Abstract
We report on the correlation of the outcomes for two cohorts of patients who had been treated for candidemia (126 episodes) or oropharyngeal candidiasis (110 episodes) with various doses of fluconazole and the MIC of fluconazole obtained by using the EUCAST standard for fermentative yeasts. Of 145 episodes caused by an isolate with a fluconazole MIC ≤2 mg/liter, 93.7% (136 of 145) responded to fluconazole treatment. The response for those infected with a strain with a MIC of 4 mg/liter was 66% but reached 100% when the dose was greater than 100 mg/day, whereas the response for those infected with strains with MICs ≥8 mg/liter was only 12%. Hence, a MIC of 2 mg/liter or 4 mg/liter was able to predict successful treatment. A cure rate of 93.9% (140 of 149) was achieved when the dose/MIC ratio was ≥100 but fell to 14.6% (16 of 109) when the ratio was less. The dose/MIC required to achieve a response rate of 50% (the 50% effective concentration) was 43.7 for the cohort of patients with oropharyngeal candidiasis. Classification and regression analysis indicated that a dose/MIC of 35.5 was the threshold for the prediction of cure or failure. However, an increase in exposure above this threshold further increased the probability of cure, and all patients were cured when the dose/MIC exceeded 100. Monte Carlo simulations showed a probability of target attainment of 99% at MICs ≤2 mg/liter and a pharmacodynamic target of a dose/MIC ratio of 100, which was equivalent to an unbound fraction of the fluconazole area under the curve versus the MIC of 79.
The setting of breakpoints for antimicrobial agents has evolved considerably in recent years. However, the development of breakpoints for antifungals has received less attention than that for antibacterials partly due to the complex nature of invasive fungal diseases and partly due to the lack of a proper standard for susceptibility tests. Publication M27-A2 of the CLSI (formerly the NCCLS) for testing the susceptibilities of yeasts to antifungal agents, including fluconazole, was an important step (24); but the process was not in line with the process for setting breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), which takes into account pharmacokinetic (PK)-pharmacodynamic (PD) data and other factors, such as dosing regimens, toxicology, resistance mechanisms, wild type MIC distributions, and clinical outcome data (15).
The Antifungal Susceptibility Testing Subcommittee (AFST) of EUCAST was charged with applying this process to antifungal drugs and recently achieved the first step in publishing a standard method (29) that differs slightly from that of the CLSI but that results in essentially the same MICs (9, 8, 30). Both methods produce MICs up to 2 mg/liter. Above this value, the CLSI method generates MICs twofold higher than those generated by the EUCAST AFST method (30).
The interpatient variability in population PK parameter estimates has only recently been recognized as a key factor in predicting the outcome for individual patients and establishing breakpoints and targets for clinical susceptibility. Monte Carlo simulation is used to address this (5), as it can be used to determine the probability of target attainment for PD indices by taking the inherent variation within different populations into account (2, 11, 12, 20, 22, 23). Indeed, this statistical technique forms an integral part of the breakpoint-setting process for antibacterials of both the CLSI and the EUCAST. Mouton used Monte Carlo simulation to determine the variations in exposure for fluconazole (21), but the PD target required to attain the breakpoint was not clear. The aim of the current study was to determine the correlation of MICs to the clinical outcomes for patients with candidemia and oropharyngeal candidiasis (OPC) who had been treated with fluconazole and also to determine the PK-PD parameter relation that best predicted this outcome. We then used these data to establish a PD target and subsequently determine the probability of target attainment for fluconazole and the clinical breakpoint for fluconazole by way of proving the concept for establishing the clinical breakpoints of antifungals.
MATERIALS AND METHODS
Patients. (i) Candidemia.
One hundred twenty-six candidemia patients treated with fluconazole were recruited from a population-based surveillance study performed in Barcelona, Spain, during 2002 and 2003 (1). A case was defined by the recovery of any Candida species from blood cultures. A case of candidemia that occurred >30 days after the initial case was considered a new case. Cure was defined by eradication of the candidemia and resolution of the associated signs and symptoms. Failure was defined as persistent candidemia, despite 4 days of fluconazole treatment. The recommended dose of fluconazole for candidemia is 400 mg/day, but the dose was adjusted to 200 mg/day when the creatinine clearance was between 10 and 50 ml/min and to 100 mg/day when the creatinine clearance was <10 ml/min.
(ii) OPC.
One hundred ten patients with human immunodeficiency virus (HIV) infection had been treated in another study with fluconazole for a total of 132 episodes of oral thrush caused by Candida albicans (16). Clinical resolution was defined as the absence of lesions compatible with oral thrush after 10 days of therapy. Mycological cure was not evaluated. All episodes were used to evaluate the clinical outcome, irrespective of the dose of fluconazole given.
Hence, a total of 258 episodes of Candida infection were available for analysis.
Antifungal susceptibility testing.
All isolates were sent to the Mycology Reference Laboratory, National Centre for Microbiology, Madrid, Spain, for species confirmation and antifungal susceptibility testing. The MICs of fluconazole for each of the isolates were determined by the standard of the AFST of EUCAST for fermentative yeasts (AFST-EUCAST discussion document 7.1 [29]). Briefly, fluconazole was distributed in RPMI supplemented with 2% glucose in flat-bottomed microtitration trays which were inoculated with 105 CFU/ml of yeast, incubated at 35°C, and then read after 24 h at 530 nm by use of a spectrophotometer. The end point was defined as the concentration that resulted in 50% inhibition of growth compared with the growth in the control well. Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019 were included for quality control. These results were not available to any of the participants until the end of the clinical studies.
Statistical analysis.
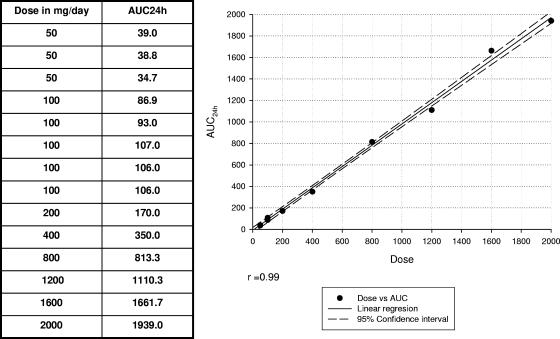
When required, data were transformed to log2 or log10 to approximate a normal distribution. The area under the curve (AUC) at 24 h (AUC24) and the corresponding dose were obtained from selected references (6, 10, 13, 14, 19, 26). Linear regression analysis was undertaken to determine the correlation coefficient and the relationship between the dose administered to each patient and the corresponding AUC24.
This enabled us to calculate the exact equivalencies between dose/MIC, AUC/MIC, and the unbound fraction (f) of fluconazole AUC versus the MIC (fAUC/MIC) by considering an unbound fraction of 88%. All these parameters were correlated with the outcomes for the two patient cohorts (Fig. 1; see also Table 3) (6, 10, 13, 14, 19, 26).
FIG. 1.
Linear regression of dose versus AUC24. Data were obtained from previous reports (6, 10, 13, 14, 19, 26).
TABLE 3.
Correlation of dose/MIC ratios and their conversion to AUC/MICa and fAUC/MICb ratios versus outcomes for patients with candidemia or OPC for EUCAST discussion document 7.1
| Dose/MIC | AUC/MIC | fAUC/MIC | Range of doses (mg/day) administered | Range of MICs (mg/liter) of isolates | % Clinical success (no. cured/total no.) in patients with:
|
||
|---|---|---|---|---|---|---|---|
| Candidemia | OPC | All | |||||
| 400-4,800 | 359-4,678 | 316-4,117 | 100-800 | 0.12-1.0 | 92 (102/111) | 100 (5/5) | 92 (107/116) |
| 150-200 | 146-189 | 129-166 | 100-600 | 0.5-4.0 | 100 (3/3) | 100 (21/21) | 100 (24/24) |
| 100 | 90 | 79 | 100-800 | 1.0-8.0 | 100 (4/4) | 100 (5/5) | 100 (9/9) |
| 50 | 45 | 40 | 100-400 | 2.0-8.0 | 33 (1/3) | 57 (4/7) | 50 (5/10) |
| 25 | 22 | 20 | 100-200 | 4.0-16.0 | 50 (1/2) | 21 (8/38) | 22.5 (9/40) |
| 12.5 | 11 | 10 | 100-400 | 8.0-32.0 | 100 (1/1) | 0 (0/37) | 2.6 (1/38) |
| ≤6.25 | 6 | 5 | 200 | 32.0 | 50 (1/2) | 0 (0/19) | 4.7 (1/21) |
The AUC/MIC values were obtained after application of the following equation: AUC = 0.99 × dose − 9.2.
fAUC/MIC was calculated by taking into consideration that the f of fluconazole is 88%.
A sigmoidal dose-response curve (maximum-effect model) with variable slope (Prism software, version 3.0; Graphpad Inc., San Diego, CA) was fitted to the outcome data for the OPC patients. The goodness of fit of the curve was judged by the R2 value. R2 is a fraction between 0.0 and 1.0 and has no units. Higher values indicate that the curve comes closer to the data. The dose/MIC that provokes a response halfway between the baseline and the maximum effect is called the 50% effective concentration (EC50). Logistic regression analysis was performed with SAS software (SAS, Cary, NC), and classification and regression tree (CART) analysis was undertaken by using WEKA software (32). Briefly, the program determines the optimal CART analysis-derived value that discriminates between patients with a higher likelihood of success and those with a likelihood of a poor outcome. Since failures may still occur when the values are above this value, we also determined the value that distinguished between those who had an almost 100% probability of cure and those who had a lower probability of cure.
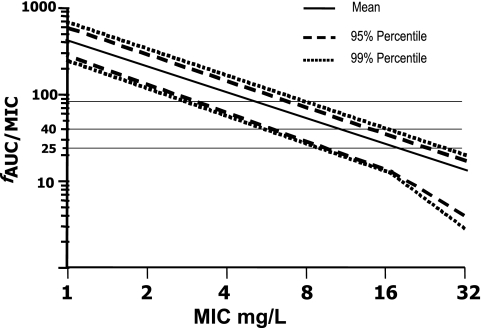
Monte Carlo simulations were performed by using the MicLab program (version 2.35; Medimatics, Maastricht, The Netherlands), as described earlier (22, 23). Briefly, a dosing regimen of 400 mg/day was simulated by using a population of 10,000 subjects and a lognormal distribution of pharmacokinetic parameters, assuming a volume of distribution of 45 liters (coefficient of variation [CV], 12%) and a half-life of 32 h (CV, 15%) of fluconazole. f was assumed to be 0.88 and was not varied (6, 10, 13, 14, 19, 26). The output consisted of a probability distribution, a cumulative probability distribution, and selected confidence intervals for the fAUC/MIC ratios. The fAUC/MIC probability distribution was determined for MICs of 1 to 32 mg/liter (see Fig. 3).
FIG. 3.
Monte Carlo simulation for a target attainment of 400 mg/day of fluconazole with the following pharmacokinetic data: volume of distribution of 45 liters (CV, 12%), a half-life of 32 h (CV, 15%), and an f of 88%. The horizontal lines indicate fAUC/MICs of 79, 40, and 25, respectively.
RESULTS
Patients.
The cohort with oral thrush has been described previously (16). Briefly, these were HIV-positive patients with OPC due to C. albicans.
The demographics and clinical data for the candidemia patients were as follows. Eighty-one patients (64.3%) were males, and 45 (35.7%) were females. Their ages ranged from 16 to 90 years, with a mean ± standard deviation age of 62.1 ± 15.8 years. Forty-seven patients (37.3%) had cancer (34 with solid cancers, 7 with lymphoma, 4 with leukemia, and 2 with multiple myeloma), 6 had HIV infection, 27 (21.4%) had diabetes, and 71 (56.3%) had undergone surgery in the 3 months before they developed candidemia. Four patients had received a solid organ transplant, and three had received a hematopoietic stem cell transplant. Only nine patients (7.1%) were neutropenic. These patients experienced a total of 126 (40.5%) episodes of candidemia. The mortality rate was 30.8%, but death was attributed to candidemia in only 6.9% of the cases.
Microorganisms.
Table 1 shows the etiologic agents of candidemia and mucosal candidosis. C. albicans was responsible for 79.4% of the episodes.
TABLE 1.
Species causing fungal infections
| Species | No. (%) of species causing:
|
||
|---|---|---|---|
| Candidemia | OPC | All | |
| Candida albicans | 73 | 132 | 205 (79.4) |
| Candida parapsilosis | 27 | 27 (10.4) | |
| Candida tropicalis | 12 | 12 (4.6) | |
| Candida glabrata | 9 | 9 (3.4) | |
| Other yeastsa | 5 | 5 (1.9) | |
| All | 126 (48.8) | 132 (51.2) | 258 |
Other yeasts comprised one isolate each of the following species: C. krusei, C. guilliermondii, C. kefyr, C. dubliniensis, and Geotrichum capitatum.
Doses.
Sixty-five episodes of OPC were treated with 100 mg/day fluconazole, 44 with a dose of 200 mg/day, and 23 with 400 mg/day. Four episodes of candidemia were treated with 100 mg/day, 25 with 200 mg/day, 92 with 400 mg/day, 2 with 600, and 3 with 800 mg/day. The doses were considered to be equivalent to the AUC, even though some patients had renal dysfunction.
Exposure-response analysis.
Overall, 93.7% (136 of 145 episodes) of the infections due to isolates with fluconazole MICs ≤2 mg/liter responded to fluconazole treatment (Table 2). A response of 66% (8 of 12 episodes) was observed when the infections were caused by isolates with MICs of 4 mg/liter, and a response of 11.8% (12 of 101 episodes) was observed when the infection was caused by isolates with fluconazole MICs ≥8 mg/liter. However, when the dose administered was taken into consideration, 93.4% (114 of 122) of the patients infected with isolates with fluconazole MICs of ≤4 mg/liter responded to >100 mg/day fluconazole, whereas only 17.9% (12 of 67 episodes) of those episodes caused by strains with fluconazole MICs ≥8 mg/liter were cured.
TABLE 2.
Correlation of MIC data with fluconazole treatment adjusted by dose administered
| MIC (mg/liter) | % Clinical success (no. cured/total no.) for the following doses of fluconazole and patients with the indicated conditions:
|
||||||
|---|---|---|---|---|---|---|---|
| 100 mg/day
|
>100 mg/day
|
All doses
|
All cases | ||||
| Candidemia | OPC | Candidemia | OPC | Candidemia | OPC | ||
| ≤0.5 | 75 (3/4) | 100 (21/21) | 92 (95/103) | 100 (5/5) | 91 (98/107) | 100 (26/26) | 93 (124/133) |
| 1 | 100 (4/4) | 100 (6/6) | 100 (6/6) | 100 (4/4) | 100 (10/10) | ||
| 2 | 100 (1/1) | 100 (1/1) | 100 (1/1) | 100 (1/1) | 100 (2/2) | ||
| 4 | 20 (1/5) | 100 (3/3) | 100 (4/4) | 100 (3/3) | 69 (5/9) | 66 (8/12) | |
| 8 | 0 (0/15) | 40 (2/5) | 41 (7/17) | 40 (2/5) | 26 (7/32) | 24 (9/37) | |
| ≥16 | 0 (0/19) | 75 (3/4) | 0 (0/41) | 75 (3/4) | 2 (0/60) | 4 (3/64) | |
The results were similar when the data were analyzed according to the disease entity (Table 2).
In total, 93.9% (140 of 149) of the patients were cured when the dose/MIC was ≥100, whereas when the dose/MIC was a lower value, only 14.6% (16 of 109) of the patients were cured. For a dose/MIC of 50, 50% (5 of 10 episodes) (Table 3) were cured. However, the response rate for a dose/MIC of ≥50 was 91.2% (145 of 159).
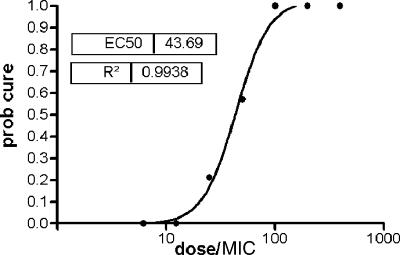
The number of episodes of OPC and the comparable numbers of successes and failures in this group made a separate analysis possible. The relationship between the dose/MIC and the probability of cure showed that all patients were cured when the dose/MIC was ≥100 and all failed when it was ≤10 (Fig. 2). An EC50 of 43.7 (95% confidence interval, 33.8 to 56.6) was estimated, and CART analysis indicated that 35.5 was the value that best separated the groups into those who were cured and those who were not. However, a number of failures occurred when the dose/MIC was greater than 35.5, although all patients were cured when the dose/MIC exceeded 100.
FIG. 2.
Probability (prob) of cure after treatment of 132 episodes of oropharyngeal candidiasis with fluconazole.
Monte Carlo simulations were performed by use of a 400-mg dose of fluconazole per day. The formula AUC = 0.99 × dose − 9.2 was used to calculate the corresponding AUC/MIC and fAUC/MIC of the dose/MICs obtained in this work (Fig. 2 and Table 3). These Monte Carlo simulations showed a probability of target attainment of 99% at MICs ≤2 mg/liter and a dose/MIC ratio of 100 (equivalent to fAUC/MIC of 79) to be the optimum PD target. For MICs ≤4 mg/liter, a PD target of 50 (equivalent to a fAUC/MIC of 40) would be attained with a 99% probability.
DISCUSSION
The results presented here showed that there was a correlation between the outcome and the results of antifungal susceptibility testing, as others have found previously (7, 17, 27, 28, 31). However, as 79.4% of the isolates were C. albicans, the results should be interpreted with this limitation in mind.
More than 90% of the patients responded to fluconazole when the isolate had a fluconazole MIC ≤2 mg/liter. For those isolates with fluconazole MICs of 4 mg/liter the response was 66%, although this reached 100% when >100 mg/day fluconazole was given. Only 11.8% (12 of 101) patients responded when the fluconazole MIC was ≥8 mg/liter. However, since few such isolates were responsible for candidemia, the data from this population were useful only as support of the results obtained for patients with OPC. In fact, an MIC of either 2 mg/liter or 4 mg/liter could be used to discriminate the success and the failure of fluconazole treatment.
The PDs of fluconazole for Candida infections have been investigated and have demonstrated that there is a correlation between the dose, the MIC of the yeast, and outcome (4, 18, 19). AUC divided by MIC, i.e., AUC/MIC, is the PD parameter that best predicts efficacy (4, 18, 19). However, the dose of fluconazole can be used as a surrogate for AUC, as the dose and AUC have been shown to be virtually equivalent (19) (Fig. 1). However, Monte Carlo simulations were performed for the fAUC/MIC of a 400-mg/day dose. Therefore, the interpretation of the data in the same units would make the exercise straightforward (Table 3). Thus, the AUC24 and the corresponding dose were obtained from selected references (6, 10, 13, 14, 19, 26). A linear regression was calculated, and then the equation obtained was used to calculate with accuracy of the equivalencies between the dose and the AUC. Then, fAUC/MIC was calculated by assuming that f was 88% (6, 10, 13, 14, 19, 26).
A dose/MIC of ≥50 achieved a response rate above 90% (145 of 159 patients) (Table 3). However, 5 of the 10 cases for which the dose/MIC was exactly 50 failed treatment (Table 3). This represents a high failure rate, albeit for a small number of patients. The probability of cure was shown to be a function of the dose/MIC and the EC50 was 43.7, but nonetheless, a dose/MIC just short of 100 was required to achieve a probability of cure of 90% (Fig. 2). In murine models of systemic candidosis, the AUC24/MIC required to achieve 50% of the maximum effect varied from 25 to 44 (4, 18), although an AUC/MIC of ≥500 was required to attain a 2-log10 reduction in the numbers of CFU (4). Preventing the emergence of resistance among Candida isolates is related to the time that the yeast populations are exposed to sub-MICs, which is reflected by the time at which the concentration remains greater than the MIC and AUC24/MIC. Since an AUC24/MIC of <32 has been associated with increases in the levels of the CDR1 transcripts of C. albicans (3, 4), high dose/MICs would be expected to increase the response to fluconazole and help prevent the emergence of resistance.
Pfaller et al. (27) did a similar analysis using the MICs obtained by the CLSI M27-A2 methodology and showed that a dose/MIC ≥25 corresponded to a response rate of 97% (308 of 318) for patients with mucosal candidiasis. The response rate fell to 70% (65 of 93) when the ratio was <25. By contrast, in our population, only 56.5% patients (43 of 76) with OPC responded when the dose/MIC was ≥25 (Table 3). The results for patients with candidemia shown by Pfaller et al. were different (27), with a response rate of 88% when dose/MIC was ≥400. For dose/MICs below this, the response fell to 66% (196 of 297); and those for a dose/MICs ≥25, ≥50, or ≥100 were between 70 and 75%. Overall, the response rate for a dose/MIC of ≥25 was 85% (489 of 575 episodes), but this declined to 61% when the dose/MIC was less than 25 (27). Only when the dose/MIC was ≥50 was a response rate that exceeded 90% achieved. In the present study, the overall response rates were 77% (154 of 199 episodes) for a dose/MIC of ≥25 and 91% (145 of 159 episodes) for a dose/MIC of ≥50 (Table 3).
Recently, Pai et al. (25) correlated the fluconazole dose/MIC and AUC/MIC with mortality in nonneutropenic patients with candidemia. However, they used weight-normalized doses (dosewn) instead of the dose in mg/day, making direct comparison of their results and those of this study difficult. Nevertheless, their results showed that the rate of mortality was significantly lower for those patients when the dosewn/MICs were 13.3 ± 10.5. Thus, for an isolate with a fluconazole MIC of 1 mg/liter and a patient weighing 70 kg, the dose/MICs would have ranged from 196 to 1,666. CART analysis showed a breakpoint of 12.0 for dosewn/MIC, so the estimated dose/MIC would be 840.
For these forms of candidiasis and these definitions of response, a goal of 90% success seems appropriate and the dose/MIC target for EUCAST would thus be between 50 and 100. However, we support a higher dose/MIC of 100, given the failure rate in our series for a dose/MIC of 50.
Moreover, given that dose/MIC and fAUC/MIC are equivalent, an fAUC/MIC of 100 would provide therapeutic coverage to 99% of the population infected with a Candida isolate with a MIC of ≤2 mg/liter (Fig. 3). Given that a dose of 400 mg/day would yield an AUC of about 386 mg · h/liter (Fig. 2) and that this is essentially equivalent to an fAUC of 340 mg · h/liter for an f of 88%, this would yield an fAUC/MIC in excess of 85 for all isolates with MICs ≤4 mg/liter. By extension, the MIC target would have to be less than 4 mg/liter to cover 99% of the infected population (Fig. 3). This is in contrast to the dose/MIC of 25 proposed by the CLSI, as this value would cover 99% of the population infected with isolates with MICs of ≤8 mg/liter, which is their breakpoint of susceptibility (Fig. 3). This disparity might be explained by the differences between the EUCAST and the CLSI targets, since the use of EUCAST discussion document 7.1 and CLSI publication M27-A2 results in equivalent MICs up to 2 mg/liter. Above this value, the CLSI method generates MICs twofold higher than those generated by the EUCAST AFST method (30). Moreover, the definitions of success and failure used are not identical, frustrating the ability to readily compare the results.
In summary, the MICs of fluconazole obtained by using the method described in EUCAST discussion document 7.1 correlated with the clinical outcomes of candidemia and OPC. This makes it possible to develop breakpoints for fluconazole in line with the guidelines adopted by EUCAST.
Acknowledgments
This work has been supported in part by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III; the Spanish Network of Infection in Transplantation (RESITRA G03/075); and the Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008).
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Almirante, B., D. Rodriguez, B. J. Park, M. Cuenca-Estrella, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, P. Saballs, S. K. Fridkin, J. Morgan, J. L. Rodriguez-Tudela, D. W. Warnock, and A. Pahissa. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., A. Forrest, A. Lepak, J. Nett, K. Marchillo, and L. Lincoln. 2006. Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob. Agents Chemother. 50:2374-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonate, P. L. 2001. A brief introduction to Monte Carlo simulation. Clin. Pharmacokinet. 40:15-22. [DOI] [PubMed] [Google Scholar]
- 6.Brammer, K. W., P. R. Farrow, and J. K. Faulkner. 1990. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 12(Suppl. 3):S318-S326. [DOI] [PubMed] [Google Scholar]
- 7.Clancy, C. J., V. L. Yu, A. J. Morris, D. R. Snydman, and M. H. Nguyen. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Influence of glucose supplementation and inoculum size on growth kinetics and antifungal susceptibility testing of Candida spp. J. Clin. Microbiol. 39:525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuenca-Estrella, M., W. Lee-Yang, M. A. Ciblak, B. A. Arthington-Skaggs, E. Mellado, D. W. Warnock, and J. L. Rodriguez-Tudela. 2002. Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of Candida species. Antimicrob. Agents Chemother. 46:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debruyne, D., and J. P. Ryckelynck. 1993. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 24:10-27. [DOI] [PubMed] [Google Scholar]
- 11.Drusano, G. L., D. Z. D'Argenio, S. L. Preston, C. Barone, W. Symonds, S. Lafon, M. Rogers, W. Prince, A. Bye, and J. A. Bilello. 2000. Use of drug effect interaction modeling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir. Antimicrob. Agents Chemother. 44:1655-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goa, K. L., and L. B. Barradell. 1995. Fluconazole. An update of its pharmacodynamic and pharmacokinetic properties and therapeutic use in major superficial and systemic mycoses in immunocompromised patients. Drugs 50:658-690. [DOI] [PubMed] [Google Scholar]
- 14.Grant, S. M., and S. P. Clissold. 1990. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 39:877-916. [DOI] [PubMed] [Google Scholar]
- 15.Kahlmeter, G., D. F. J. Brown, F. W. Goldstein, A. P. MacGowan, J. W. Mouton, A. Osterlund, A. Rodloff, M. Steinbakk, P. Urbaskova, and A. Vatopoulos. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 52:145-148. [DOI] [PubMed] [Google Scholar]
- 16.Laguna, F., J. L. Rodriguez-Tudela, J. V. Martinez-Suarez, R. Polo, E. Valencia, T. M. Diaz-Guerra, F. Dronda, and F. Pulido. 1997. Patterns of fluconazole susceptibility in isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis due to Candida albicans. Clin. Infect. Dis. 24:124-130. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, H. Y. Chen, N. Lee, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, P. Kaw, M. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie, A., Q. F. Liu, G. L. Drusano, W. Liu, M. Mayers, E. Anaissie, and M. H. Miller. 1998. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob. Agents Chemother. 42:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery, M. J., P. M. Beringer, A. Aminimanizani, S. G. Louie, B. J. Shapiro, R. Jelliffe, and M. A. Gill. 2001. Population pharmacokinetics and use of Monte Carlo simulation to evaluate currently recommended dosing regimens of ciprofloxacin in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:3468-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouton, J. W. 2005. Antifungals and children: pharmacokinetic and pharmacodynamics issues. Trends in medical mycology, 2nd ed. Blackwell Publishing Ltd., Berlin, Germany.
- 22.Mouton, J. W., N. Punt, and A. A. Vinks. 2005. A retrospective analysis using Monte Carlo simulation to evaluate recommended ceftazidime dosing regimens in healthy volunteers, patients with cystic fibrosis, and patients in the intensive care unit. Clin. Ther. 27:762-772. [DOI] [PubMed] [Google Scholar]
- 23.Mouton, J. W., A. Schmitt-Hoffmann, S. Shapiro, N. Nashed, and N. C. Punt. 2004. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48:1713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 25.Pai, M. P., R. S. Turpin, and K. W. Garey. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry, C. M., R. Whittington, and D. McTavish. 1995. Fluconazole. An update of its antimicrobial activity, pharmacokinetic properties, and therapeutic use in vaginal candidiasis. Drugs 49:984-1006. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Tudela, J. L., F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, D. Denning, J. P. Donnelly, B. Dupont, W. Fegeler, C. Moore, M. Richardson, P. E. Verweij, and Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 9:I-VIII. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Tudela, J. L., J. P. Donnelly, M. A. Pfaller, E. Chryssantou, P. Warn, D. W. Denning, A. Espinel-Ingroff, F. Barchiesi, and M. Cuenca-Estrella. 2007. Statistical analyses of correlation between fluconazole MICs for Candida spp. assessed by standard methods set forth by the European Committee on Antimicrobial Susceptibility Testing (E.Dis. 7.1) and CLSI (M27-A2). J. Clin. Microbiol. 45:109-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takakura, S., N. Fujihara, T. Saito, T. Kudo, Y. Iinuma, and S. Ichiyama. 2004. Clinical factors associated with fluconazole resistance and short-term survival in patients with Candida bloodstream infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:380-388. [DOI] [PubMed] [Google Scholar]
- 32.Witten, I. H., and E. Frank. 2005. Data mining: practical machine learning tools and techniques. Morgan Kaufmann, San Francisco, CA.