Voriconazole (VRC; Vfend [Pfizer, United Kingdom]) is a broad-spectrum triazole with activity against fungi including non-albicans Candida, Aspergillus, Fusarium, and Scedosporium species. Favorable therapeutic responses in patients with plasma VRC levels above 2.05 μg/ml were found previously (9). The main adverse effects of VRC are hepatotoxicity and blurred vision (1, 2, 4, 6, 11). Prolonged exposure to high plasma VRC concentrations (trough blood VRC levels of >5.5 μg/ml for more than 7 days) was found to be associated with an enhanced risk of serious neurological adverse events (5).
A 45-year-old male (body weight, 100 kg) with fatty-liver cirrhosis (Child-Pugh class C; model of end-stage liver disease score, 20) who was listed for liver transplantation and showed signs of portal hypertension (esophageal varices and ascites) and cholestasis (plasma bilirubin level, 20.26 mg/dl, or 346 μmol/liter) received 2 mg of VRC/kg of body weight orally twice a day because of suspected pulmonary aspergillosis. At day 30 of clinical treatment with VRC, he was transferred to the intensive care unit because of unconsciousness (Glasgow Coma Scale score, 5 of 15) and hyperventilation. Plasma VRC concentrations were determined by high-performance liquid chromatography and UV detection (3). Pharmacokinetic parameters were calculated using a noncompartmental model.
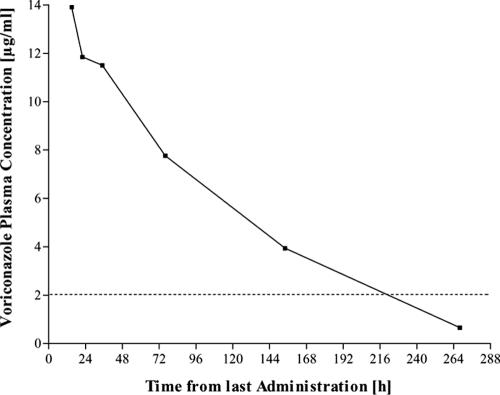
Upon admission to the intensive care unit (15 h after the last intake of VRC), the plasma VRC level amounted to 13.9 μg/ml (Fig. 1). Therefore, VRC therapy was discontinued. The patient's condition, particularly the central nervous system symptoms, gradually improved as VRC levels slowly declined, and he could be transferred to the ward after 2 days (VRC concentration, 10.0 μg/ml). A half-life of 53.1 h (half-life in healthy volunteers, 4.7 h [7]), an apparent volume of distribution at steady state of 0.13 liters/kg (volume of distribution in healthy volunteers, 2.04 liters/kg [7]), and a VRC clearance rate as low as 1.4 ml/h/kg (clearance rate in healthy volunteers, 253.9 ml/h/kg [7]) were calculated. Even after 11 days, VRC was detectable in the plasma (0.66 μg/ml). Since diagnostic reevaluation did not reveal any signs of fungal infection, there was no need to continue antimycotic treatment at that time. The patient underwent a successful liver transplantation 1 month later.
FIG. 1.
Voriconazole elimination after discontinuation of voriconazole. The dashed line indicates the concentration limit for favorable responses (9).
The elimination of VRC appears to be markedly prolonged in patients with decompensated liver cirrhosis, and this delay leads to potentially toxic levels of VRC in the plasma. After cytochrome P450 (2C9, 2C19, and 3A4)-dependent hepatic metabolism, about 80% of VRC is eliminated via the kidneys. Biliary elimination accounts for 20%. VRC displays nonlinear pharmacokinetics, with a prolonged half-life at higher concentrations (8). In patients with moderate liver cirrhosis (Child-Pugh class B), the VRC clearance rate is approximately half that in patients with normal hepatic function after oral intake. A reduction of the maintenance dose by 50% is recommended for patients with mild to moderate hepatic insufficiency (10). For patients with severely impaired liver function, a dose reduction of more than 50% appears to be required, and therapeutic drug monitoring will greatly improve therapeutic safety. Pharmacokinetic studies in patients with severe hepatic impairment should be performed in order to establish reliable dose recommendations for this group of patients, who are at high risk of developing invasive fungal infections.
Acknowledgments
We thank Pfizer Austria for financial support.
Footnotes
Published ahead of print on 2 July 2007.
REFERENCES
- 1.Den Hollander, J. G., C. van Arkel, B. J. Rijnders, P. J. Lugtenburg, S. de Marie, and M. D. Levin. 2006. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J. Antimicrob. Chemother. 57:1248-1250. [DOI] [PubMed] [Google Scholar]
- 2.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 3.Khoschsorur, G., F. Fruehwirth, and S. Zelzer. 2005. Isocratic high-performance liquid chromatographic method with ultraviolet detection for simultaneous determination of levels of voriconazole and itraconazole and its hydroxy metabolite in human serum. Antimicrob. Agents Chemother. 49:3569-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. Church, J. M. Brown, H. T. Schlamm, I. T. Oborska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 5.Pascual, A. A., S. Bolay, and O. Marchetti. 2006. Monitoring of voriconazole (VRC) blood levels for prevention of serious neurological adverse events (SNAE). Int. J. Infect. Dis. 10:S67. [Google Scholar]
- 6.Potoski, B. A., and J. Brown. 2002. The safety of voriconazole. Clin. Infect. Dis. 35:1273-1275. [DOI] [PubMed] [Google Scholar]
- 7.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 9.Smith, J., N. Safdar, V. Knasinski, W. Simmons, S. M. Bhavnani, P. G. Ambrose, and D. Andes. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan, K., N. Brayshaw, K. Tomaszweski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 11.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petersen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, E. Anaissie, and J. Lee. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]