Abstract
Antifungal agents may differ in their fungicidal activities against Aspergillus spp. In order to compare the fungicidal activities of voriconazole and amphotericin B against 40 isolates of Aspergillus fumigatus, A. flavus, and A. terreus, we developed a new microbroth colorimetric method for assessing fungicidal activities and determining minimal fungicidal concentrations (MFCs). This methodology follows the antifungal susceptibility testing reference method M-38A for MIC determination. After drug removal and addition of fresh medium, growth of viable conidia adhering to the bottoms of the microtitration wells was assessed by a colorimetric assay of metabolic activity after 24 h of incubation. The new method was faster (six times), reproducible (92 to 97%), and in agreement with culture-based MFCs (91 to 100%). Differential fungicidal activities of voriconazole and amphotericin B were found among the three Aspergillus species, with A. fumigatus and A. flavus having the lowest (1 and 2 mg/liter, respectively) and A. terreus the highest (>16 mg/liter) median amphotericin B MFCs; A. flavus had a lower median voriconazole MFC (4 mg/liter) than the other species (>8 mg/liter; P < 0.05). Amphotericin B was fungicidal (MFC/MIC ≤ 4) against all A. fumigatus and A. flavus isolates but no A. terreus isolates, whereas voriconazole was fungicidal against 82% of A. flavus isolates and fungistatic (MFC/MIC > 4) against 94% of A. fumigatus and 84% of A. terreus isolates. The new methodology revealed a concentration-dependent sigmoid pattern of fungicidal effects, indicating that fungicidal activity is not an all-or-nothing phenomenon and that some degree of fungicidal action can be found even for agents considered fungistatic based on the MFC/MIC ratio.
Invasive aspergillosis in immunocompromised patients is often associated with poor therapeutic outcome (20). Successful treatment of invasive aspergillosis in immunocompromised patients may require fungicidal therapy, which will ultimately achieve eradication of the organism and clinical cure (21). Amphotericin B and voriconazole are commonly used to treat patients with invasive aspergillosis with the hope not only of preventing dissemination from the site of infection, but also of eradicating Aspergillus hyphae. However, antifungal agents may differ in their fungicidal activities against Aspergillus spp. (6).
The lethal activities of antifungal agents are usually measured in vitro by determination of minimal fungicidal concentrations (MFCs) (7, 21). Conventional MFC determination is based on subculturing the supernatants from the wells where no growth was observed in broth microdilution plates in antifungal susceptibility testing (7, 9). However, the culture-based methodologies are time-consuming and prone to carryover errors. These methods provide all-or-nothing results, depending on the volume subcultured and the cutoff of the percentage of killing used to determine the MFCs. These disadvantages make MFC determination methodologies challenging (21). Thus, laboratory investigations of the in vitro activities of antifungal agents are usually limited to MICs. Other methods for measuring fungicidal activities, such as time-kill curves, flow cytometry, vitality/mortality probe assays, and measurements of intracellular ATP (21), are cumbersome or require special equipment.
In the present study, we developed an easy and reproducible in vitro microbroth colorimetric method for determination of MFCs of amphotericin B and voriconazole against a panel of Aspergillus isolates. This methodology is based on our initial hypothesis, subsequently confirmed by preliminary experiments, that viable Aspergillus conidia are strongly attached to the bottoms of microtitration wells during standard growth-inhibitory susceptibility testing and can potentially grow after the removal of antifungal agents, enabling the determination of MFCs. Thus, following the widely used M38-A reference method of the Clinical and Laboratory Standards Institute (CLSI) (18), the contents of the wells were removed, leaving the viable conidia attached to the bottoms of the wells. The conidia were then washed in order to remove the drug and incubated with fresh medium. Finally, growth was detected with an XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] colorimetric assay (14). Using this methodology, the fungicidal activities of amphotericin B and voriconazole were analyzed and concentration-effect relationships were described in detail for each species, demonstrating differential fungicidal activities against Aspergillus species.
MATERIALS AND METHODS
Isolates.
A collection of 40 clinical isolates of Aspergillus spp. was used: 16 Aspergillus fumigatus, 11 A. flavus, and 13 A. terreus. Isolates kept frozen on potato dextrose agar at −70°C were cultured on potato dextrose agar slants and incubated at 35°C for 5 to 7 days. Conidia were then obtained by scraping the agar slants with a sterile pipette and were suspended in sterile normal saline containing 0.025% Tween 20. The conidial suspensions were adjusted with a hemacytometer to 2.5 × 104 conidia/ml in order to obtain two times the final inoculum, which ranged from 0.5 × 104 to 5 × 104 CFU/ml in the assay medium (23). The reference strains Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, A. fumigatus ATCC MYA-3626, A. flavus ATCC MYA-3631, and A. terreus ATCC MYA-3633 were used as quality controls (8). MFC data from the quality control Aspergillus strains were included in the overall analysis.
Medium.
RPMI 1640 medium with l-glutamine and without bicarbonate, buffered to pH 7.0 with 0.165 M 3-N-morpholinopropanesulfonic acid (Cambrex Bio Science, Inc., Walkersville, MD), was provided as unconcentrated solution and was used as the assay medium throughout.
XTT and menadione.
XTT (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline at a concentration of 1 mg/ml. Menadione (Sigma-Aldrich, St. Louis, MO) was initially dissolved in absolute ethanol at a concentration of 10 mg/ml. A working solution of 0.5 mg/ml of XTT with 125 μM of menadione was prepared in saline.
Antifungal agents.
Voriconazole (Pfizer Pharmaceuticals, New York, NY) was obtained in a 10-mg/ml vial of clinical formulation. An amphotericin B deoxycholate (Apothecon Ben Venue Laboratories, Inc., Bedford, OH) stock concentration of 5 mg/ml was prepared in sterile water. Twofold serial dilutions of amphotericin B and voriconazole were prepared in 100 μl of the medium in 96-well flat-bottom microtitration plates (Costar 3596; Corning Inc., Corning, NY). We used flat-bottom microplates because they are more amenable to microscopic determination of MFCs and spectrophotometric readings for the XTT assay than the round-bottom wells recommended by CLSI for visual determination of MICs. The flat-bottom microplates used in the present study have been previously used for susceptibility testing of Aspergillus species and have a minimal effect on the results, which are comparable with those of previous studies in which round-bottom plates were used (1, 14). The final concentrations of amphotericin B and voriconazole after the addition of inoculum ranged from 0.015 to 16 mg/liter and from 0.008 to 8 mg/liter, respectively.
Susceptibility testing.
The susceptibilities of all 40 Aspergillus isolates to amphotericin B and voriconazole were tested according to the CLSI M38-A method (18). After the addition of 100 μl of assay medium containing two times the final inoculum size to 100 μl of assay medium containing two times the final drug concentrations, two duplicate microtitration plates per experiment were incubated at 37°C for 48 h, and the MIC was determined as the lowest drug concentration showing no visible growth. All experiments were performed twice.
Fungicidal-activity testing.
After MIC determination, the MFCs of amphotericin B and voriconazole were determined from two duplicate microtitration plates by a conventional culture-based method (7, 9) and the new microbroth colorimetric method as follows.
(i) Culture-based CFU method.
From the first duplicate plate, 20 μl and 100 μl from all visually clear wells and the first well with the highest drug concentration showing growth (0.5× MIC) were removed with a micropipette after pipetting up and down five times and were subcultured onto Sabouraud dextrose agar (SDA) plates. The SDA plates were incubated at 37°C for 24 to 48 h, and the CFU were counted for each drug concentration. The MFCs of voriconazole and amphotericin B were defined as the lowest drug concentration yielding no growth using 20 μl (CFU20 MFC) and 100 μl (CFU100 MFC).
Because conidia may adhere to the well bottoms despite vigorous pipetting, we also quantified the residual amount of viable conidia remaining on the well by this methodology. Thus, the remaining contents of the microtitration wells were carefully aspirated, the wells were washed twice with 200 μl of prewarmed (37°C) normal saline, and 200 μl of fresh medium was added. After an additional incubation at 37°C for 24 h, the wells were inspected under an inverted microscope and the mycelia (up to a maximum of 20) on the bottoms of the wells were counted for each drug concentration. The presence of fungal growth inside the wells was used to correct the CFU MFCs (corrected CFU MFC) when growth was observed at drug concentrations higher than the CFU20 or CFU100 MFCs.
(ii) Microbroth colorimetric XTT (MBX) method.
The contents of all clear wells of the second duplicate plate and the first well with the highest drug concentration showing growth (0.5× MIC) were carefully aspirated, and 200 μl of prewarmed (37°C) normal saline was added. After gentle agitation, the wells were again washed with saline, and 200 μl of fresh medium was added to each well. The microtitration plates were incubated at 37°C for 24 h, and the wells were then inspected under an inverted microscope and the mycelia (up to a maximum of 20) inside the wells were counted. Subsequently, 50 μl of the XTT-menadione solution was added to each well, yielding a final concentration of 0.1 mg/ml of XTT and 25 μM of menadione, and the plates were incubated for another 2 h (Fig. 1). After the plates were shaken for 1 to 2 min (Wallac Plate Shake 1296-004; Wallac OY, Turku, Finland) until the formazan derivatives were completely dissolved, the color absorbance was measured spectrophotometrically at 450 and 630 nm (Elx808; Bio-Tek Instruments, Winooski, VT). The background absorbance was measured for each microtitration plate from five conidia-free wells processed in the same way as the inoculated wells. The XTT absorbance values after the subtraction of the background absorbance quantified the amount of fungal growth at each drug concentration and could be used as a measure of fungicidal activity at that particular concentration of amphotericin B or voriconazole. The lower the XTT absorbance value (reflecting less viable biomass) at a particular drug concentration, the stronger was the fungicidal action at that concentration.
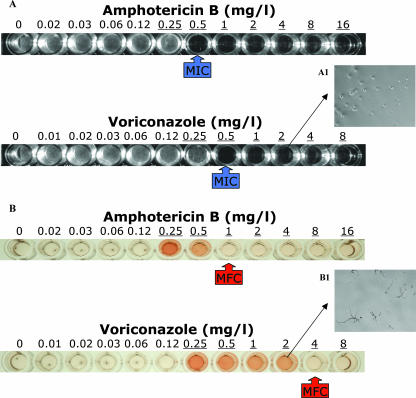
FIG. 1.
Photomicrographs of microtitration wells showing the new microbroth colorimetric method for testing the fungicidal activities of amphotericin B and voriconazole against an A. fumigatus isolate. After 48 h of susceptibility testing with the CLSI M38-A reference broth microdilution method (18) and MIC determination (A), all visually clear wells and the well corresponding to 0.5× MIC determination (underlined drug concentrations) were washed, and fresh medium was added and incubated for 24 h, followed by additional 2-h incubation with 0.1 mg/liter XTT plus 25 μM MEN (B). The MFC was determined as the lowest drug concentration corresponding to an XTT absorbance of <0.01. The MICs of voriconazole and amphotericin B were 0.5 mg/liter. The MFCs of amphotericin B and voriconazole were 1 and 4 mg/liter, respectively. Inset photograph A1 (magnification, ×200) shows the adhered conidia after aspiration of the supernatant and washing with normal saline, and B1 (magnification, ×50) shows that viable conidia produce hyphae after 24 h of incubation.
The MBX MFC was determined as the lowest drug concentration corresponding to an absorbance higher than a cutoff value. In order to find the appropriate absorbance cutoff value for determining MBX MFCs, receiver-operator characteristic (ROC) curves were constructed (see below) for each drug based on the presence of fungal growth (≥1 mycelium) assessed microscopically. The cutoff absorbance value that gave high sensitivity and specificity for both drugs (>90%) was chosen as the cutoff for determining the MBX MFCs. In order to account for delayed growth, MBX MFCs were also determined after incubation for 48 h.
In order to ensure that conidia were not aspirated during the washes, 100 μl of the supernatant (the drug-containing assay medium and washing solutions) from the wells with the highest and lowest drug concentrations processed for the MBX method was subcultured on SDA plates, and the presence of conidia on the bottoms of the wells was confirmed under an inverted microscope before and after the plates were washed. Cross-contamination from well to well was checked every five wells by dipping the aspirating needle into a well containing only the assay medium. Furthermore, the aspirating needle was subcultured on SDA plates after aspiration of all wells of a microtitration plate. Preliminary experiments also showed that centrifuging the microtitration plates before aspirations did not increase the yield of viable conidia. These quality control tests confirmed our initial hypothesis that viable conidia strongly adhered to the bottoms of the wells and that aspiration avoided cross-contamination between wells, as well as preventing the detachment of conidia.
Analysis. (i) Sensitivity and specificity.
The sensitivities and specificities were calculated for the MBX, CFU20, and CFU100 methods and for each drug using the ROC curves. The ROC curves were constructed using Prism 4 for Macintosh (GraphPad Software Inc., San Diego, CA) by plotting the percent sensitivity against 100% minus the percent specificity for different cutoff values (i.e., the absorbance value for the MBX method and the number of CFU for the CFU methods). The sensitivities and specificities of the CFU and MBX methods were calculated based on the presence or absence of growing mycelia on the bottoms of the wells of the CFU and MBX plates, respectively, assessed microscopically under an inverted microscope. The area under the ROC curve quantifies the overall ability of each method to discriminate between wells with growth (≥1 mycelium) and those without growth. A useless test would have an area under the ROC curve of 0.5, whereas, a perfect test would have an area of 1. The 95% confidence interval (CI) and a P value testing the null hypothesis that the area under the curve really equaled 0.50 were also reported (15).
(ii) Agreement, reproducibility, and consistency.
The agreement between the MBX and the CFU methods was calculated for each drug and species as the percentage of MBX MFCs that fell within 1 dilution of the CFU20 and CFU100 MFCs. The differences between log2-transformed MBX and CFU MFCs were analyzed statistically using a paired t test. The reproducibility of MBX MFCs was calculated as the percentage of MBX MFCs of the first replicate experiment that fell within 1 dilution of the second replicate experiment. Furthermore, the consistency of MBX results over time was assessed by calculating the percentage of MBX MFCs after 24 h that remained the same after 48 h of incubation.
(iii) Regression analysis.
In order to test whether the XTT absorbance data obtained with the MBX method followed a concentration-dependent pattern, the sigmoid Emax model was fitted with nonweighted, nonlinear regression analysis with Prism 4.0 software (GraphPad Inc., San Diego, CA). The Emax model is described by the following equation: E = (Emax − B)/[1 + 10(logEC50 − logC)m] + B (1), where E is the measured fungal growth (the XTT absorbance or the number of mycelia; the dependent variable) at the drug concentration C (the independent variable), Emax is the maximum measured fungal growth, B is the minimum measured fungal growth observed at a vast drug concentration, EC50 is the drug concentration producing 50% of the Emax, and m is the slope (the Hill coefficient). The goodness of fit was assessed for each isolate/replicate based on R2, run tests, and visual inspection of the concentration-effect curves. This analysis was also performed for all isolates of each species together after global fitting, sharing the Emax among the isolates of each species and keeping B constant at 0 (population regression analysis) (16).
Finally, the relationship between XTT conversion and the number of mycelia was explored by plotting the absorbance value of each well against the number of mycelia counted microscopically on the bottom of the well, and the relationship was analyzed by linear regression analysis (Prism 4.0 software). The slopes and their statistically significant deviations from zero were reported for each species and drug.
Interspecies differences of fungicidal activities.
The interspecies differences of fungicidal activities were assessed based on MBX and CFU MFCs with an analysis of variance, followed by Tukey's multiple-comparison test after log2 transformation and passing Bartlett's test for equal variances (15). Furthermore, the MBX MFC/MIC ratios were calculated for each isolate, and the number of isolates with MFC/MIC ratios of ≤4 was reported for each species. By extrapolation from the conventional definition used for bacterial testing, a compound was considered fungicidal when the MFC/MIC ratio was ≤4 and fungistatic when the MFC/MIC ratio was >4 (21).
Finally, differential fungicidal activities were also assessed based on the results of the population regression analysis of the XTT absorbance data obtained by the MBX method. The differences among the three species of the slopes m and the EC50s of the Emax model obtained for amphotericin B and voriconazole were analyzed using analysis of variance.
RESULTS
The MICs for the quality control yeast and mold strains were within the reference ranges. Table 1 shows the MICs and the MFCs for the quality control mold strains. The MICs of amphotericin B and voriconazole for all isolates of each Aspergillus species are summarized in Table 2. The median MICs of amphotericin B were 1, 1, and 2 mg/liter for A. fumigatus, A. flavus, and A. terreus, respectively. The median MICs of voriconazole were 0.5, 0.5, and 1 mg/liter for A. fumigatus, A. flavus, and A. terreus, respectively. These MICs for Aspergillus isolates are similar to previously published MICs where pure powders of voriconazole and amphotericin B were used (9). Thus, the excipients of the clinical formulations used in the present study did not significantly alter the activities of antifungal drugs.
TABLE 1.
Quality control Aspergillus strains and reference MIC ranges for amphotericin B and voriconazole
| Quality control strain | Agent | MIC range
|
MFC rangea
|
||
|---|---|---|---|---|---|
| Referenceb | Present study | CFU MFCs | MBX MFCs | ||
| A. fumigatus (ATCC MYA-3626) | Amphotericin B | 0.5-4 | 1-1 | 1-2 | 1-2 |
| Voriconazole | 0.25-1 | 1-1 | 4->8 | 8->8 | |
| A. flavus (ATCC MYA-3631) | Amphotericin B | 1-8 | 1-2 | 1-4 | 2-4 |
| Voriconazole | 0.5-2 | 0.5-1 | 0.5-2 | 4->8 | |
| A. terreus (ATCC MYA-3633) | Amphotericin B | 2-8 | 2-2 | 2->16 | 16->16 |
| Voriconazole | 0.25-1 | 0.5-0.5 | 2-4 | 8->8 | |
MFCs determined by the culture-based (CFU20 and CFU100 MFCs) and the new MBX (MBX MFCs) methods are presented.
From reference 8.
TABLE 2.
MICs and MFCs of amphotericin B and voriconazole for Aspergillus spp. determined by M38A (MIC) and CFU20 or CFU100 and MBX (MFC) methods
| Species (no. of isolates) | Amphotericin B (median, range)
|
Voriconazole (median, range)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) | MFC (mg/liter) by:
|
MBX MFC/MIC ratiob | MIC (mg/liter) | MFC (mg/liter) by:
|
MBX MFC/MIC ratio | |||||
| CFU20a | CFU100a | MBX | CFU20 | CFU100 | MBX | |||||
| A. fumigatus (16) | 1 (0.5-1) | 1 (0.5-2) | 2 (0.5-2) | 1 (0.5-2) | 2 (1-4) [100] | 0.5 (0.5-1) | 2 (0.25->8) | >8 (8->8) | >8 (2->8) | >16 (2->16) [6] |
| A. flavus (11) | 1 (1-2) | 1 (0.5-2) | 2 (0.5->16) | 2 (1-8) | 2 (1-4) [100] | 0.5 (0.5-1) | 0.5 (0.5-4) | 1 (0.5->8) | 4 (2->8) | 4 (1->16) [82] |
| A. terreus (13) | 2 (1-4) | >16 (2->16) | >16 | >16 (8->16) | 16 (4-32) [0] | 1 (0.25-16) | 8 (0.25->8) | >8 (4->8) | >8 (4->8) | 8 (1->16) [15] |
CFU MFCs may underestimate the true MFC, because when fresh medium was added to the wells, 20 μl and 100 μl of which was subcultured in order to determine the CFU MFCs, growth was detected after 24 h of incubation at concentrations higher than the CFU MFCs, indicating that pipetting from the wells is not adequate to detach viable conidia from the bottoms of the wells.
Numbers in brackets represent percentages of isolates with median (between the replicates) MBX MFC/MIC ratios of ≤4, indicating fungicidal activity.
Cultured-based CFU20 and CFU100 methods.
The areas under the ROC curve for the CFU20 and CFU100 methods were 0.79 (95% CI, 0.72 to 0.87; P < 0.001) and 0.87 (95% CI, 0.79 to 0.92; P < 0.001) for amphotericin B and 0.80 (95% CI, 0.74 to 0.87; P < 0.001) and 0.92 (95% CI, 0.88 to 0.97; P < 0.001) for voriconazole, respectively. Growth was detected microscopically on the bottoms of wells at drug concentrations equal to or higher than the CFU MFCs despite the pipetting manipulations of CFU plates, indicating strong adherence of conidia to the bottoms of the wells. Similar areas under the ROC curves were obtained for CFU methods (0.77 to 0.92) when they were compared with the growth detected microscopically on the bottoms of the nonmanipulated wells of the MBX plates. The sensitivities and specificities of the CFU methods ranged from 69% to 85% for both drugs. The median CFU100 MFCs of amphotericin B were 2 mg/liter for A. fumigatus and A. flavus and >16 mg/liter for A. terreus (Table 2). The median CFU100 MFCs of voriconazole were >8 mg/liter for A. fumigatus and A. terreus and 1 mg/liter for A. flavus. Overall, the CFU100 MFCs of amphotericin B and voriconazole were 1 to 2 dilutions higher than the CFU20 MFCs (P < 0.001), emphasizing the dependence of CFU MFCs on the volume subcultured.
MBX method.
For the MBX method, the area under the ROC curve was 0.99 (95% CI, 0.97 to 1.00; P < 0.001) for amphotericin B and 0.97 (95% CI, 0.93 to 1.00; P < 0.001) for voriconazole, indicating an excellent ability to detect fungal growth inside the microtitration wells (viable mycelia, wherever they were present inside the wells, could be detected by color change of the well content) (Fig. 1). Low absorbance values at high drug concentrations, corresponding to a small amount of fungal growth, can be detected by the MBX method, increasing the sensitivity of the assay. The cutoff absorbance of 0.01 for MBX MFC determination resulted in high levels of sensitivity and specificity for amphotericin B and voriconazole in detecting fungal growth inside the wells (Fig. 2). Using this cutoff, the sensitivities of the MBX method were 97% and 99% and the specificities were 95% and 90% for amphotericin B and voriconazole, respectively. The MBX method was very reproducible (93 to 97%) and consistent after incubation for 24 h and 48 h (90% of MBX MFCs were exactly the same, with the remaining 8% and 2% within 1 and 2 dilutions, respectively). The MBX method was faster than the CFU methods, since the time required for processing 25 wells was six times shorter (15 min with the MBX method versus 1.5 h with the CFU methods).
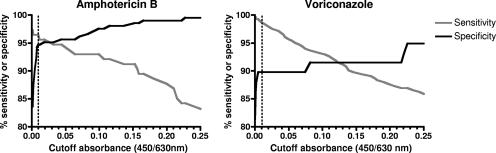
FIG. 2.
Sensitivity and specificity curves of the MBX method for amphotericin B and voriconazole. The sensitivity and specificity of the MBX method were calculated based on different absorbance values used as cutoffs to determine the MBX MFCs in relation to the presence of mycelia growing inside the wells assessed microscopically. The cutoff of 0.01 was chosen because it gave very high sensitivity (97% and 99% for amphotericin B and voriconazole, respectively) and high levels of specificity (95% and 90% for amphotericin B and voriconazole, respectively). Higher cutoffs significantly decreased the sensitivity (<95%) without increasing the specificity considerably, whereas lower cutoffs resulted in poor specificity (90%).
The data obtained by the MBX method showed a concentration-dependent sigmoid pattern for amphotericin B and voriconazole, as shown in Fig. 3. The Emax model described well the concentration-effect relationship between XTT absorbance and drug concentrations of voriconazole and amphotericin B with an R2 of >0.84 for 90% of the isolates/replicates (Fig. 3A and B). The relationship between the number of mycelia and the drug concentration also followed a sigmoid pattern described well by the Emax model with an R2 of >0.92 for 90% of isolates/replicates (Fig. 3C and D). A linear relationship between XTT absorbance and the number of mycelia was found (slopes, 0.014 to 0.044; P < 0.001) (Fig. 4). However, this relationship was weak (r2 = 0.09 to 0.58 for amphotericin B and 0.08 to 0.63 for voriconazole), indicating that factors other than the number of mycelia also contribute to XTT absorbance (e.g., the sizes of mycelia). Variation in the numbers and sizes of mycelia may be due to interconidial and interstrain differences in drug susceptibilities, postantifungal effects (recovery of conidia after drug removal), and growth rates.
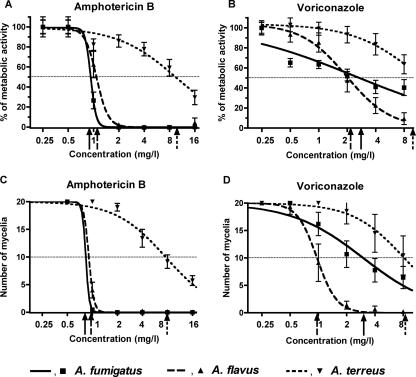
FIG. 3.
Concentration-dependent fungicidal activities of voriconazole and amphotericin B by the MBX method, indicated by the sigmoid pattern of percent XTT absorbance (A and B) and the number of mycelia (C and D) with increasing drug concentrations. The symbols represent the means, and the error bars indicate the standard errors for all isolates of each species. The curves were obtained with nonlinear regression analysis with the Emax model. The top of the Emax model was 100% (the XTT conversion at 0.5× MIC) for panels A and B and 20 mycelia (the maximum number of mycelia countable under the inverted microscope) for panels C and D. The bottom of the Emax model was kept constant at 0% (A and B) and zero mycelia (C and D). The horizontal dotted lines represent 50% of the Emax (i.e., 50% metabolic activity for panels A and B and 10 mycelia for panels C and D). The arrows on the x axes represent the EC50s of the concentration-effect curves of A. fumitgatus (solid arrow), A. flavus (dashed arrow), and A. terreus (dotted arrow). Note the strong fungicidal activities of amphotericin B for A. fumigatus (EC50 = 0.95 mg/liter) and A. flavus (EC50 = 1.10 mg/liter) isolates but not for A. terreus (EC50 = 9.55 mg/liter) isolates and the weaker fungicidal activities of voriconazole for A. fumigatus (EC50 = 2.34 mg/liter), A. flavus (EC50 = 1.96 mg/liter), and particularly A. terreus (EC50 = 11.69 mg/liter) isolates.
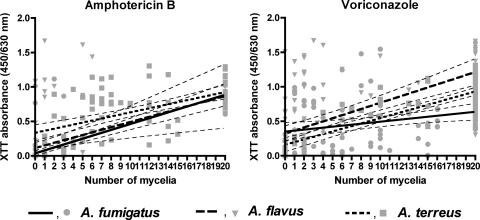
FIG. 4.
Relationship between metabolic activities (expressed as A450/A630 values) and the number of mycelia for the MBX method. The XTT absorbance data were linearly but weakly related to the number of mycelia for each species and drug (slopes, 0.014 to 0.044; P < 0.001; r2 = 0.08 to 0.63). The thin dotted lines around the thick solid, dotted, and dashed regression lines represent 95% confidence bands of linear regression for each species. Factors other than the number of mycelia also contribute to XTT absorbance (e.g., the sizes of mycelia). Variation in the numbers and sizes of mycelia may be due to interconidial and interstrain differences in drug susceptibilities, postantifungal effects (recovery of conidia after drug removal), and growth rates.
Agreement between MBX and CFU methods.
The agreement between the MBX and CFU100 methods was very good for all Aspergillus species for amphotericin B (82 to 100%). For voriconazole, the agreement between the MBX and the CFU100 was very good (85 to 100%) for A. fumigatus and A. terreus, but not for A. flavus (<45%) (Table 3). Lower levels of agreement between the MBX and the CFU20 methods were found, particularly for voriconazole. However, the CFU methods underestimated the actual MFCs because growth was detected at concentrations higher than the CFU MFCs up to MBX MFCs when the remaining contents of the wells that were processed for the CFU methods were aspirated and fresh medium was added. The low levels of agreement between the MBX and CFU methods for voriconazole and A. flavus may be due to strong adherence of viable A. flavus conidia to the bottoms of the wells, which were not easily transferred and subcultured. When the CFU MFCs were corrected based on these findings, the agreement between the new colorimetric microbroth method and the CFU method was 91% for voriconazole and A. flavus. Such corrections increased the levels of agreement between the MBX and CFU methods up to 100% for the other species and both drugs (Table 3).
TABLE 3.
Average agreement between culture-based (CFU20 and CFU100) and microbroth colorimetric (MBX) methods for each antifungal agent and Aspergillus species
| Species (no. of isolates) | Avg (%) agreement (two replicates) for MBX vs.:
|
|||||
|---|---|---|---|---|---|---|
| Amphotericin B
|
Voriconazole
|
|||||
| CFU20 | CFU100 | Corrected CFUa | CFU20 | CFU100 | Corrected CFUa | |
| A. fumigatus (16) | 91 (88, 94) | 91 (88, 94) | 100 (100, 100) | 22 (18, 25) | 97 (94, 100) | 100 (100, 100) |
| A. flavus (11) | 60 (55, 67) | 85 (82, 89) | 100 (100, 100) | 5 (0, 9) | 27 (9, 45) | 91 (88, 94) |
| A. terreus (13) | 86 (83, 90) | 91 (83, 100) | 100 (100, 100) | 68 (61, 75) | 92 (85, 100) | 100 (100, 100) |
The corrected CFU MFCs were obtained after CFU MFCs were corrected based on the presence of growth in the microtitration wells, 20 μl and 100 μl of which was subcultured for the CFU methods after fresh medium was added and incubated for 24 h (see footnote a to Table 2).
Interspecies differences in fungicidal activities.
There were significant differences in amphotericin B MBX MFCs among all three Aspergillus species (Tukey's test, P < 0.05), with A. fumigatus having the lowest MBX MFCs (median, 1 mg/liter; range, 0.5 to 2 mg/liter), A. flavus intermediate MBX MFCs (median, 2 mg/liter; range, 1 to 8 mg/liter), and A. terreus the highest MBX MFCs (median, >16 mg/liter; range, 8 to >16 mg/liter) (Table 2). Notably, CFU methods resulted in statistically significant differences in amphotericin B MFCs only between A. terreus and the other two Aspergillus species and not between A. fumigatus and A. flavus. The MBX MFC/MIC ratio was ≤4 for all A. fumigatus and A. flavus isolates and no A. terreus isolate.
Interspecies significant differences (Tukey's test, P < 0.05) in voriconazole MBX MFCs were found between A. flavus (median, 4 mg/liter; range, 2 to >8 mg/liter) and the other two Aspergillus species, which had higher MFCs (median, >8 mg/liter; range, 2 to >8 mg/liter). Comparable results were obtained with the CFU100 method, but not with the CFU20 method, which did not show significantly different fungicidal activities of voriconazole against A. flavus and A. terreus (Table 2). The MBX MFC/MIC ratio was ≤4 for most A. flavus isolates (82%) and a few A. fumigatus (6%) and A. terreus (15%) isolates.
Assessing fungicidal activities based on the MFC/MIC ratio is a crude and arbitrary approach, based on which agents are classified either as fungicidal, killing the entire inoculum at concentrations near the MIC, or fungistatic, not exhibiting any fungicidal activity near the MIC. However, regression analysis of the XTT absorbance data for the MBX method showed that fungicidal activity is concentration dependent, following a sigmoidal pattern with variable slopes, and that even “fungistatic” agents based on an MFC/MIC ratio of >4 exhibit some degree of fungicidal activity at concentrations lower than the MFC. The fungicidal effects over a range of concentrations of amphotericin B and voriconazole against all Aspergillus species can be summarized by the EC50 and slope parameters of the Emax model. The EC50 of the Emax model is a measure of the potency of fungicidal activity (the higher the EC50, the lower the fungicidal potency), while the negative slope, m, of the Emax model is a measure of the steepness of the concentration-fungicidal-effect curve (the higher the slope, the shallower the curve, i.e., there are small changes in fungicidal effects with increasing drug concentrations).
A more detailed comparison of the fungicidal activities of voriconazole and amphotericin B was obtained with population regression analysis of XTT absorbance data, where differential fungicidal activities of amphotericin B and voriconazole against Aspergillus species were also found (Fig. 3). The population EC50s (95% CI) of drug concentration-XTT absorbance relationships of amphotericin B differed significantly among the species (P < 0.001) and were 0.95 (0.34 to 2.46) mg/liter for A. fumigatus, 1.10 (0.87 to 1.38) mg/liter for A. flavus, and 9.55 (7.2 to 12.67) mg/liter for A. terreus. Differential fungicidal activities were also found for voriconazole, with population EC50s (95% CI) of 2.34 (1.42 to 4.06) mg/liter for A. fumigatus, 1.96 (1.48 to 2.58) mg/liter for A. flavus, and 11.69 (6.21 to 22.02) mg/liter for A. terreus. Interspecies significant differences were also found for the slopes of the drug concentration-XTT absorbance curves (P < 0.001). The slopes ranged from −0.38 to −2.63 for all species and drugs, indicating shallow curves, except for A. fumigatus and A. flavus with amphotericin B, for which the slopes were −6.25 and −7.48, indicating steep curves. A significant correlation was found between slopes of the drug concentration-XTT absorbance curves and MBX MFC/MIC ratios (r = 0.62; P < 0.001), with slopes of >−3 being strongly associated with MBX MFC/MIC ratios of >4 (χ2 = 26.67; P <0.001). Ninety-six percent of all isolates/replicates with shallow (slope, >−3) drug concentration-XTT absorbance curves had MFC/MIC ratios of >4. Sixty percent of all isolates/replicates with steep (slope, <−3) drug concentration-XTT absorbance curves had MFC/MIC ratios of ≤4.
Because the cutoff absorbance (0.01 XTT absorbance) for MBX MFC determination corresponded to 1% of maximal metabolic activity (∼1.0 XTT absorbance), the MBX MFCs were closer to the EC1s (the drug concentrations associated with 1% of maximal metabolic activity) of the drug concentration-XTT absorbance curves than the EC50s, which were lower than the EC1s (Fig. 3A and B) and therefore lower than the MBX MFCs (Table 2). The levels of agreement between MBX MFCs and EC1s were 92%, higher than the levels of agreement between MBX MFCs and EC25s (the drug concentrations corresponding to 25% of maximal metabolic activity) or EC50s, which were 73% and 61%, respectively.
DISCUSSION
Amphotericin B was fungicidal against A. fumigatus and A. flavus (median MBX MFC/MIC ratio, ≤4) but not against A. terreus (median MBX MFC/MIC ratio, >4) isolates. This is consistent with the nonlinear regression analysis of metabolic activity, which resulted in strong fungicidal activities for A. fumigatus and A. flavus isolates (low population EC50s, ranging from 0.34 to 2.46 mg/liter) and weak fungicidal activities for A. terreus isolates (high population EC50s, ranging from 7.2 to 12.67 mg/liter). The amphotericin B fungicidal activity is in agreement with previous in vitro studies, where it was found that amphotericin B is fungicidal against A. fumigatus and A. flavus by culture-based techniques (3, 5, 6, 11), flow cytometry (22), or time-kill assays (13, 25). In agreement with the findings of the present study, a weaker fungicidal activity of amphotericin B was previously found for A. terreus than for other Aspergillus species (5, 6, 24). Furthermore, in a recently developed rapid susceptibility testing microbroth colorimetric methodology, significant metabolic activity was detected after 8 h of incubation for A. terreus (16% of the drug-free control), but not for A. fumigatus (3%) and A. flavus (3%) isolates at high concentrations of amphotericin B, indicating differential amphotericin B activities against Aspergillus species, similar to that observed in the present study (1). Previous in vivo studies using amphotericin B for treatment of experimental invasive aspergillosis with A. fumigatus, A. flavus, and A. terreus appear to be correlated with the MBX MFCs described here (19, 25, 26).
Voriconazole was fungistatic against most A. fumigatus (94%) and A. terreus (84%) isolates (median MBX MFC/MIC ratio, ≥8) and fungicidal against most (82%) A. flavus isolates (median MBX MFC/MIC ratio, 4). The high voriconazole MBX MFCs for A. terreus are concordant with previous findings (5, 24). The voriconazole MBX MFCs for A. flavus and A. fumigatus determined in the present study are higher than the MFCs previously obtained by culture-based techniques, where MFC90s ranged from 0.25 to 4 mg/liter, implying that voriconazole is fungicidal against these Aspergillus species (5, 11, 27). However, in those studies, <100% of the volume was used to determine voriconazole MFCs, as the volume subcultured ranged from 10 to 100 μl and the percentage of killing ranged from 90% to 99.97% compared to our study, where the entire well (200 μl) was assessed and 100% killing was used for the MBX MFC determination. Furthermore, because the results of culture-based methods depend on the adherence of conidia to the bottoms of wells, as demonstrated in the present study, MFC determination by culturing the contents of the wells may overestimate the lethal activities of antifungal drugs. The intensity and duration of pipetting before subculturing the well content, the sampling error, inadequate spread onto agar plates, and drug carryover may increase variation in MFC determination by the culture-based methods. As discussed in a comprehensive review of the determination of fungicidal activities (21), “the criterion used to define MFCs (99.9% killing) is completely arbitrary” and “has probably no biological or clinical significance” while “the use of a less stringent criterion for defining the MFC poses the risk of ‘very major’ errors by classifying an agent as fungicidal when in fact it is not.” Thus, the inability of voriconazole to kill all the conidia of the relatively small inoculum used in susceptibility-testing methodologies and in the present study may call its fungicidal activity into question.
An MFC determined using either the culture-based or the MBX method suggests an all-or-nothing situation and corresponds to a single drug concentration. However, the MBX method provides additional quantitative information about fungicidal effects over a range of drug concentrations. Nonlinear regression analysis revealed a sigmoid pattern of voriconazole fungicidal actions for all species, with increasing fungicidal effects (small percentages of metabolic activity) at higher concentrations. Notably the previously published culture-based MFCs based on 90 to 99.97% killing levels (5, 11, 27) for A. fumigatus and A. flavus are close to the voriconazole population EC50s obtained from the nonlinear regression analysis of the XTT absorbance data (2.34 and 1.96 mg/liter, respectively), while MBX MFCs are closer to the EC1s. Thus, the MFC represents a single point in the concentration-fungicidal-effect curve.
Whether fungicidal activity should be assessed using an MFC associated with the EC50, the EC1, or another endpoint is unknown. Although for steep curves like those obtained for amphotericin B against A. flavus and A. fumigatus the difference between the EC50, the EC1, and other endpoints would be minimal, for shallow curves like those obtained for voriconazole these differences would be large (Fig. 3). Rather than using MFCs for classifying an agent as fungicidal, an alternative approach would be to consider an agent fungicidal based on the slope of the concentration-fungicidal-effect curve; a fungistatic agent would have a shallow concentration-fungicidal- effect curve (e.g., slopes of >−3). Thus, amphotericin B would be considered fungicidal against A. fumigatus and A. flavus (e.g., slopes of <−3), whereas amphotericin B against A. terreus and voriconazole against all Aspergillus species would be considered fungistatic (slopes of >−3). This approach is distinct from the conventional approach of classifying agents as fungicidal when the MFC is ≤4× MIC (21), as the slope is determined from a range of drug concentrations. It has been proposed that the assessment of the fungicidal action of a drug should be based on whether a drug eradicates the infecting organism in a neutropenic-animal model of disseminated infection (21). On this line, voriconazole therapy for experimental aspergillosis caused by A. fumigatus (2, 4, 10, 12, 17) in immunocompromised animals improved survival and reduced the fungal burden but never eradicated the organisms from all animals, even at high dosages (40 mg/kg of body weight). Whether this is related to inadequate drug exposure, altered host defense factors, or the weaker fungicidal activity of voriconazole requires further investigation.
In agreement with the voriconazole population EC50s of the drug concentration-XTT absorbance curve for A. fumigatus found in the present study (1.42 to 4.06 mg/liter), Ramani et al. demonstrated by flow cytometry only a 50% decrease in viable A. fumigatus conidia at 1 to 8 mg/liter of voriconazole after 48 h of incubation (22). Furthermore, time-kill assays showed that 10 mg/liter of voriconazole could not completely kill A. fumigatus conidia, and a stronger fungicidal activity was found for A. flavus than for A. fumigatus after 24 h of incubation (13), in agreement with the findings of our study. Finally, exposure of Aspergillus conidia to voriconazole for 6 to 8 h resulted in significant metabolic activity for A. terreus isolates (36% of that of the drug-free control), followed by A. flavus (16%) and A. fumigatus (14%) isolates, at high concentrations of voriconazole (1), indicating differential antifungal activities of voriconazole against Aspergillus species, similar to the differential fungicidal activities observed in the present study.
In conclusion, the new microbroth colorimetric assay for measuring the fungicidal activities of antifungal agents is easy, rapid, and reproducible. It also is amenable to automation, standardization, and large-scale multicenter studies of the fungicidal activities of single agents and combinations. It provides quantitative and detailed information about concentration- dependent fungicidal effects that may be useful to understand the antifungal pharmacodynamics beyond growth-inhibitory effects. Differential fungicidal activities were found against Aspergillus species, with amphotericin B possessing fungicidal activity against A. fumigatus and A. flavus but not against A. terreus and voriconazole exhibiting fungicidal activities against most A. flavus isolates and fungistatic activities against most A. fumigatus and A. terreus isolates. Finally, the new assay showed that fungicidal activity is not an all-or-nothing phenomenon, as the MFC determination implies, and that fungistatic agents based on the MFC/MIC ratio may exhibit fungicidal effects at high drug concentrations.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
Published ahead of print on 18 June 2007.
REFERENCES
- 1.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2006. Use of high inoculum for early metabolic signalling and rapid susceptibility testing of Aspergillus species. J. Antimicrob. Chemother. 59:230-237. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekar, P. H., J. Cutright, and E. Manavathu. 2000. Efficacy of voriconazole against invasive pulmonary aspergillosis in a guinea-pig model. J. Antimicrob. Chemother. 45:673-676. [DOI] [PubMed] [Google Scholar]
- 3.Clancy, C. J., and M. H. Nguyen. 1998. In vitro efficacy and fungicidal activity of voriconazole against Aspergillus and Fusarium species. Eur. J. Clin. Microbiol. Infect. Dis. 17:573-575. [DOI] [PubMed] [Google Scholar]
- 4.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob. Agents Chemother. 49:4867-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A. 2001. Germinated and nongerminated conidial suspensions for testing of susceptibilities of Aspergillus spp. to amphotericin B, itraconazole, posaconazole, ravuconazole, and voriconazole. Antimicrob. Agents Chemother. 45:605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., A. Fothergill, M. Ghannoum, E. Manavathu, L. Ostrosky-Zeichner, M. Pfaller, M. Rinaldi, W. Schell, and T. Walsh. 2005. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M 38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 43:5243-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George, D., P. Miniter, and V. T. Andriole. 1996. Efficacy of UK-109496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob. Agents Chemother. 40:86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, E. M., A. Szekely, and D. W. Warnock. 1998. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J. Antimicrob. Chemother. 42:741-745. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob. Agents Chemother. 44:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manavathu, E. K., J. L. Cutright, and P. H. Chandrasekar. 1998. Organism-dependent fungicidal activities of azoles. Antimicrob. Agents Chemother. 42:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motulsky, H. 2003. Prism 4 statistics guide—statistical analyses for laboratory and clinical researchers, p. 122-126. GraphPad Software Inc., San Diego, CA.
- 16.Motulsky, H., and A. Christopoulos. 2003. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. GraphPad Software Inc., San Diego, CA.
- 17.Murphy, M., E. M. Bernard, T. Ishimaru, and D. Armstrong. 1997. Activity of voriconazole (UK-109,496) against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. NCCLS, Wayne, PA.
- 19.Odds, F. C., F. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, J. R. Graybill, et al. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramani, R., M. Gangwar, and V. Chaturvedi. 2003. Flow cytometry antifungal susceptibility testing of Aspergillus fumigatus and comparison of mode of action of voriconazole vis-a-vis amphotericin B and itraconazole. Antimicrob. Agents Chemother. 47:3627-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Tudela, J. L., E. Chryssanthou, E. Petrikkou, J. Mosquera, D. W. Denning, and M. Cuenca-Estrella. 2003. Interlaboratory evaluation of hematocytometer method of inoculum preparation for testing antifungal susceptibilities of filamentous fungi. J. Clin. Microbiol. 41:5236-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton, D. A., S. E. Sanche, S. G. Revankar, A. W. Fothergill, and M. G. Rinaldi. 1999. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J. Clin. Microbiol. 37:2343-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, T. J., V. Petraitis, R. Petraitiene, A. Field-Ridley, D. Sutton, M. Ghannoum, T. Sein, R. Schaufele, J. Peter, J. Bacher, H. Casler, D. Armstrong, A. Espinel-Ingroff, M. G. Rinaldi, and C. A. Lyman. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305-319. [DOI] [PubMed] [Google Scholar]
- 26.Warn, P. A., G. Morrissey, J. Morrissey, and D. W. Denning. 2003. Activity of micafungin (FK463) against an itraconazole-resistant strain of Aspergillus fumigatus and a strain of Aspergillus terreus demonstrating in vivo resistance to amphotericin B. J. Antimicrob. Chemother. 51:913-919. [DOI] [PubMed] [Google Scholar]
- 27.Warn, P. A., A. Sharp, and D. W. Denning. 2006. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J. Antimicrob. Chemother. 57:135-138. [DOI] [PubMed] [Google Scholar]