Abstract
Amphotericin B and flucytosine (5FC) have an additive effect when used for disseminated candidiasis. Here, we bridge the results of an experimental pharmacodynamic study to humans and demonstrate that a 5FC dosage of 25 mg/kg of body weight/day in four divided doses in combination with amphotericin B produces near-maximal effect.
Bridging from experimental systems to humans is increasingly used as a tool to further explore the clinical implications of experimental data (3, 8, 9). The combination of amphotericin B and flucytosine (5FC) is used to treat disseminated candidiasis. The standard dosage of 5FC of 150 mg/kg of body weight/day in combination with amphotericin B at 0.5 to 1.0 mg/kg/day frequently results in peak 5FC levels greater than 100 mg/liter, which are associated with toxicity (6). Recently, we demonstrated that the combination of amphotericin B and 5FC is additive in a murine model of disseminated candidiasis (10). Here, we bridge these experimental results to humans to generate hypotheses regarding the optimal clinical dosages of these antifungal agents when administered concomitantly.
(This work was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 30 October to 2 November 2 2004.)
The steps undertaken in the in vivo-to-human bridging process are summarized in Table 1. The Greco equation was used for the interaction effect modeling (7, 10). This model takes the form
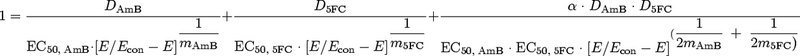 |
where Econ is the fungal burden in the absence of therapy; DAmB and D5FC are the drug exposures of amphotericin B and 5FC, respectively, producing the effect E; EC50, AmB and EC50, 5FC are the drug concentrations producing 50% of the maximum effects of amphotericin B and 5FC, respectively; mAmB and m5FC are the respective Hill (slope) constants; and α is the interaction parameter. The amphotericin B MIC of the experimental strain was 0.03 mg/liter (determined using a microdilution modification of CLSI [formerly NCCLS] methodology [12] with the addition of antibiotic medium 3; the MIC90 for 4,247 Candida albicans strains in our laboratory using this method is 0.125 mg/liter). The 5FC MIC using CLSI methodology (12) was 0.125 mg/liter, and the published MIC90 using this methodology is 1 mg/liter (13).
TABLE 1.
Techniques, assumptions, and pertinent issues when bridging from in vivo systems to humans
| Step | Approach and technique(s) | Comments and assumptions |
|---|---|---|
| 1 | Define the experimental dose-response relationships. | The experimental model represents a biologically plausible model which will allow valid inferences to be drawn. |
| 2 | Using a pharmacokinetic model, the dose-response relationships are transformed to the pharmacokinetic/pharmacodynamic ratioa-response relationship. | The MIC serves as a standard measure of potency of the antimicrobial agent for the microbiological target. The pharmacokinetic/pharmacodynamic ratio which best links drug exposure to effect must be determined experimentally. |
| 3 | Define the behavior of a drug within the population of interest. Population pharmacokinetic modeling techniques are used to define both measures of central tendency and the variance of drug handling within a population. | An assumption is made that the original population from which the population model is developed is representative of a larger population of interest. |
| 4 | Monte Carlo simulation is used to reconstruct a much larger population, using the information contained within the population pharmacokinetic model related to measures of central tendency, variance, and covariance. | The extent to which the simulated population is representative of the original population pharmacokinetic model should be determined. A log-normal distribution of the pharmacokinetic parameters is usually used in the simulation process. |
| 5 | Calculate the predicted effect of antimicrobial therapy for each simulated patient, using the transformed exposure-response relationship derived in step 2. The effect of human pharmacokinetic variability on the extent of microbiological kill can then be estimated. | The bridging process makes the assumption that the microbiological target (i.e., the organism in question) in the experimental model is the same as in humans. |
| 6 | Derive an expectation of target attainment. The proportion of simulated patients that attain a predefined endpoint can be defined. | The predicted effect can be used to further guide rational dosing strategies, as a basis for clinical trial design, and to generate further hypotheses of anti-infective therapy. |
Depending on the drug, this may be the AUC/MIC ratio, Cmax/MIC ratio, or %TMIC.
To bridge the results from experimental models to humans, drug exposure must be transformed from a measure quantified with respect to the host (i.e., dose) to one made with respect to the common microbiological target; the latter is achieved by using the pharmacokinetic/pharmacodynamic ratio that best links drug exposure to the observed effect (Table 1). In this process, the MIC serves as a measure of antifungal drug potency for the microbiological target for both the experimental system and simulated humans. In the case of amphotericin B, we used the area under the concentration-time curve (AUC)/MIC ratio as the dynamically linked variable. While we could have used the maximum concentration of drug in serum (Cmax)/MIC ratio (1, 14), in our experimental model, amphotericin B was administered only once, thus ensuring complete colinearity between the Cmax/MIC and AUC/MIC ratios (10). The administration of more than one dose (as occurred in the simulations [described below]) may potentially lead to a degree of dissociation between the Cmax/MIC and AUC/MIC ratios; consequently, we may have induced a degree of bias if the former is truly linked with outcome. For 5FC, the percentage of time above the MIC (%TMIC) was employed as the dynamically linked variable, as previously described (2).
Nath et al. (11) described the population pharmacokinetics of amphotericin B in children. The pharmacokinetic parameters from this model were scaled and applied to a 70-kg human. In the case of 5FC, the model of Ette et al. was used (5).
For the Monte Carlo simulations, the mean pharmacokinetic parameter values were embedded within ADAPT II (4). The simulations suggested that 25 mg/kg/day administered in four divided dosages resulted in a %TMIC of 100% for all patients—this was the case for isolates with MICs of ≤1 mg/liter. Notably, this dosage is significantly less than the currently recommended 100 to 150 mg/kg/day. Subsequently, the AUC from 0 to 24 h (AUC0-24)/MIC ratio at steady state for 800 simulated patients receiving amphotericin B at 0.1, 0.3, and 0.6 mg/kg/day was also determined.
The mean parameter estimates from the drug interaction model were inserted into ADAPT II. The AUC0-24/MIC and %TMIC values (for amphotericin B and 5FC, respectively) at steady state that developed following the administration of the drugs alone and in combination to each of the 800 simulated patients were calculated and then inserted into the Greco equation. The residual fungal burden in each individual was calculated, and the overall effect for the simulated population was determined.
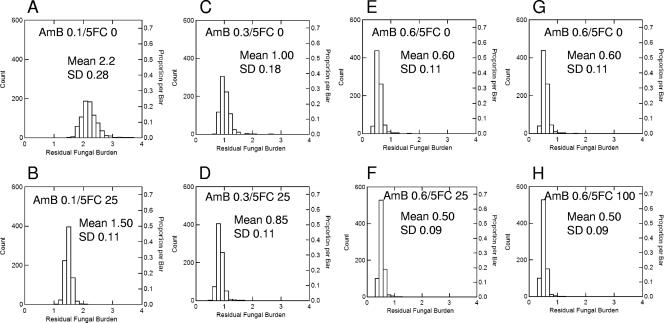
Amphotericin B at 0.1, 0.3, and 0.6 mg/kg/day administered as monotherapy resulted in mean (± standard deviation) residual fungal burdens of 2.2 ± 0.28, 1.0 ± 0.18, and 0.6 ± 0.11 log10 CFU/g, respectively (Fig. 1A, C, and E). The concomitant administration of 5FC at 25 mg/kg in four divided dosages with amphotericin B resulted in a small additional fungal kill relative to that observed with amphotericin B alone (Fig. 1B, D, and F). This additional fungicidal effect was largest for the combination of amphotericin B at 0.1 mg/kg with 5FC at 25 mg/kg (i.e., the decline was 0.7 log10 CFU/g); for amphotericin B at 0.3 and 0.6 mg/kg, the additional effect was smaller (0.15 and 0.1 log10 CFU/g, respectively); this was because these higher dosages of amphotericin B had induced a near-maximal reduction in fungal burden.
FIG. 1.
Residual fungal burden from the Monte Carlo simulations following the administration of amphotericin B alone at 0.1, 0.3, and 0.6 mg/kg (A, C, and E) and the additional fungicidal effect induced by combination with 25 mg/kg 5FC (B and D) and 100 mg/kg 5FC (E). The addition of 5FC at 25 mg/kg to amphotericin B at 0.1, 0.3, and 0.6 mg/kg resulted in further declines in fungal burden of 0.7, 0.15, and 0.1 log10 CFU/g, respectively (B, D, and F). The effect induced by the addition of 5FC becomes progressively smaller with higher dosages of amphotericin B with the induction of near-maximal effect. The use of 5FC at 100 mg/kg did not result in a further decline in fungal burden to that observed with 5FC at 25 mg/kg because %TMIC is 100% for both regimens (G and H). SD, standard deviation.
Thus, two conclusions are possible from these simulations: (i) the addition of 5FC to amphotericin B administered in a standard clinical dosage for disseminated candidiasis (i.e., 0.6 mg/kg) results in relatively little additional fungicidal effect since this human dosage results in drug exposures which induce near-maximal reduction in fungal burden; (ii) if 5FC is to be used, dosages in excess of 25 mg/kg/day are not associated with additional fungicidal effect for the vast majority of Candida isolates and may unnecessarily expose patients to the risk of drug-related toxicity.
Therefore, the principal advantage of the bridging process is the ability to generate a number of refined and clinically pertinent hypotheses which are suitable for further study at the bedside. Importantly, the conclusions of this paper do not necessarily apply to isolates of C. albicans which exhibit high-level amphotericin B and/or 5FC resistance, infections within sanctuary sites, and non-Candida yeasts such as Cryptococcus neoformans. The findings of this study should prompt a reevaluation of the dosage of 5FC used in combination with amphotericin B for the treatment of disseminated candidiasis.
Acknowledgments
This study was supported by Valeant Pharmaceuticals and the Fungal Research Trust. W.H. was supported by an unrestricted educational grant from Merck and Co.
Footnotes
Published ahead of print on 6 August 2007.
REFERENCES
- 1.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and M. van Ogtrop. 2000. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine disseminated candidiasis model. Antimicrob. Agents Chemother. 44:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booker, B. M., P. F. Smith, A. Forrest, J. Bullock, P. Kelchlin, S. M. Bhavnani, R. N. Jones, and P. G. Ambrose. 2005. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype Typhi. Antimicrob. Agents Chemother. 49:1775-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles. http://bmsr.esc.edu/.
- 5.Ette, E. I., P. J. Williams, Y. H. Kim, J. R. Lane, M. J. Liu, and E. V. Capparelli. 2003. Model appropriateness and population pharmacokinetic modeling. J. Clin. Pharmacol. 43:610-623. [PubMed] [Google Scholar]
- 6.Francis, P., and T. J. Walsh. 1992. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin. Infect. Dis. 15:1003-1018. [DOI] [PubMed] [Google Scholar]
- 7.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 8.Hope, W. W., M. J. Kruhlak, C. A. Lyman, R. Petraitiene, V. Petraitis, A. Francesconi, M. Kasai, D. Mickiene, T. Sein, J. Peter, A. M. Kelaher, J. E. Hughes, M. P. Cotton, C. J. Cotten, J. Bacher, S. Tripathi, L. Bermudez, T. K. Maugel, P. M. Zerfas, J. R. Wingard, G. L. Drusano, and T. J. Walsh. 2007. Pathogenesis of Aspergillus fumigatus and the kinetics of galactomannan in an in vitro model of early invasive pulmonary aspergillosis: implications for antifungal therapy. J. Infect. Dis. 195:455-466. [DOI] [PubMed] [Google Scholar]
- 9.Hope, W. W., P. A. Warn, A. Sharp, S. Howard, M. Kasai, A. Louie, T. J. Walsh, G. L. Drusano, and D. W. Denning. 2006. Derivation of an in vivo drug exposure breakpoint for flucytosine against Candida albicans and impact of the MIC, growth rate, and resistance genotype on the antifungal effect. Antimicrob. Agents Chemother. 50:3680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hope, W. W., P. A. Warn, A. Sharp, P. Reed, B. Keevil, A. Louie, D. W. Denning, and G. L. Drusano. 2005. Surface response modeling to examine the combination of amphotericin B deoxycholate and 5-fluorocytosine for treatment of invasive candidiasis. J. Infect. Dis. 192:673-680. [DOI] [PubMed] [Google Scholar]
- 11.Nath, C. E., A. J. McLachlan, P. J. Shaw, R. Gunning, and J. W. Earl. 2001. Population pharmacokinetics of amphotericin B in children with malignant diseases. Br. J. Clin. Pharmacol. 52:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 13.Pfaller, M. A., S. A. Messer, L. Boyken, H. Huynh, R. J. Hollis, and D. J. Diekema. 2002. In vitro activities of 5-fluorocytosine against 8,803 clinical isolates of Candida spp.: global assessment of primary resistance using National Committee for Clinical Laboratory Standards susceptibility testing methods. Antimicrob. Agents Chemother. 46:3518-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiederhold, N. P., V. H. Tam, J. Chi, R. A. Prince, D. P. Kontoyiannis, and R. E. Lewis. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]