Abstract
The relevance of apoptosis to killing of intracellular Mycobacterium tuberculosis was studied with phorbol-differentiated THP-1 cells. Microparticles containing isoniazid and rifabutin induced intrinsic apoptosis and bacterial killing equivalent to that with dissolved drugs and maximally enhanced purinergic P2 receptor activity, while drug-free microparticles induced apoptosis via a different mechanism without killing bacteria.
Host macrophage death induced by intracellular Mycobacterium tuberculosis correlates with virulence and microbial burden (12). At a low multiplicity of infection (MOI), virulent strains inhibit apoptosis while attenuated strains, such as H37Ra, induce it (3, 13). At a high MOI, apoptosis is caspase independent and shows necrosis-like features (7). While acknowledged as a key host defense strategy, induction of host macrophage apoptosis has not been considered as a therapeutic strategy for tuberculosis (TB).
Recently highly efficacious, inhalable drug delivery systems targeting macrophage-resident TB bacteria have been proposed (9, 10, 15-17). Their efficacy has been ascribed to efficient drug targeting, which is not fully consonant with the experience of high-dose chemotherapy for TB (1, 6). Inhaled biodegradable microparticles containing a large payload of anti-TB drugs (8) yield high intracellular drug levels (16) and evoke free radicals and tumor necrosis factor alpha secretion by murine macrophages (15). Since microparticle phagocytosis might activate infected human macrophages (11, 14), whether apoptosis would be a consequence of such activation and whether it would be relevant to killing of the resident pathogen were examined. Kornfeld, Remold, and colleagues have addressed several phenomena relevant to innate antimycobacterial activity and apoptosis of infected macrophages (2, 3, 7, 13), which were revisited to determine whether microparticle treatment would enhance the host defense strategy of macrophage apoptosis.
THP-1 cells maintained in RPMI-10% fetal bovine serum (Gibco BRL) were treated with 20 nM of phorbol myristate acetate (PMA) (Sigma) for 14 h. Cells were washed and incubated in fresh medium for another 10 h. PMA-induced differentiation yields cells established to behave like primary human macrophages (4, 13). These cells were infected with M. tuberculosis strain H37Ra grown to log phase on Lowenstein-Jensen medium. A scraping was suspended in RPMI and the optical density at 600 nm used to adjust the concentration. After infection at 20 MOI for 2 h, cells were washed to remove extracellular bacteria and treated for 2 h with drugs in solution, drug-containing microparticles (3 μg of each agent/ml of medium; all materials were a gift from Lupin Laboratories Ltd., Pune, India), or drug-free microparticles (15). Wells were washed free of drugs or microparticles and fresh medium replaced.
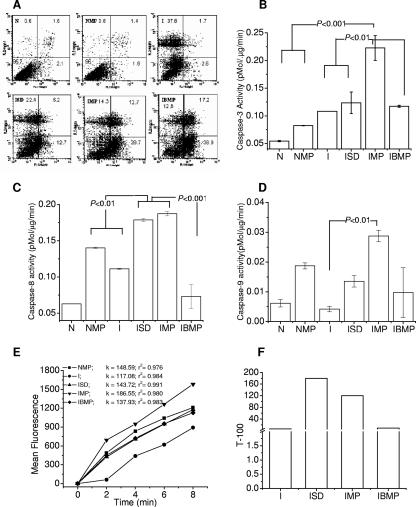
Flow cytometry (FACSCalibur, Cell Quest; BD Biosciences) was used to assess apoptosis and necrosis at 14 h posttreatment with 106 cells double stained with annexin V conjugated to fluorescein isothiocyanate plus propidium iodide according to the kit manufacturer's instructions (Oncogen). Quadrants were marked according to the pattern exhibited by uninfected, untreated cells. Drug-containing microparticles induced minimal apoptosis in uninfected cells, while infection under the experimental conditions employed primarily caused necrosis. Treatment with dissolved drugs, drug-containing microparticles, and blank microparticles increased the proportion of apoptotic cells by approximately 5, 11, and 15 times, respectively, compared to results for untreated infection (n = 2; P < 0.05).
Since microparticles are phagocytosed, in contrast to intracellular diffusion of dissolved drugs, the impact of treatment modalities on the caspase cascade was investigated. Fluorogenic assays for caspase activity were performed with cell lysates prepared in digitonin-Tris-EDTA lysis buffer (5). Activities of caspase-3, -8, and -9 were measured in equal amounts of total cell protein, using specific substrates (Sigma).
Background levels of caspase-3 (Fig. 1B) induced by PMA in uninfected cells were not enhanced significantly (n = 3; analysis of variance, P > 0.05) either on infection or on phagocytosis of drug-containing microparticles by uninfected cells. In terms of enzyme activity (in pmol/ml/min), drug-containing microparticles administered to infected cells significantly (P < 0.001) induced caspase-3 (0.223 ± 0.022), while dissolved drugs (0.124 ± 0.019) did not induce higher activity than infection itself (0.108 ± 0.002) or treatment with drug-free microparticles (0.117 ± 0.002). As shown in Fig. 1C, drug-containing microparticles and dissolved drugs induced equivalent caspase-8, while drug-free microparticles resulted in marginal downregulation of the response to infection. Caspase-9 (Fig. 1D) was induced significantly (n = 3; P<0.01) only when infected cells were treated with drug-loaded microparticles. It was inferred that despite the common mechanism of internalization, caspase induction followed different pathways depending on whether microparticles did or did not contain anti-TB drugs.
FIG. 1.
(A) Necrotic (propidium iodide positive; y axis) versus apoptotic (annexin V positive; x axis) ungated differentiated THP-1 populations upon infection and/or treatment. Inset numbers show percentages of cells in the quadrant. Uninfected cells (N) treated with microparticles (NMP) did not show significant cell death, whereas infection primarily induced necrosis (I). Treatment of infected cells with drugs in solution (ISD), drug-containing microparticles (IMP), or drug-free microparticles (IBMP) induced increasing extents of apoptosis in comparison to necrosis (n = 2; P < 0.05). Induction of caspase-3 (B), caspase-8 (C), and caspase-9 (D) indicated differences in apoptotic pathways and effector caspase levels upon treatment with drugs in solution or microparticles. Drug-containing microparticles induced caspase-3, -8, and -9 activity. Drugs in solution induced significant caspase-9 activity, but drug-free microparticles did not induce high levels of caspases. One-way analysis of variance (n = 3) followed by Bonferroni's posttest to compare group means was used to test significance. Error bars indicate standard deviations. (E) Purinergic P2X receptor-mediated ethidium influx. Slopes (k) of kinetic curves showed the regression coefficients (r2) indicated. All treatments enhanced P2XR activity in comparison to that observed after infection, with drug-containing microparticles eliciting the highest activity. Group nomenclature is as described above. (F) Survival of M. tuberculosis in cell lysate is expressed as time required to attain a growth index of 100 (T-100).
Since intrinsic apoptosis is associated with mitochondrial membrane integrity (3), mitochondrial membrane potential (Δψm) was measured 6, 12, and 18 h posttreatment (data not shown). Reduction in Δψm was discernible only at 12 h. The difference between drug-containing and drug-free microparticles was statistically insignificant. This suggested that mitochondrial membrane permeability alterations did not correlate with caspase induction or extents of apoptosis.
Inhibition of apoptosis by stabilization of Δψm promotes macrophage mycobactericidal activity through purinergic P2X receptor (P2XR)-mediated alteration of the inflammatory state of the affected cells (3, 18). P2XR was assayed in about 106 cells 14 h after treatment. Cells were dispersed in 840 μl sheath fluid for flow cytometry and 50 μl of 0.5 mM ethidium bromide plus 10 μl of 100 mM ATP added for flow cytometric evaluation every 2 min (3). Figure 1E shows ATP-dependent ethidium influx through P2XR. Infection significantly slowed influx, while treatment with dissolved drugs or drug-free microparticles yielded influx rates comparable to the uninfected cells' response to drug-containing microparticles. Treatment of infected cells with drug-containing microparticles enhanced ethidium flux maximally (P < 0.01).
Drug-containing microparticles enhanced caspase-8-dependent (and probably P2XR-mediated) apoptosis, while drug-free microparticles induced apoptosis through other mechanisms. The relevance of these two modes of cell death to bacterial survival was addressed.
Viability of bacteria in different groups was assessed 48 h after treatment using the BACTEC 460 TB system. Cells were lysed in 100 μl of 0.1% saponin, and the lysate was inoculated into BACTEC B12 vials. The bacterial growth index was monitored every 12 h, and the time required to reach a growth index of 100 was calculated (2). Figure 1F shows results of the Bactec assay. Comparable efficacies were observed when dissolved drugs or microparticles were administered to infected cells. Blank microparticles did not affect bacterial survival despite their greater efficiency in inducing apoptosis. It was concluded that drug-containing microparticles are more efficient than dissolved drugs at enlisting host defense responses to M. tuberculosis. Caspase-independent apoptosis and P2XR activation induced by blank microparticles are not sufficient for direct mycobactericidal activity.
Acknowledgments
Financial support from CSIR grant 5/258/6/2001-NMITLI and a senior research fellowship (A.B.Y.) is acknowledged.
We thank A. L. Vishwakarma for flow cytometry and Sandeep Sharma for Bactec.
This is CDRI Communication 7123.
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Cynamon, M. H., Y. Zhang, T. Harpster, S. Cheng, and M. S. DeStefano. 1999. High-dose isoniazid therapy for isoniazid-resistant murine Mycobacterium tuberculosis infection. Antimicrob. Agents Chemother. 43:2922-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fratazzi, C., R. D. Arbeit, C. Carini, and H. G. Remold. 1997. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 158:4320-4327. [PubMed] [Google Scholar]
- 3.Gan, H., X. He, L. Duan, E. Mirabile-Levens, H. Kornfeld, and H. G. Remold. 2005. Enhancement of antimycobacterial activity of macrophages by stabilization of inner mitochondrial membrane potential. J. Infect. Dis. 191:1292-1300. [DOI] [PubMed] [Google Scholar]
- 4.Hestvik, A. L., Z. Hmama, and Y. Av-Gay. 2005. Mycobacterial manipulation of the host cell. FEMS Microbiol. Rev. 29:1041-1050. [DOI] [PubMed] [Google Scholar]
- 5.Kanuka, H., S. Hisahara, K. Sawamoto, S. Shoji, H. Okano, and M. Miura. 1999. Proapoptotic activity of Caenorhabditis elegans CED-4 protein in Drosophila: implicated mechanisms for caspase activation. Proc. Natl. Acad. Sci. USA 96:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langton, M. E., and R. L. Cowie. 1985. Failure of a prothionamide-containing oral antituberculosis regimen. S. Afr. Med. J. 68:881. [PubMed] [Google Scholar]
- 7.Lee, J., H. G. Remold, M. H. Ieong, and H. Kornfeld. 2006. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J. Immunol. 176:4267-4274. [DOI] [PubMed] [Google Scholar]
- 8.Muttil, P., J. Kaur, K. Kumar, A. B. Yadav, R. Sharma, and A. Misra. 4 July 2007, posting date. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur. J. Pharm. Sci. doi: 10.1016/j.ejps.2007.06.006. [DOI] [PubMed]
- 9.O'Hara, P., and A. J. Hickey. 2000. Respirable PLGA microspheres containing rifamycin for the treatment of tuberculosis: manufacture and characterization. Pharm. Res. 17:955-961. [DOI] [PubMed] [Google Scholar]
- 10.Pandey, R., and G. K. Khuller. 2005. Antitubercular inhaled therapy: opportunities, progress and challenges. J. Antimicrob. Chemother. 55:430-435. [DOI] [PubMed] [Google Scholar]
- 11.Prior, S., B. Gander, N. Blarer, H. Merkle, M. Subira, J. Irache, and C. Gamazo. 2002. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur. J. Pharm. Sci. 15:197-207. [DOI] [PubMed] [Google Scholar]
- 12.Rajavelu, P., and S. D. Das. 2007. A correlation between phagocytosis and apoptosis in THP-1 cells infected with prevalent strains of Mycobacterium tuberculosis. Microbiol. Immunol. 51:201-210. [DOI] [PubMed] [Google Scholar]
- 13.Riendeau, C. J., and H. Kornfeld. 2003. THP-1 cell apoptosis in response to mycobacterial infection. Infect. Immun. 71:254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnyder, J., and M. Baggiolini. 1978. Role of phagocytosis in the activation of macrophages. J. Exp. Med. 148:1449-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma, R., P. Muttil, A. B. Yadav, S. K. Rath, V. K. Bajpai, U. Mani, and A. Misra. 2007. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 59:499-506. [DOI] [PubMed] [Google Scholar]
- 16.Sharma, R., D. Saxena, A. K. Dwivedi, and A. Misra. 2001. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm. Res. 18:1405-1410. [DOI] [PubMed] [Google Scholar]
- 17.Suarez, S., P. O'Hara, M. Kazantseva, C. E. Newcomer, R. Hopfer, D. N. McMurray, and A. J. Hickey. 2001. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm. Res. 18:1315-1319. [DOI] [PubMed] [Google Scholar]
- 18.Wilson, H. L., R. W. Varcoe, L. Stokes, K. L. Holland, S. E. Francis, S. K. Dower, A. Surprenant, and D. C. Crossman. 2007. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. Br. J. Pharmacol. 151:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]