Abstract
The paradoxical growth (PG) of Candida sp. biofilms in the presence of high caspofungin (CAS) concentrations was previously unknown. We sought to characterize the PG at supra-MICs of CAS among clinical Candida sp. isolates grown as biofilms in 96-well polystyrene microtiter plates. The MICs of CAS were determined for 30 clinical Candida sp. isolates (4 Candida albicans, 6 C. tropicalis, 7 C. parapsilosis, 8 C. orthopsilosis, and 5 C. metapsilosis isolates) when they were grown as planktonic cells and biofilms and were defined as the lowest drug concentrations that resulted in a prominent decrease in growth and a 50% reduction in metabolic activity, respectively. PG was defined as a resurgence of growth (>50% of that in the drug-free growth control well) at drug concentrations above the MIC. With the exception of C. tropicalis, all isolates displayed PG more frequently when they were grown as biofilms than when they grown as planktonic cells. PG was undetectable among C. metapsilosis isolates in planktonic cell MIC tests but was present in 100% of the isolates in biofilm MIC tests. The drug concentration and the number of drug dilutions supporting PG were higher for biofilms than for planktonic cells. Microscopic changes in cell morphology were observed among both planktonic and biofilm cells with PG. Specifically, the accumulation of enlarged, globose cells was associated with PG, and we hypothesize that CAS-induced changes in the cell wall composition may be the explanation.
Paradoxical growth (PG) in Candida has been described as growth in the presence of echinocandin concentrations above the MIC in broth microdilution susceptibility tests performed according to Clinical Laboratory Standards Institute (CLSI; formerly NCCLS) guidelines (25, 26, 27). The evidence to date suggests that PG is most commonly observed with caspofungin (CAS) and less so with other echinocandin antifungals (2). The echinocandin antifungals exert their antifungal effect by inhibiting cell wall biogenesis through inhibition of β-1,3-glucan synthesis (13). Candida sp. isolates are generally susceptible to CAS in vitro, with the majority of isolates inhibited at a MIC of ≤1 μg/ml (20). Despite the growth-inhibitory activity of CAS at low concentrations, PG at supra-MICs, followed by growth inhibition in the presence of the highest drug concentrations, has been observed for some Candida sp. isolates. Cells growing in the presence of high CAS concentrations are not resistant on retesting but show the PG effect of the parent, i.e., inhibition at low CAS concentrations and a resurgence of growth at supra-MICs (25). PG is distinct from the trailing growth phenotype described for some Candida albicans and Candida tropicalis isolates in broth microdilution azole antifungal susceptibility tests; trailing growth refers to the reduced but persistent growth of cells in the presence of drug concentrations above the MIC, whereas PG is a resurgence of growth in the presence of supra-MICs of a drug to nearly the levels achieved in the absence of drug. To date, studies describing PG in Candida isolates have focused on planktonic cell cultures (2, 4, 25, 26, 27), and the in vivo significance of PG remains uncertain (4).
We report here for the first time the ability of Candida sp. biofilms to display PG when they are exposed to high concentrations of CAS in vitro. Biofilms are commonly defined as a structured community of microbial cells attached to a surface and encased in a self-produced organic extracellular matrix (6). A clinically significant characteristic of microbial biofilms is their markedly enhanced resistance to antimicrobial agents (7, 12). Many manifestations of candidiasis are associated with the formation of biofilms on host tissue (e.g., oral thrush) and indwelling medical devices (e.g., central venous catheter-associated candidemia) (1). Because of the extreme difficulty in treating catheter-associated candidemia with systemic antifungal therapy, current national guidelines recommend the removal of catheters infected with Candida in order to eradicate a potential nidus of bloodstream infection (15). Catheter removal, however, is not always feasible for extremely ill patients, and therefore, treatment strategies which enable catheter salvage, such as antifungal lock therapy, are desirable (19). Antifungal lock therapy consists of filling the catheter lumen with an antibiotic at high concentrations and allowing it to dwell in the device for a period of time in order to sterilize the device. With this method, a high local concentration of an appropriate antibiotic can be applied in the catheter lumen while avoiding systemic toxicity and the need to monitor serum drug levels. CAS and lipid amphotericin B are the only antifungal drugs with demonstrable activities against Candida biofilms in vitro (5, 11) and in vivo (21, 22) and represent promising candidates for antifungal lock therapy for catheter-associated candidemia. Therefore, as antifungal lock therapy with high concentrations of CAS continues to evolve as a realistic treatment strategy for catheter-associated candidemia, it is pertinent to understand the phenomenon of biofilm PG in the presence of high CAS concentrations.
Clinical isolates from five Candida spp. were studied, including two newly recognized species, Candida metapsilosis and Candida orthopsilosis. These new species are phenotypically identical but genotypically distinct from Candida parapsilosis and are capable of causing bloodstream infections (14, 28). The in vitro susceptibilities of Candida sp. biofilms to CAS and the frequency of PG in biofilms compared with the frequency of PG in planktonic cells was studied. Microscopic examination of the cells with PG was performed and provided clues to the underlying mechanism(s) of PG. These studies are necessary to understand the contribution of PG to the recalcitrant nature of Candida biofilms in vivo and any potential risk that antifungal lock therapy with high CAS concentrations, e.g., concentrations associated with PG in vitro, poses to patients who may be candidates for this treatment strategy.
MATERIALS AND METHODS
Isolates.
Thirty clinical Candida sp. isolates, including four C. albicans, six C. tropicalis, seven Candida parapsilosis, eight Candida orthopsilosis, and five Candida metapsilosis isolates, were used. The strains were from patients from different Brazilian cities and were isolated from different clinical specimens, including blood, oropharyngeal, vaginal, urine, skin, and nail specimens. They were selected from among strains previously identified and maintained at −70°C in the yeast stock collection of the Special Mycology Laboratory, Universidade Federal de Sao Paulo. For species identification, the strains were plated onto CHROMagar Candida (CHROMagar Microbiology, Paris, France) to check the purity and viability of all original yeast cultures. All isolates were identified on the basis of their micromorphology on cornmeal-Tween 80 agar and biochemical tests performed with the commercial ID32C system (bioMerieux Marcy l'Etoile, France). Since phenotypic methods do not differentiate C. orthopsilosis and C. metapsilosis from C. parapsilosis, the isolates identified as C. parapsilosis were submitted to molecular testing by randomly amplified polymorphic DNA (RAPD) analysis (with primer 1253 [5′-GTT TCC GCC C-3′]) and internal transcribed spacer (ITS) region sequencing for species identification. Reference strains C. parapsilosis ATCC 90018, C. orthopsilosis ATCC 96141, and C. metapsilosis ATCC 96143 were used as controls to interpret the RAPD and ITS sequencing results.
Planktonic cell MICs.
CAS pure powder was received as a gift from Merck & Co., Inc. (Rahway, NJ) and was solubilized in sterile water. The susceptibility of planktonic cells was determined by the M27-A2 method, as described previously (18). MIC endpoints were determined after 24 h on the basis of a prominent decrease in growth compared to that of the drug-free growth control. CLSI-recommended quality control strains (C. krusei ATCC 6258 and C. parapsilosis ATCC 22019) were included on each day of testing, and the MICs were within the recommended range. MIC endpoints were also determined by using the colorimetric indicator 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) with menadione as an electron-coupling agent to parallel the biofilm MIC endpoint determination method. The XTT-menadione solution was prepared fresh on each day of testing by adding 1.5 ml of XTT (1 mg/ml in sterile saline; Sigma Chemicals, St. Louis, MO) to 300 μl menadione solution (0.4 mM in acetone; Sigma Chemicals). After 48 h, the MICs were determined visually; 12 μl of XTT-menadione solution was added to each well of the microtiter plate, including both the drug-free growth control and the sterility control wells; and the contents of each well were mixed by pipetting them up and down with a multichannel pipette. The plates were incubated for 1 h at 35°C in the dark. Following incubation, the microtiter plate was covered with tape and centrifuged at 4,000 rpm for 7 min to pellet the yeast cells. One hundred microliters of supernatant was transferred to a fresh flat-bottom microtiter plate, and the optical density was read with a spectrophotometer at 490 nm. The MIC obtained by the use of XTT was defined as the lowest drug concentration that caused a 50% decrease in metabolic activity relative to that in the drug-free growth control. PG in planktonic MIC tests was defined as metabolic activity (20% or more of that in the drug-free control well) in the presence of drug concentrations above the MIC.
Biofilm formation.
The biofilm formation and the growth conditions used in this study were adapted from those described elsewhere (9). Yeast strains were cultured on Sabouraud dextrose agar at 30°C for 24 h and then subcultured into RPMI 1640 broth medium with l-glutamine, without bicarbonate (Sigma Chemicals), buffered to pH 7.0 with 3-(N-morpholino)propanesulfonic acid (165 M; Sigma Chemicals) and were grown overnight (18 h) with shaking at 120 rpm at 35°C. The cell cultures were harvested, washed twice with phosphate-buffered saline (PBS), and adjusted to a concentration of approximately 1 × 107 cells/ml in RPMI 1640 medium. Biofilms were produced on sterilized, polystyrene, flat-bottomed, 96-well microtiter plates (Costar; Corning Incorporated, Corning, NY). For the attachment phase, 100 μl of the adjusted cell suspension was transferred to each well. No cells were added to the final column to be used as the negative control. The plate was incubated at 37°C for 1.5 h with shaking at 75 rpm so that the cells could attach to the surfaces of the wells. Following the attachment phase, unattached cells were removed, the wells were washed with 150 μl of PBS, and 100 μl of fresh RPMI 1640 medium was added. The plate was incubated at 37°C for 66 to 72 h with shaking at 75 rpm to allow biofilm growth.
Biofilm quantitation by XTT reduction assay.
Biofilm metabolic activity was measured by the XTT reduction assay. After biofilm formation, the wells were washed two times with 200 μl PBS. To each well, 200 μl of PBS and 12 μl of XTT-menadione solution (prepared as described above) were added. The culture plate was incubated in the dark for 1 h at 35°C, and afterward, 100 μl of the reaction solution was transferred to a new flat-bottomed microtiter plate and the absorbance was measured with a spectrophotometer plate reader at 490 nm. The experiments were performed with five replicates for each strain. The absorbance values of the negative control wells (containing no cells) were subtracted from the values of the test wells.
Biofilm MICs.
Biofilms were formed in RPMI 1640 medium as described above. After 24 h of biofilm growth, the biofilms were washed two times with PBS and the plates were inverted onto absorbent paper to remove residual amounts of PBS prior to challenge with CAS. CAS was diluted in RPMI 1640 medium to yield 10 doubling serial dilutions ranging from 2 to 1,024 μg/ml. Two hundred microliters of each of the drug concentrations was added to the respective wells of the microtiter plate. The biofilms were incubated with CAS for 48 h at 35°C with shaking at 75 rpm. Wells containing biofilm but no drug served as positive controls for each strain tested. After 48 h of drug exposure, the medium was removed and the biofilms were washed two times with PBS. Biofilm activity was then measured by the XTT reduction assay exactly as described above. Biofilm MICs were calculated on the basis of a 50% reduction in metabolic activity compared with the activity of the drug-free control. Isolates were tested in duplicate.
Microscopy.
Cells from the planktonic cell MIC tests were removed from the drug-containing and the drug-free control wells and visualized with a BX40 light microscope (Olympus, Central Valley, PA) at ×400 magnification. Evaluation of biofilm morphology with and without CAS exposure was carried out with an inverted light microscope (Leitz-Diavert). The flat-bottomed microtiter plates were placed on the microscope platform and examined at ×200 magnification. Photographs were taken with a digital camera (Nikon Corp., Melville, NY).
RESULTS
CAS susceptibility. (i) Planktonic cell MICs.
Broth microdilution susceptibility testing of planktonic cells by use of the CLSI guidelines revealed that the CAS MICs were ≤2 μg/ml for all isolates tested (Table 1). In addition to visual MIC readings, the MICs of planktonic cells were also determined by the XTT reduction assay to ensure that the differences in MICs between planktonic and biofilm cells were not influenced by the use of different endpoint determination methods. In all cases, the MICs determined by the XTT reduction assay were the same as or 1 dilution higher than those determined visually (data not shown). The median CAS MICs were the same for the C. parapsilosis, C. orthopsilosis, and C. metapsilosis isolates (median MIC = 2 μg/ml) and were consistently 1 to 2 dilutions higher than the MICs for the C. albicans (median MIC = 0.5 μg/ml) and the C. tropicalis (median MIC = 0.75 μg/ml) isolates (Table 1).
TABLE 1.
Caspofungin MICs and PG characteristics for 30 clinical Candida sp. isolates in planktonic and biofilm growth forms
| Isolate no. | Species | Planktonic cells
|
Biofilms
|
||
|---|---|---|---|---|---|
| MIC (μg/ml)a | Drug concn range (μg/ml) producing PGb | MIC (μg/ml)c | Drug concn range (μg/ml) producing PG | ||
| BR-A1 | C. albicans | 0.5 | NAd | 4 | 8-128 |
| BR-A2 | C. albicans | 0.5 | 16 | 2 | 8-128 |
| BR-A4 | C. albicans | 0.5 | NA | 2 | 16-128 |
| BR-A7 | C. albicans | 0.5 | NA | 2 | 16-128 |
| BR-A8 | C. tropicalis | 1 | 8 | 2 | 4-32 |
| BR-A9 | C. tropicalis | 1 | 8 | 2 | 4-32 |
| BR-A14 | C. tropicalis | 0.5 | 8 | 256 | NA |
| BR-A18 | C. tropicalis | 0.5 | NA | 128 | NA |
| BR-A20 | C. tropicalis | 0.5 | NA | 2 | 8-64 |
| BR-A24 | C. tropicalis | 1 | NA | 2 | 8-64 |
| BR-A25 | C. parapsilosis | 2 | 32 | 2 | 16-256 |
| BR-A26 | C. parapsilosis | 2 | 16-32 | 256 | NA |
| BR-A27 | C. parapsilosis | 2 | NA | 256 | NA |
| BR-A28 | C. parapsilosis | 2 | NA | 2 | 16-128 |
| BR-A29 | C. parapsilosis | 2 | 32-64 | 4 | 16-128 |
| BR-A30 | C. parapsilosis | 2 | NA | 256 | NA |
| BR-A31 | C. parapsilosis | 2 | NA | 4 | 32-64 |
| BR-A32 | C. orthopsilosis | 2 | NA | 2 | 32-64 |
| BR-A33 | C. orthopsilosis | 1 | NA | 2 | 32-64 |
| BR-A34 | C. orthopsilosis | 1 | 4 | 2 | 32-128 |
| BR-A35 | C. orthopsilosis | 1 | NA | 2 | 16-128 |
| BR-A36 | C. orthopsilosis | 1 | NA | 2 | 16-128 |
| BR-A37 | C. orthopsilosis | 2 | 32-64 | 2 | 32-64 |
| BR-A38 | C. orthopsilosis | 2 | 16-64 | 512 | NA |
| BR-A39 | C. orthopsilosis | 2 | NA | 2 | 32-128 |
| BR-A40 | C. metapsilosis | 1 | NA | 2 | 16-64, 512 |
| BR-A41 | C. metapsilosis | 2 | NA | 2 | 16 |
| BR-A42 | C. metapsilosis | 1 | NA | 2 | 32-64 |
| BR-A43 | C. metapsilosis | 2 | NA | 2 | 16 |
| BR-A44 | C. metapsilosis | 2 | NA | 2 | 16 |
| 22019 | C. parapsilosis | 2 | 8 | 2 | 16-128 |
MIC endpoint based on visual determination of the lowest drug concentration that produced a prominent decrease in growth relative to that for the drug-free growth control well.
Drug concentration(s) above the MIC with visible growth ≥20% for planktonic cells and ≥50% for biofilm cells relative to the growth for the drug-free growth control.
MIC endpoint based on the lowest drug concentration producing a 50% reduction in metabolic activity relative to that for the drug-free growth control, as measured by the XTT reduction assay.
NA, no PG observed.
(ii) Biofilm MICs.
CAS MICs for 24-h biofilms (with 48 h of drug exposure) ranged from 2 to 512 μg/ml (Table 1). CAS appeared to have excellent activity against the biofilms from all five species tested; the median biofilm MIC was 2 μg/ml for each species, which was consistent with the planktonic cell MICs for the same isolates. Biofilm MICs >2 μg/ml were observed for 6 of 30 (20%) isolates tested, including 2 C. tropicalis isolates, 3 C. parapsilosis isolates, and 1 C. orthopsilosis isolate (Table 1). Low run-to-run variability was observed among the XTT assay results for the replicate samples tested; standard deviations were ≤20% of the mean values (data not shown).
PG.
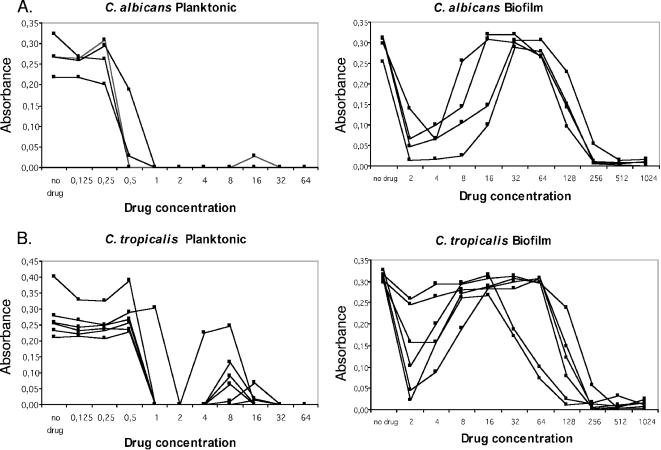
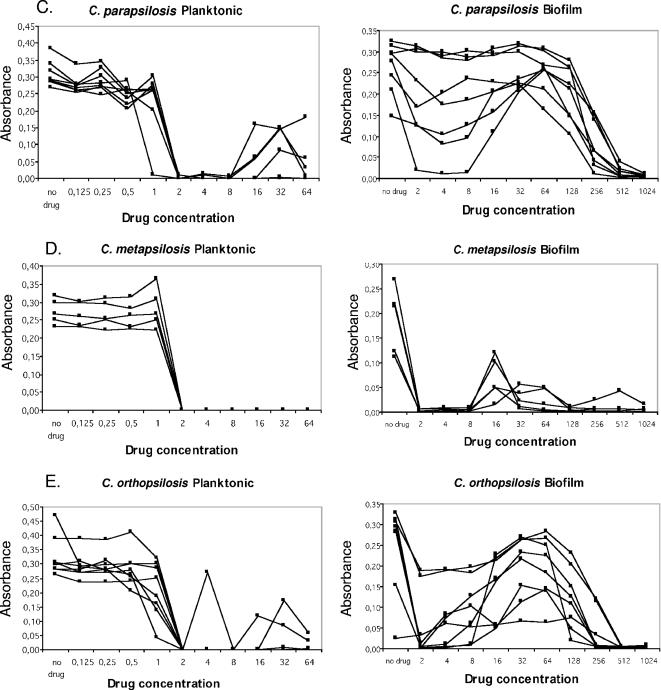
PG, or the reemergence of growth in the presence of drug concentrations above the MIC, was observed for planktonic and/or biofilm cells of each species tested. PG was specific for CAS and was not observed in amphotericin B or fluconazole MIC tests for planktonic or biofilm cells (data not shown). The PG effect of Candida sp. biofilms in micafungin and anidulafungin MIC tests was unable to be determined due to an inability to obtain drug powders. PG was more frequently observed in biofilm MIC tests (24 of 30 isolates; 80%) than in planktonic cell MIC tests (12 of 30 isolates; 40%). The frequency of PG among planktonic cells differed by species, as follows: C. tropicalis (five of six isolates; 83%) > C. parapsilosis (three of seven isolates; 43%) > C. orthopsilosis (three of eight isolates; 37.5%) >, C. albicans (one of four isolates; 25%). C. metapsilosis isolates did not display PG in planktonic cell CAS MIC tests. PG was more prevalent in biofilm MIC tests; and species-specific differences in frequency were also noted: C. albicans (four of four isolates; 100%) = C. metapsilosis (five of five isolates; 100%) > C. orthopsilosis (seven of eight; 87%) > C. tropicalis (four of six isolates; 67%) > C. parapsilosis (four of seven isolates; 57%). The lower frequency of PG among C. tropicalis and C. parapsilosis isolates in biofilm MIC tests is due to the fact that these biofilms were less susceptible to CAS in vitro. There was, however, evidence of a “mini-PG” effect for the two C. tropicalis and the three C. parapsilosis isolates with high CAS biofilm MICs. The “mini-PG” was defined as an initial decrease in metabolic activity at a lower CAS concentration (although it did not meet the criteria for the MIC), followed by an increase in metabolic activity at a higher CAS concentration. For these isolates, the “mini-PG” effect occurred over the drug concentration range of 8 to 128 μg/ml (Fig. 1B and C). Interestingly, PG in planktonic cell MIC tests did not predict PG in biofilm MIC tests. Of the 30 isolates studied, 3 isolates (1 isolate each of C. tropicalis, C. parapsilosis, and C. orthopsilosis) displayed PG in planktonic cell tests but not in biofilm tests and 15 isolates (3 C. albicans, 2 C. parapsilosis, 5 C. orthopsilosis, and 5 C. metapsilosis isolates) displayed PG in biofilm tests but not in planktonic cell tests (Table 1).
FIG. 1.
Metabolic activity of Candida sp. isolates as measured by XTT reduction assay of planktonic and biofilm cells following CAS challenge for 48 h. (A) C. albicans; (B) C. tropicalis; (C) C. parapsilosis; (D) C. metapsilosis; (E) C. orthopsilosis.
In addition to differences in the frequency of PG between planktonic cells and biofilms, biofilms displayed a distinct pattern of PG. Specifically, biofilms displayed PG over a wider range of CAS concentrations (3 to 4 twofold drug dilutions) than planktonic cells (1 to 2 twofold drug dilutions) and at higher drug concentrations (16 to 128 μg/ml) than planktonic cells (4 to 32 μg/ml) (Table 1 and Fig. 1). Finally, the growth in the PG wells relative to that in the drug-free control well for a given isolate was higher when the isolate was grown as a biofilm than when it was grown as planktonic cells, even though under both growth conditions the magnitude of growth inhibition at the MIC was similar (Fig. 1). That is, PG in biofilm cells increased to levels near that of the drug-free growth control, whereas for planktonic cells, the metabolic activity in the PG wells increased to 20 to 50% of that in the drug-free control (Fig. 1).
Microscopy.
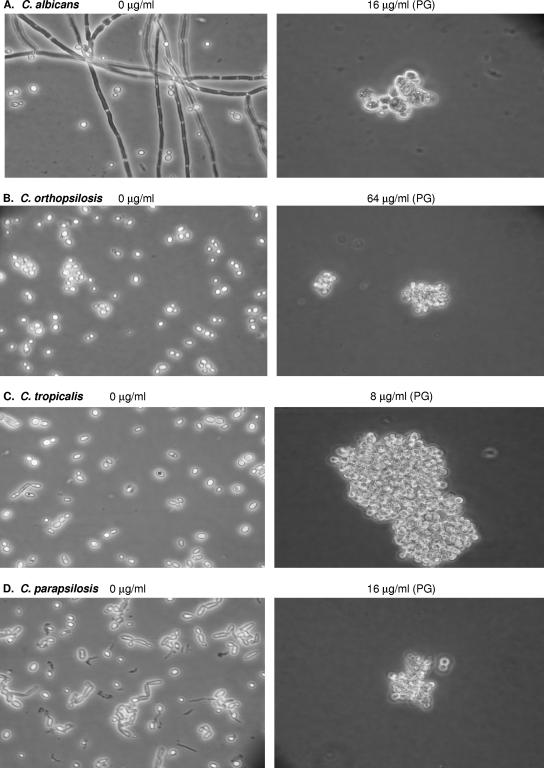
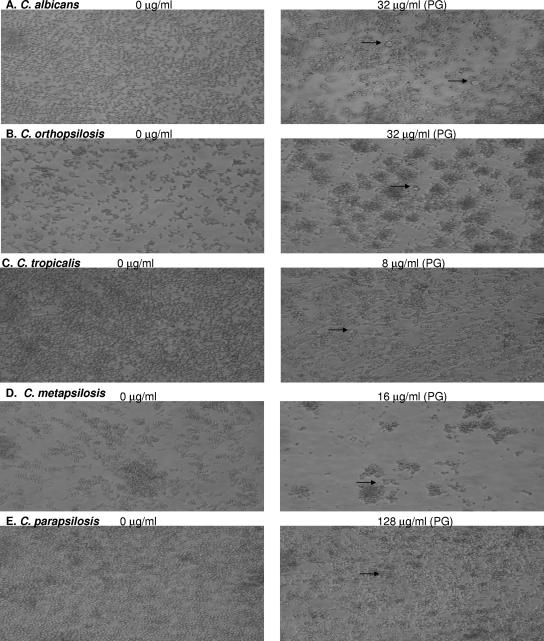
To investigate whether PG was associated with changes in cellular morphology, we studied planktonic cells (Fig. 2) and biofilms (Fig. 3) in the presence and absence of CAS using light microscopy. CAS exposure was associated with the inhibition of hyphae and pseudohyphae and the rounding and clumping of yeast cells in both the planktonic and the biofilm growth modes (Fig. 2 and 3). The appearance of enlarged and globose “giant cells” was correlated with PG for both biofilm and planktonic cells. The viability of the giant cells was not investigated directly in this study, but these cells appeared to be viable on the basis of microscopic evidence of budding in some cases. Furthermore, the number of giant cells in a given well with PG corresponded to the level of metabolic activity in that well with PG; that is, the well with PG and the highest metabolic activity corresponded to the well with the greatest number of giant cells. As the metabolic activity dropped off in the presence of higher CAS concentrations, the number of giant cells also declined.
FIG. 2.
Light microscopy of representative isolates of each of the Candida spp. tested which displayed PG in planktonic MIC tests. Cells from the drug-free control well and cells displaying PG in the presence of high CAS concentrations after 48 h incubation are shown. (A) C. albicans; (B) C. orthopsilosis; (C) C. tropicalis; (D) C. parapsilosis.
FIG. 3.
Light microscopy of representative biofilms of each of the Candida sp. tested which displayed PG in biofilm MIC tests. Biofilms in the drug-free control well and biofilms displaying PG in the presence of high CAS concentrations after 48 h incubation are shown. (A) C. albicans; (B) C. orthopsilosis; (C) C. tropicalis; (D) C. metapsilosis; (E) C. parapsilosis. Arrows point to the “globose” cells frequently observed in wells displaying PG.
DISCUSSION
We have described biofilm formation, CAS susceptibility, and prevalence of PG among isolates from five medically relevant Candida species, including C. albicans, C. tropicalis, C. parapsilosis, C. orthopsilosis, and C. metapsilosis. Previously, Song and colleagues reported that C. orthopsilosis and C. metapsilosis, also known as group II and group III C. parapsilosis, respectively, did not form biofilms, suggesting that the biofilm-forming phenotype ascribed to C. parapsilosis was limited to group I isolates (23). One explanation for the discrepancy in results is the difference in the biofilm growth and quantitation methods between the two studies. In our study, we used the chemically defined RPMI 1640 medium (pH 7.0) containing minimal (0.2%) glucose, whereas Song et al. used a complex medium, Sabouraud dextrose broth, supplemented with 8% glucose (23). Studies of bacterial biofilms have shown that nutrient density and medium osmolarity are important environmental factors which regulate biofilm attachment, growth, and maturation (24); and for most microbial species, moderate nutrient limitation favors biofilm growth relative to the growth achieved under nutrient-rich and nutrient-poor conditions (24). Another important difference that may have contributed to our result was our use of the XTT reduction assay to measure biofilm metabolic activity rather than spectrophotometric measurement of the decreased light transmission caused by biofilm formation on microtiter plate wells. XTT reduction has been widely used to measure biofilm activity and allows the detection of small differences in metabolic activity between strains when the colorimetric reaction is measured during the linear phase of the reaction (8, 11, 16).
MIC results indicated that CAS has excellent in vitro activity against all isolates tested in the planktonic growth mode and against many isolates grown as biofilms. Interestingly, C. parapsilosis biofilms were more resistant to CAS than C. orthopsilosis and C. metapsilosis biofilms, even though they had the same MICs in planktonic cell tests. Since CAS acts by disrupting β-1,3-glucan synthesis, the intrinsic ability of a given strain to compensate for the reduction of this polymer may promote reduced caspofungin susceptibility. β-1,3-Glucan has also been shown to be an important constituent of the Candida biofilm extracellular matrix, where it contributes to substrate and/or cell-cell adherence and overall biofilm stability (3, 10). The CAS-induced reduction of β-1,3-glucan in the biofilm matrix may increase the fragility of the biofilm and, likewise, the susceptibility to antifungal killing. Furthermore, Candida strains that have adapted to life with reduced amounts of β-1,3-glucan in the cell wall and biofilm matrix may be less susceptible to the antifungal effects of CAS. Quantitative comparisons of the β-1,3-glucan content in the cell wall and the extracellular matrix among various Candida species represent an interesting area for further exploration.
PG following exposure to supra-MICs of CAS has previously been described for Candida sp. isolates grown as planktonic cells but not as biofilms (25, 27). We observed PG in 40% of the isolates when they were tested with CAS as planktonic cells and twice that (80%) when the same isolates were tested as biofilms, suggesting that PG is not an uncommon phenomenon.
In this study, we used simple light microscopy to visualize the morphological changes in planktonic and biofilm cells associated with CAS exposure and PG. Cell clumping and the appearance of enlarged, globose cells were hallmarks of PG. One explanation is the fungal cell wall changes due to the reduced β-1,3- and β-1,6-glucan contents and increased chitin content. A study by Stevens et al. (26) reported that cell wall preparations from a C. albicans isolate capable of PG and grown in the presence of supra-MICs of CAS had 81% and 73% reductions in β-1,3- and β-1,6-glucans contents, respectively, and an 898% increase in chitin content compared to the contents in cells grown in the absence of CAS. Furthermore, Nakai et al. reported that the PG of Candida spp. in broth microdilution tests with micafungin was dependent on the osmotic conditions of the growth medium; PG was produced only under hyperosmotic conditions (17). Understanding of the links between PG and (i) the presence of large, rounded cells in biofilms, (ii) the shift in the contents of the key components of the fungal cell wall, and (iii) the dependency on the osmolarity of the medium will be key to understanding the basis of echinocandin-associated PG among Candida sp. isolates and the clinical significance of PG.
In conclusion, Candida sp. biofilms can display PG in the presence of high concentrations of CAS and do so more readily then planktonic cells of the same strains. The cellular morphological changes associated with PG can be observed microscopically and are likely due to alterations in the fungal cell wall. The clinical significance of PG remains unclear, yet the proposed use of high concentrations of echinocandin antifungals as catheter lock therapy for the treatment and prevention of catheter-associated candidemia may be thwarted by the stimulation of Candida biofilm growth at CAS concentrations above the MIC. On the basis of these findings, further studies to determine the occurrence of PG in vivo with animal models of catheter-associated infection and antifungal lock therapy are warranted.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
Footnotes
Published ahead of print on 25 June 2007.
REFERENCES
- 1.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 2.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echninocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons, K. V., M. Espiritu, R. Parmer, and D. A. Stevens. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocuaud, C., M.-H. Rodier, G. Daniault, and C. Imbert. 2005. Anti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilms. J. Antimicrob. Chemother. 56:507-512. [DOI] [PubMed] [Google Scholar]
- 6.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawser, S. P., and L. J. Douglas. 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 62:915-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin, Y., H. K. Yip, Y. H. Samaranayake, J. Y. Yau, and L. P. Samaranayake. 2003. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 41:2961-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn, D. M., and M. A. Ghannoum. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186-197. [PubMed] [Google Scholar]
- 13.Letscher-Bru, V., and R. Herbrecht. 2003. Caspofungin: the first representative of a new antifungal class. J. Antimicrob. Chemother. 51:513-521. [DOI] [PubMed] [Google Scholar]
- 14.Lin, D., L. C. Wu, M. G. Rinaldi, and P. F. Lehmann. 1995. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 33:1815-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee, P. K., and J. Chandra. 2004. Candida biofilm resistance. Drug Resist. Updat. 7:301-309. [DOI] [PubMed] [Google Scholar]
- 17.Nakai, T., T. Suzuki, I. M., K. Hatano, S. Matsumoto, F. Ikeda, and K. Maki. 2006. Paradoxical growth of Candida tropicalis in response to micafungin, abstr. M-1761, p. 435. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 18.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Nucci, M., and E. J. Anaissie. 2002. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin. Infect. Dis. 34:591-599. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schinabeck, M. K., L. A. Long, M. A. Hossain, J. Chandra, P. K. Mukherjee, S. Mohamed, and M. A. Ghannoum. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuford, J. A., M. S. Rouse, K. E. Piper, J. M. Steckelberg, and R. Patel. 2006. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catherter infection model. J. Infect. Dis. 194:710-713. [DOI] [PubMed] [Google Scholar]
- 23.Song, J. W., J. H. Shin, D. H. Shin, S. Jung, D. Cho, S. J. Kee, M. G. Shin, S. P. Suh, and D. W. Ryang. 2005. Differences in biofilm production by three genotypes of Candida parapsilosis from clinical sources. Med. Mycol. 43:657-661. [DOI] [PubMed] [Google Scholar]
- 24.Stanley, N. R., and B. A. Lazazzera. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. 52:917-924. [DOI] [PubMed] [Google Scholar]
- 25.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin: evidence for B-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens, D. A., T. C. White, D. Perlin, and C. P. Selitrennikoff. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173-178. [DOI] [PubMed] [Google Scholar]
- 28.Tavanti, A., A. D. Davidson, N. A. Gow, M. C. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]