzer, S. W. Christianson, B. Gott, I. B. Schweitzer, B. Tennent, S. McKenna, L. Mobraaten, T. V. Rajan, D. L. Greiner, and E. H. Leiter. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154:180-191. [PubMed] [Google Scholar]
- 34.Takaya, A., M. Suzuki, H. Matsui, T. Tomoyasu, H. Sashinami, A. Nakane, and T. Yamamoto. 2003. Lon, a stress-induced ATP-dependent protease, is critically important for systemic Salmonella enterica serovar Typhimurium infection of mice. Infect. Immun. 71:690-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiegs, G., J. Hentschel, and A. Wendel. 1992. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 90:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, J. H., Y. C. Liu, S. S. Lee, M. Y. Yen, Y. S. Chen, J. H. Wang, S. R. Wann, and H. H. Lin. 1998. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 26:1434-1438. [DOI] [PubMed] [Google Scholar]
- 37.Xu, X., H. Wei, Z. Dong, Y. Chen, and Z. Tian. 2006. The differential effects of low dose and high dose concanavalin A on cytokine profile and their importance in liver injury. Inflamm. Res. 55:144-152. [DOI] [PubMed] [Google Scholar]