Abstract
Fluconazole-FK506 or fluconazole-cyclosporine drug combinations were tested in an ex vivo Trichophyton mentagrophytes human skin infection model. Conidia colonization was monitored by scanning electron microscopy over a 7-day treatment period. The fluconazole-FK506 combination demonstrated the most obvious advantage over single-drug therapy by clearing conidia and protecting skin from damage at low drug concentrations.
Dermatophyte fungal pathogens cause substantial morbidity in otherwise healthy individuals (15). Trichophyton species cause ∼70% of all dermatophyte infections (16). Terbinafine and fluconazole are ergosterol biosynthesis inhibitors with antifungal activity against Trichophyton species (1, 4, 5), but azoles are used less frequently than terbinafine for management (8). The calcineurin inhibitors cyclosporine A (CsA) and FK506 are synergistic with ergosterol biosynthesis inhibitors against Candida albicans (2, 9, 10, 12, 13). We hypothesize that this drug synergy could extend to Trichophyton mentagrophytes. We determined MICs and tested the therapeutic potential of proposed drug combinations in an ex vivo human skin infection model (3).
T. mentagrophytes strains were grown on potato dextrose agar (30°C, 7 days). Conidia were diluted to 103 CFU/ml in liquid RPMI 1640 (Gibco) buffered to pH 7 with 0.165 M morpholinepropanesulfonic acid and NaOH. In vitro susceptibility assays were performed according to a modified Clinical and Laboratory Standards Institute (CLSI) M38-A protocol. Each drug concentration was prepared at 100× and diluted 10-fold, and 0.9 ml of inoculum was added to 0.1 ml of 10× drug solution. Drugs were dissolved in 100% dimethyl sulfoxide or ethanol and provided by LC Biolabs, Novartis, and Duke Pharmacy. Drug concentrations were 0.125 μg/ml to 64 μg/ml (CsA and fluconazole), 0.03125 μg/ml to 16 μg/ml (FK506), and 0.004 μg/ml to 2 μg/ml (terbinafine). Both strains exhibited moderate fluconazole sensitivity and potent terbinafine susceptibility (Table 1).
TABLE 1.
MICs of drugs for T. mentagrophytes strains
| Straina | MIC (μg/ml) of drugb:
|
|||
|---|---|---|---|---|
| CsA | FK506 | FLCc | TRB | |
| DUMC112.02 | 2 | 1 | 8-16 | 0.004 |
| DUMC160.03 | 1 | 1 | 8-16 | 0.0312 |
Strains were from the Duke University Medical Center stock collection.
FLC, fluconazole; TRB, terbinafine.
The lowest MIC (8 μg/ml) was used for ex vivo studies.
Checkerboard synergy assays with drug concentrations from 0.125 to 8 μg/ml (CsA) and 0.5 to 16 μg/ml (fluconazole) or 0.064 to 4 μg/ml (FK506) and 0.5 to 16 μg/ml (fluconazole) were performed. Terbinafine was excluded due to potency (Table 1). The fractional inhibitory concentration index (ΣFIC) was calculated, and values of ≤0.5 revealed synergy, those of >0.5 but <4 indicated no interaction, and those of >4 were antagonistic (11). The fluconazole-FK506 combination was synergistic against strain DUMC112.02 (ΣFIC = 0.28). The fluconazole-CsA combination demonstrated synergy against both strains, decreasing MICs by 4- to 32-fold (Table 2). These drug combinations were tested against strain DUMC160.03 in a skin infection model.
TABLE 2.
T. mentagrophytes MICs and ΣFICs for combination therapy
| Strain | MIC80a (μg/ml)
|
FLCb-CsA
|
FLC-FK506
|
||||
|---|---|---|---|---|---|---|---|
| FLC | CsA | FK506 | Combined MICc | ΣFICd | Combined MICc | ΣFICd | |
| DUMC112.02 | 16 | 2 | 1 | 4/0.5 | 0.5 | 4/0.03 | 0.28 |
| DUMC160.03 | 16 | 8 | 1 | 0.5/2 | 0.28 | 4/0.35 | 0.6 |
MIC at which 80% of the isolates tested are inhibited.
FLC, fluconazole.
Shown as MIC of first drug/MIC of second drug, both in micrograms per milliliter.
ΣFIC values represent the median values from two or more experiments.
Excess full-thickness (0.5 to 1 cm diameter) skin sections from plastic surgery procedures were maintained in sterile skin graft fluid at pH 6.4 for 2 to 3 weeks (3, 14). Samples were inoculated with 1 × 107 to 5 × 107 conidia/ml, incubated at 30°C, and monitored by digital scanning electron microscopy; images represent at least five replicates from two independent experiments (3, 6, 7). Drugs were applied 30 minutes after inoculation and then every 2 days. By days 1, 3, and 7 postinfection (p.i.), each sample received one, two, or four treatments, respectively. No agent was deleterious to the skin after 7 days (Fig. 1A). T. mentagrophytes rapidly colonized untreated skin, and 1 day after inoculation, hyphae were visible (Fig. 1B). After 3 days, skin appeared damaged with abundant hyphae that further increased by day 7 p.i. (Fig. 1B).
FIG. 1.
Experimental and treatment controls for the ex vivo skin infection model. (A) In the absence of T. mentagrophytes, antifungal agents were applied at 2× MIC to skin samples every 2 days for 7 days with no damage to skin integrity. Scanning electron microscopy images show the skin surface. Samples were fixed following 7 days of treatment. Bars, 5 μm. (B) T. mentagrophytes conidia begin to colonize skin samples at day 1 p.i. and produce a dense collection of hyphae by day 7 p.i. on untreated skin samples. D0, D1, D3, and D7 refer to the number of days p.i.
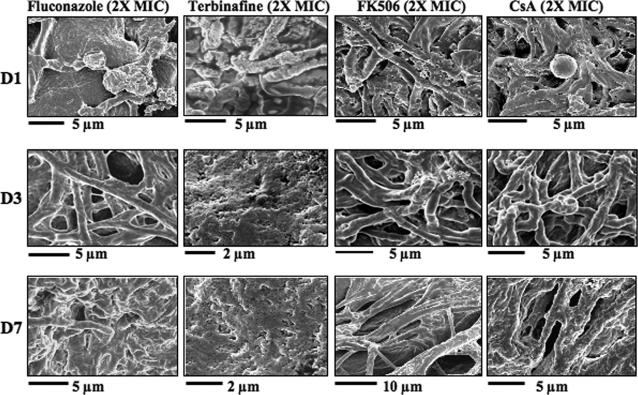
Single-dose fluconazole at 2× MIC caused minimal growth inhibition (Fig. 2). Damaged skin samples with prominent conidia were observed on day 3 (Fig. 2). By day 7, hyphae were rare and skin sections appeared normal (Fig. 2). At 2× MIC of terbinafine, germinating conidia and damaged hyphae were visible on day 1 p.i. and rare by day 3 p.i. and skin appeared normal on day 7 p.i. (Fig. 3). These findings support the in vitro assays where terbinafine was more potent than fluconazole (Table 1). At 2× MIC, FK506- and/or CsA-treated samples resembled the untreated controls (compare Fig. 1B and 2).
FIG. 2.
The ex vivo dermatophyte skin infection model responds to drug treatment. Skin samples colonized with T. mentagrophytes (DUMC160.03) were treated every 2 days (days 0, 2, 4, and 6) with 2× MIC of fluconazole, terbinafine, FK506, or CsA. Samples were fixed at the indicated times. A significant reduction in hyphal colonization and intact skin was observed by day 7 p.i., demonstrating the efficacy of fluconazole or terbinafine. FK506 or CsA treatment did not result in hyphal clearing or improved skin integrity.
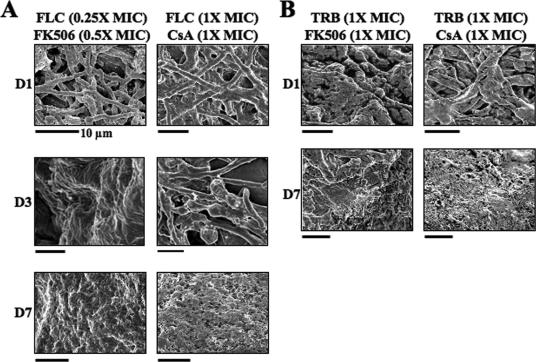
FIG. 3.
T. mentagrophytes is susceptible to drug combinations in the ex vivo infection model. The antifungal activity of fluconazole was enhanced by FK506 or CsA. (A) Fluconazole was more effective by day 3 p.i. when combined with FK506 or CsA (compare with Fig. 2). The accelerated hyphal clearing observed when drugs were combined at lower concentrations was indicative of drug synergy. (B) Samples treated with terbinafine-CsA or terbinafine-FK506 combinations were indistinguishable from those treated with terbinafine alone (Fig. 2). FLC, fluconazole; TRB, terbinafine. Bars, 5 μm (unless otherwise indicated).
Fluconazole or terbinafine was combined with each calcineurin inhibitor in ex vivo drug studies. For each drug, MICs or fractional MICs were applied to skin (Table 1). The fluconazole (0.25× MIC)-FK506 (0.5× MIC) combination had little effect on hyphal growth after one dose (Fig. 3A). However, by day 3 p.i., skin appeared normal with no hyphae observed (Fig. 3A). This differed from skin receiving 2× MIC fluconazole alone, which still appeared damaged on day 3 p.i., with hyphae present on day 7 p.i. (Fig. 2). The fluconazole-CsA (1× MIC each) combination resulted in hyphal collapse on day 3 p.i. (Fig. 3A). Hyphae cleared by day 7 p.i., and skin appeared normal (Fig. 3A). Compared to single-drug therapy, the fluconazole-CsA combination achieved an effective dose when the MIC was reduced 50% (Fig. 2).
The terbinafine-FK506 (1× MIC each) combination protected skin from damage; in most fields, no adhering hyphae were observed from days 1 to 7 p.i. (Fig. 3B). These samples resembled those receiving 2× MIC terbinafine monotherapy (Fig. 2). Similarly, no hyphae were observed 7 days after terbinafine-CsA (1× MIC each) treatment (Fig. 3).
As anticipated from the in vitro studies, fluconazole and terbinafine were effective in inhibiting T. mentagrophytes colonization (Fig. 2). We were unable to evaluate positive interactions with terbinafine and CsA and/or FK506 since T. mentagrophytes was highly sensitive to terbinafine alone. The fluconazole-FK506 combination exhibited synergism against DUMC112.02, while the fluconazole-CsA combination was synergistic against DUMC160.03 (Table 2). However, in the infection model, the fluconazole (0.25× MIC)-FK506 (0.5× MIC) combination cleared hyphae more rapidly than the fluconazole-CsA (1× MIC each) combination did, and both combinations were more effective than fluconazole alone at 2× MIC (compare Fig. 2 and 3). The ability of these agents to eliminate colonizing hyphae more rapidly when combined at lower concentrations was indicative of a positive interaction in this ex vivo model.
The proposed drug combinations have in vivo implications since calcineurin inhibitor anti-inflammatory activity might accelerate symptom resolution. Calcineurin inhibitors alone did not improve disease resolution (Fig. 2), but as adjuncts to topical antifungal therapies, they may facilitate healing by reducing inflammatory mediators at infection sites. Rapidly healing lesions could result in improved patient compliance, decreased morbidity, and avoidance of adverse effects from some oral therapies. However, these immunosuppressants could also negatively affect local immune processes.
This dermatophyte infection model simulates human infection and allows evaluation of host-pathogen interactions (3). It could facilitate drug studies because skin can be inoculated, treated with multiple drugs, and processed efficiently with implications for understanding pathogenesis and establishing new treatment. Skin specimens from different patient populations can be investigated for genetic contributions to drug responses and disease susceptibility.
Acknowledgments
We are grateful to N. Isham and M. A. Ghannoum for strains and advice.
This work was supported by RO1 grant NIH/NIAID AI50438, a Howard Hughes Research Training Fellowship to Emily Eads, a Technion V.P.R. Fund grant to Israela Berdicevsky, and a UNCF/Merck Graduate Fellowship to Chiatogu Onyewu.
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Cetinkaya, Z., N. Kiraz, S. Karaca, M. Kulac, I. H. Ciftci, O. C. Aktepe, M. Altindis, N. Kiyildi, and M. Piyade. 2005. Antifungal susceptibilities of dermatophytic agents isolated from clinical specimens. Eur. J. Dermatol. 15:258-261. [PubMed] [Google Scholar]
- 2.Cruz, M. C., A. L. Goldstein, J. R. Blankenship, M. Del Poeta, D. Davis, M. E. Cardenas, J. R. Perfect, J. H. McCusker, and J. Heitman. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21:546-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duek, L., G. Kaufman, Y. Ulman, and I. Berdicevsky. 2004. The pathogenesis of dermatophyte infections in human skin sections. J. Infect. 48:175-180. [DOI] [PubMed] [Google Scholar]
- 4.Gupta, A. K., Y. Kohli, and R. Batra. 2005. In vitro activities of posaconazole, ravuconazole, terbinafine, itraconazole and fluconazole against dermatophyte, yeast and non-dermatophyte species. Med. Mycol. 43:179-185. [DOI] [PubMed] [Google Scholar]
- 5.Gupta, A. K., J. E. Ryder, and A. M. Johnson. 2004. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br. J. Dermatol. 150:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman, G., I. Berdicevsky, J. A. Woodfolk, and B. A. Horwitz. 2005. Markers for host-induced gene expression in Trichophyton dermatophytosis. Infect. Immun. 73:6584-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman, G., B. A. Horwitz, R. Hadar, Y. Ulman, and I. Berdicevsky. 2004. Green fluorescent protein (GFP) as a vital marker for pathogenic development of the dermatophyte Trichophyton mentagrophytes. Microbiology 150:2785-2790. [DOI] [PubMed] [Google Scholar]
- 8.Loo, D. S. 2006. Systemic antifungal agents: an update of established and new therapies. Adv. Dermatol. 22:101-102. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti, O., J. M. Entenza, D. Sanglard, J. Bille, M. P. Glauser, and P. Moreillon. 2000. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 44:2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti, O., P. Moreillon, M. P. Glauser, J. Bille, and D. Sanglard. 2000. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 44:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 12.Onyewu, C., N. A. Afshari, and J. Heitman. 2006. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob. Agents Chemother. 50:3963-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peled, I. N., and E. Lindenbaum. 1991. Prolonged skin graft preservation with keratinocyte culture medium. Eur. J. Plast. Surg. 14:232-234. [Google Scholar]
- 15.Vander Straten, M. R., M. A. Hossain, and M. A. Ghannoum. 2003. Cutaneous infections dermatophytosis, onychomycosis, and tinea versicolor. Infect. Dis. Clin. N. Am. 17:87-112. [DOI] [PubMed] [Google Scholar]
- 16.Weitzman, I., and R. C. Summerbell. 1995. The dermatophytes. Clin. Microbiol. Rev. 8:240-259. [DOI] [PMC free article] [PubMed] [Google Scholar]