Abstract
Although a growing number of studies have found a relationship between delayed appropriate antibiotic therapy and mortality, few have attempted to quantify the temporal association between delayed appropriate antibiotic therapy and mortality. This study was designed to measure the elapsed time associated with an increased risk of 30-day mortality among patients with Pseudomonas aeruginosa bacteremia. The retrospective cohort study was conducted among immunocompetent, adult patients with P. aeruginosa bacteremia onset at least 2 days after hospital admission between 1 January 2001 and 30 September 2006. Classification and regression tree analysis (CART) was used to identify the delay in appropriate antibiotic therapy that was associated with an increased risk of 30-day mortality. During the study period, 100 patients met the inclusion criteria. The CART-derived breakpoint between early and delayed treatment was 52 h. The delayed treatment group experienced a >2-fold significant increase in 30-day mortality compared to the early treatment group (44 and 19%, respectively, P = 0.008). Delayed appropriate therapy of >52 h (odds ratio [OR] = 4.1; 95% confidence interval [CI] 1.2 to 13.9, P = 0.03) was independently associated with 30-day mortality in the multivariate analysis. Antibiotic resistance ≥3 classes (adjusted OR [AOR] = 4.6; 95% CI = 1.9 to 11.2, P = 0.001) and chronic obstructive pulmonary disease (AOR = 5.4; 95% CI = 1.5 to 19.7, P = 0.01) were independently associated with delayed appropriate therapy of >52 h. The data strongly suggest that delaying appropriate therapy for approximately 2 days significantly increases the risk of 30-day mortality in patients with P. aeruginosa bloodstream infections.
The lifesaving benefits of prompt, appropriate therapy are well documented (1, 4-7, 9, 11-15, 17). The study by Kollef et al. (9) was one of the first to highlight the relationship between inadequate antimicrobial treatment of infection and hospital mortality for patients requiring intensive care unit (ICU) admission. The findings of Kollef et al. have been replicated by other investigations in various clinical settings. Although the literature is still developing, the data suggest that inappropriate empirical antibiotic therapy for those with Pseudomonas aeruginosa bacteremia is a key determinant of mortality risk (1, 6, 15-17).
A growing number of studies have found a relationship between delayed appropriate therapy and mortality (1, 4-7, 9, 11-15, 17), but many differentiating factors remain unexplored. Previous studies have primarily examined the appropriateness of the first antibiotic regimen, the adequacy of therapy at the time of microbiologic identification and antibiotic susceptibility profile reporting, or the selected predefined time to appropriate therapy windows (24 h, 48 h, etc.) (1, 4-7, 9, 14-17). To date, few researchers have attempted to measure the elapsed time before appropriate antibiotic therapy that is associated with increased mortality (13). Thus, the delay in appropriate antibiotics associated with an increased mortality remains unresolved and largely unknown for many types of infections or organisms, including P. aeruginosa bloodstream infections. The present study quantifies the delay before appropriate therapy associated with an increased risk of 30-day mortality among patients with P. aeruginosa bacteremia.
MATERIALS AND METHODS
Study population.
The present study was performed at the Albany Medical Center Hospital, a 651-bed teaching hospital in Albany, NY. Patients with a positive P. aeruginosa blood culture between 1 January 2001 and 30 September 2006 were included if they were (i) ≥18 years old, (ii) non-neutropenic (absolute neutrophil count of ≥1,000 cells/mm3), (ii) the P. aeruginosa bloodstream culture met the Centers for Disease Control and Prevention criteria for infection (3), (iv) infection occurred ≥2 days after hospital admission, and (v) patients did not have cystic fibrosis. We excluded neutropenic patients because we wanted to examine a homogeneous patient population. We excluded patients who developed their P. aeruginosa bloodstream outside of the hospital because it is difficult to accurately note the time between bacteremia onset and initiation of treatment for these patients. If a patient had more than one episode of P. aeruginosa during a hospitalization, only the first episode was considered.
Study design.
A retrospective cohort analysis was performed to evaluate the effect of delayed treatment on 30-day mortality secondary to P. aeruginosa bacteremia. Classification and regression tree analysis (CART) was used to identify the time delay in appropriate antibiotic therapy that was associated with an increased risk of 30-day mortality. Specifically, CART was used to analyze the duration of time that elapsed between the collection of index P. aeruginosa blood culture and the administration of appropriate antibiotic treatment and to identify the temporal breakpoint that maximized the difference in 30-day mortality, thereby dividing the study population into two groups: those with a high likelihood of 30-day mortality (delayed treatment) and those with a low likelihood of 30-day mortality (early treatment) (13, 18). Clinical characteristics associated with the delayed treatment group selected by CART were also examined to identify potentially modifiable predictors of delayed therapy.
Data.
The following data were obtained for each patient from the medical record by trained medical abstractors: age, sex, comorbidities, healthcare contact within 180 days of admission, length of hospitalization for the index admission prior to collection of P. aeruginosa blood culture (total and ICU, when applicable) hospital unit at P. aeruginosa blood culture collection (ICU versus non-ICU), mechanical ventilation at collection of P. aeruginosa blood culture, severity of illness prior to P. aeruginosa blood culture (as calculated by the acute physiology and chronic health evaluation [APACHE-II] score) (8), microbiologic data, and treatment data.
The following comorbid conditions were documented: chronic obstructive pulmonary disease (COPD), diabetes mellitus, heart failure (New York Heart Association classes II to IV), hepatic dysfunction, renal failure (as indicated by the necessity for dialysis), and presence of decubitus ulcers (stages II to IV).
Prior healthcare exposure was defined as any healthcare institution exposure for >72 h in the 6 months prior to index hospitalization. The APACHE-II score was calculated using the highest values in the 24 h prior to collection of the P. aeruginosa blood culture (8).
Microbiologic data.
Microbiologic data included the date, time, and susceptibilities for all positive P. aeruginosa cultures. Susceptibility testing was done by the Kirby-Bauer method and interpreted according to Clinical and Laboratory Standards Institute guidelines (2). Intermediate results were considered resistant.
For the purpose of this analysis, each of the following represents an antipseudomonal drug class: piperacillin-tazobactam, cefepime, imipenem/meropenem, ciprofloxacin, tobramycin, and amikacin. It should be noted that several formulary changes occurred during the study period. For example, meropenem was replaced with imipenem in February 2004 during a supply shortage, and then the hospital switched back to meropenem when the drug supply stabilized a year later. Susceptibility was based on the formulary agent from each respective class at the time of P. aeruginosa culture collection.
Treatment data.
All antimicrobials administered were noted, including the date, time, dose, route, and duration. Culture-to-antibiotic time was measured in hours and represented the difference in time between collection of the first positive P. aeruginosa blood culture and appropriate antibiotic administration.
Two factors determined the appropriateness of treatment: susceptibility and timeliness. If a patient received at least one intravenous antibiotic to which the P. aeruginosa blood isolate was susceptible within the CART-defined early time period, it was considered appropriate early treatment. An example of “delayed treatment” would be a patient with a piperacillin-tazobactam resistant, imipenem-susceptible P. aeruginosa bacteremia who was initially treated with piperacillin-tazobactam but did not receive imipenem until some time after the CART-defined breakpoint. Monotherapy with an aminoglycoside was not considered appropriate therapy. Concomitant therapy with an aminoglycoside or fluoroquinolone was deemed “combination therapy” if both agents (i) had activity against the recovered P. aeruginosa bloodstream isolate, (ii) were provided within the CART-derived breakpoint, and (iii) were administered for >24 h. We further stratified the data to evaluate the impact of time to appropriate therapy within the CART-derived breakpoint (e.g., <12 h, 13 to 24 h, and 25 to 52 h).
Outcomes assessment.
The primary outcome measure was 30-day in-hospital mortality after the index P. aeruginosa blood culture collection. Due to the retrospective nature of the study, we did not attempt to determine whether 30-day mortality was attributable to the P. aeruginosa bloodstream infection. We believed this allowed for an objective assessment of the 30-day mortality endpoint.
Data analysis plan.
Categorical variables were compared by the Pearson χ2 or Fisher exact test, and continuous variables were compared by the Student t or Mann-Whitney U tests. We used CART software (Salford Systems, San Diego, CA) to identify the time to appropriate therapy breakpoint. The CART time point was identified by using the goodness-of-split statistic (18). The c-statistic, representing the area under the receiving operating characteristic (ROC) curve, was used to assess calibration and discrimination of the CART-derived hour breakpoint compared to other time to appropriate therapy breakpoints before and after the CART-derived hour breakpoint. A higher c-statistic (area under the ROC curve) represents a more favorable discriminator (10).
Logistic regression was used to identify variables independently associated with 30-day mortality. All variables associated with 30-day mortality in the bivariate analysis (P ≤ 0.1) were included at model entry, and a stepwise approach was used to identify independent predictors of 30-day mortality. Variables were retained in the final model if the P value was ≤0.05. All calculations were performed with SPSS version 11.5 (SPSS, Chicago, IL) and CART software (Salford Systems).
RESULTS
During the study period, 100 patients met the inclusion criteria, and the clinical characteristics for this population are presented in Table 1. Of the 100 patients, 31 died within 30 days of culture collection. Overall, the antibiotic susceptibility was <70% for aztreonam, ciprofloxacin, meropenem/imipenem, and piperacillin-tazobactam; amikacin was the only agent with >90% susceptibility (Table 2). Of the 100 cases, 41 were fully susceptible to all agents studied. Over half were resistant to at least two antipseudomonal classes, 19% were resistant to at least five drugs, and two cases were resistant to all seven agents studied (Table 2).
TABLE 1.
Baseline patient characteristics
| Baseline characteristica | Valueb |
|---|---|
| Mean age in yr (SD) | 57.8 (17.9) |
| Male subjects | 55 (55) |
| Subjects with prior healthcare exposure within 180 days of admission | 62 (62.0) |
| Median length of stay in days prior to culture collection (IQR) | 17.5 (5-38) |
| Subjects in ICU at culture collection | 74 (74.0) |
| Median no. of consecutive ICU days prior culture collection (IQR) | 9 (0-31) |
| Subjects on mechanical ventilation at culture collection | 60 (60.0) |
| Median no. of days on consecutive mechanical ventilation days prior to onset (IQR) | 7.5 (0-27) |
| Mean APACHE-II score (SD) | 17.1 (7.9) |
| Subjects with diabetes mellitus | 35 (35.0) |
| Subjects with heart failure | 22 (22.0) |
| Subjects with chronic obstructive pulmonary disease | 18 (18.0) |
| Subjects with hepatic dysfunction | 11 (11.0) |
| Subjects on dialysis | 22 (22.0) |
| Subjects with decubitus ulcers | 22 (22.0) |
IQR, interquartile range.
n = 100. All data are presented as the number of subjects (with the percentage in parentheses) unless otherwise noted in column 1.
TABLE 2.
Overall antibiotic susceptibility and distribution of cross-resistance (number of resistant classes) among P. aeruginosa bloodstream infections
| Variable | Overall resistancea (n = 100) |
|---|---|
| Antibiotic | |
| Amikacin | 8 (8.0) |
| Aztreonam | 37 (37.0) |
| Cefepime | 20 (20.0) |
| Ciprofloxacin | 50 (50.0) |
| Meropenem or imipenem | 34 (34.0) |
| Piperacillin-tazobactam | 39 (39.0) |
| Tobramycin | 33 (33.0) |
| No. of resistant classes among P. aeruginosa bloodstream infections | |
| No agents (fully susceptible) | 41 (41.0) |
| Resistance to one agent | 4 (4.0) |
| Resistance to two agents | 10 (10.0) |
| Resistance to three agents | 14 (14.0) |
| Resistance to four agents | 12 (12.0) |
| Resistance to five agents | 9 (9.0) |
| Resistance to six agents | 8 (8.0) |
| Resistance to seven agents | 2 (2.0) |
All data are presented as the number of subjects (with the percentage in parentheses) unless otherwise noted in column 1.
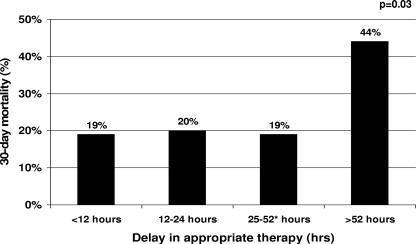
The time breakpoint derived by CART to delineate the risk of 30-day mortality was 52 h; 52 patients (52%) received appropriate treatment within 52 h of index culture collection. Patients with delayed appropriate treatment for >52 h had a >2-fold significant increase in 30-day mortality than patients who received timely appropriate therapy within 52 h (43.8 and 19.2%, respectively; P = 0.008). Examination of the different time delays to appropriate therapy within the 52-h CART-derived breakpoint is shown in Fig. 1. Of the 52 patients, 26 received appropriate antibiotics within 12 h, 5 received appropriate antibiotics between 13 and 24 h after index blood culture collection, and 21 received appropriate antibiotics between 25 and 52 h after index blood culture collection. The 30-day mortality rates were similar for all groups within the 52-h CART-derived breakpoint, and a higher 30-day mortality rate was observed when the length of delay exceeded 52 h (P = 0.03, using linear-by-linear association). Comparison of the c-statistics (areas under the ROC curves) between the CART-derived hour breakpoint and the other time-to-appropriate-therapy hour breakpoints are shown in Table 3. The CART-derived breakpoint of 52 h provided the best discriminating area under the ROC curve and was the only c-statistic that was significantly different from the null hypothesis area of 0.5. The next optimal discriminating c-statistics were all in close proximity to the CART-derived breakpoint of 52 h.
FIG. 1.
Thirty-day mortality stratified by the length of delay in receiving appropriate therapy. *, CART-derived time to appropriate therapy breakpoint.
TABLE 3.
Comparison of area under ROC curves between the CART-derived and other time-to-appropriate-therapy hour breakpoints
| Time-to-appropriate-therapy windows (in h) | Area under ROC curve (c-statistic) | 95% CI | Pa |
|---|---|---|---|
| 0-6 | 0.56 | 0.43-0.67 | 0.4 |
| 0-12 | 0.57 | 0.45-0.69 | 0.3 |
| 0-24 | 0.59 | 0.48-0.71 | 0.2 |
| 0-36 | 0.58 | 0.46-0.70 | 0.2 |
| 0-48 | 0.61 | 0.49-0.73 | 0.09 |
| 0-52b | 0.64 | 0.53-0.76 | 0.02 |
| 0-60 | 0.62 | 0.50-0.74 | 0.06 |
| 0-72 | 0.58 | 0.46-0.70 | 0.2 |
Null hypothesis: true area = 0.5.
CART-derived breakpoint.
Alteration of therapy to an appropriate regimen after the 52-h time point did not impact the outcome. Among the 48 subjects that did not receive appropriate therapy within 52 h of culture, 28 ultimately received appropriate therapy. There was no difference in 30-day mortality among delayed treatment patients that ultimately received appropriate therapy and those that did not receive appropriate therapy (39.3% versus 50.0%, P = 0.5).
The relationship between clinical features and 30-day mortality is shown in Table 4. In the bivariate analysis, length of hospitalization prior to index blood culture collection, mechanical ventilation at index blood culture collection, and APACHE-II score were significantly associated with 30-day mortality. In the logistic regression analysis, each of the following factors were independently associated with 30-day mortality: APACHE-II score (adjusted odds ratio [AOR] = 1.3; 95% confidence interval [CI] = 1.1 to 1.4; P < 0.001), delayed appropriate therapy for >52 h (AOR = 4.1; 95% CI = 1.2 to 13.9; P = 0.03), and presence of decubitus ulcers (AOR = 4.3; 95% CI = 1.1 to 16.5; P = 0.03). The APACHE-II score was modeled continuously, such that the AOR was representative of a one-point increase (Table 5).
TABLE 4.
Bivariate analysis of relationship between clinical features at culture collection and 30-day mortality
| Baseline characteristica | Subjectsb that:
|
P | |
|---|---|---|---|
| Died (n = 31) | Survived (n = 69) | ||
| Mean age in yr (SD) | 62 (15.3) | 56 (18.7) | 0.2 |
| Male subjects | 16 (51.6) | 39 (56.5) | 0.6 |
| Subjects with healthcare exposure within 180 days prior to admission | 22 (71.0) | 41 (59.4) | 0.3 |
| Median length of stay in days prior to culture collection (IQR) | 26 (16-42) | 14 (2-31) | 0.01 |
| Subjects in ICU at onset | 25 (80.6) | 44 (63.8) | 0.09 |
| Median consecutive ICU days prior to culture collection (IQR) | 11 (4-31) | 6 (0-29) | 0.2 |
| Subjects on mechanical ventilation at onset | 25 (80.6) | 35 (50.7) | 0.01 |
| Mean APACHE-II score (SD) | 24 (8.3) | 14 (5.4) | <0.001 |
| Subjects with diabetes mellitus | 11 (35.5) | 24 (34.8) | 0.9 |
| Subjects with congestive heart failure | 8 (25.8) | 14 (20.3) | 0.5 |
| Subjects with chronic obstructive pulmonary disease | 7 (22.6) | 11 (15.9) | 0.4 |
| Subjects with hepatic dysfunction | 4 (12.9) | 7 (10.1) | 0.7 |
| Subjects on dialysis | 9 (29.0) | 13 (18.8) | 0.3 |
| Subjects with decubitus ulcers | 10 (32.2) | 12 (17.4) | 0.1 |
| Subjects in antibiotic resistant classes ≥3 | 17 (54.8) | 28 (40.6) | 0.2 |
| Subjects on delayed appropriate therapy for >52 hc | 21 (67.7) | 27 (39.1) | 0.008 |
IQR, interquartile range.
All data are presented as number of subjects (with the percentage in parentheses) unless indicated otherwise in column 1.
CART-derived breakpoint.
TABLE 5.
Independent predictors of 30-day mortality in the logistic regression analysis
| Predictor of 30-day mortality | AOR | 95% CI | P |
|---|---|---|---|
| Delayed appropriate therapy for >52 h | 4.1 | 1.2-13.9 | 0.03 |
| APACHE-II score | 1.3 | 1.1-1.4 | <0.001 |
| Presence of decubitus ulcers | 4.4 | 1.1-16.5 | 0.03 |
Of the 52 patients that received early appropriate treatment, 23 (44.2%) received β-lactam with an aminoglycoside, and four patients (7.7%) received combination therapy with a β-lactam and fluoroquinolone. Twenty patients (38.5%) received β-lactam monotherapy, and five (9.6%) received fluoroquinolone monotherapy. No significant differences in 30-day mortality were detected among these regimens. Of the 23 patients who received combination therapy with a β-lactam and an aminoglycoside, 5 died. This is comparable to the 5 patients that died among the 20 patients who received β-lactam monotherapy.
The relationship between appropriate therapy within 52 h and number of resistant classes among P. aeruginosa bloodstream infections is shown in Table 6. A significant relationship (P = 0.007) was observed between the number of resistance classes and appropriate therapy within 52 h; the proportion of patients who received delayed appropriate treatment increased as the number of resistance classes increased. Among clinical features, COPD, APACHE-II score, and an antibiotic resistance of ≥3 classes were the only variables significantly associated with delayed appropriate therapy of >52 h in the bivariate analysis. In the logistic regression, antibiotic resistance ≥3 classes (AOR = 4.6; 95% CI = 1.9 to 11.2; P = 0.001) and COPD (AOR = 5.4; 95% CI = 1.5 to 19.7; P = 0.01) were independently associated with delayed appropriate therapy >52 h (Table 7). We performed an additional logistic regression analysis that included both COPD and antibiotic resistance of ≥3 classes at logistic regression model entry and delayed treatment for >52 h was still an independent predictor of 30-day mortality at the same level of significance using both a stepwise and the standard approach to identify independent predictors. Furthermore, COPD and an antibiotic resistance of ≥3 classes were not independent predictors of 30-day mortality in these supplemental logistic regression analyses.
TABLE 6.
Relationship between appropriate therapy within 52 h and number of resistant classes among P. aeruginosa bloodstream infections
| Resistant class among P. aeruginosa bloodstream infections | Appropriate therapya
|
|
|---|---|---|
| Delayed (n = 52) | Early (n = 48) | |
| Zero agents (fully susceptible) | 13 (27.1) | 28 (53.8) |
| Resistance to one agent | 2 (4.2) | 2 (3.8) |
| Resistance to two agents | 3 (6.3) | 7 (13.5) |
| Resistance to three agents | 6 (12.5) | 8 (15.4) |
| Resistance to four agents | 7 (14.6) | 5 (9.6) |
| Resistance to five agents | 8 (16.7) | 1 (1.9) |
| Resistance to six agents | 7 (14.6) | 1 (1.9) |
| Resistance to seven agents | 2 (4.2) | 0 (0) |
All data are presented as the number of subjects (with the percentage in parentheses). P = 0.007 for the number of resistance classes between early and delayed appropriate therapies.
TABLE 7.
Independent predictors of delayed appropriate therapy for >52 h in the logistic regression analysis
| Predictor of 30-day mortality | AOR | 95% CI | P |
|---|---|---|---|
| COPD | 5.4 | 1.5-19.7 | 0.01 |
| Antibiotic resistance of ≥3 classes | 4.6 | 1.9-11.2 | 0.001 |
DISCUSSION
This study sought to identify the critical delay in appropriate therapy associated with an increased risk of 30-day mortality among patients with P. aeruginosa bacteremia. Using CART, patients who experienced delayed appropriate treatment for 52 h or more were at greatest risk for 30-day mortality and had a >2-fold significant increase in 30-day mortality compared to patients who received appropriate therapy within 52 h. Delayed appropriate therapy for 52 h was independently associated with 30-day mortality in the multivariate analysis. Furthermore, examination of the different time delays to appropriate therapy within the 52 h CART-derived breakpoint revealed that 30-day mortality was relatively flat (∼19%) across all groups (Fig. 1), and no additional elevation in 30-day mortality was noted when appropriate therapy was delayed for 12, 24, or 52 h. Lastly, compared to other time-to-appropriate-therapy hour thresholds, the CART-derived 52-h breakpoint had the optimal discriminating c-statistic (area under the ROC curve). Collectively, these data strongly suggest that delaying appropriate therapy for approximately 2 days significantly increased the risk of 30-day mortality in patients with P. aeruginosa bloodstream infections.
Our results are generally consistent with three recent studies that evaluated the relationship between delayed appropriate therapy and mortality among hospitalized patients with P. aeruginosa bloodstream infections (6, 15, 16). In a study by Micek et al. of 365 subjects, inappropriate antibiotic therapy at the time of P. aeruginosa susceptibility reporting was identified as an independent predictor of hospital mortality in a multivariate analysis (15). Considering that microbiologic identification is typically available 2 to 3 days after index blood culture collection, these findings are consistent with the results of our study. Our results differ slightly from a study by Kang et al. of 136 subjects (6). These researchers found that failure to provide effective antimicrobial therapy within 24 h after blood culture samples were obtained was highly predictive of 30-day mortality (AOR = 4.6; 95% CI = 1.2 to 18.1; P = 0.03). There are several plausible explanations for the observed differences. First, these authors included patients who presented to the hospital with bacteremia (both community- and nursing home-acquired cases). We excluded patients who developed their P. aeruginosa infections outside of the hospital because it is difficult to accurately note the time between bacteremia onset and initiation of treatment for these patients. Second, Kang et al. included neutropenic patients, and we excluded this population. Third and most important, Kang et al. did not formally attempt to determine the precise delay associated with an elevation in 30-day mortality. They did, however, note that the 30-day mortality was almost identical between patients who received appropriate therapy within 24 h of blood culture collection (27.7%) and patients who received appropriate therapy 24 to 72 h after blood culture collection (31.3%). Further, Kang et al. also reported that 30-day mortality began to rise once the delay in receiving appropriate antibiotics exceeded 72 h. This finding is highly consistent with the relatively flat 30-mortality among all patient groups within the 52 h CART-derived breakpoint observed and area under ROC curve analysis noted in our study and supports the 2-day time-to-therapy window we describe here.
Our findings also differ slightly by the study by Osih et al. of 167 subjects (16). That study evaluated the effect of inappropriate empirical therapy on mortality for patients with P. aeruginosa bacteremia, where empirical was defined as the therapy received before the receipt of final antibiotic susceptibility testing results. Osih et al. further divided the empirical therapy time window into three periods and evaluated the association between inappropriate therapy in each time period with patient mortality. Although they observed a correlation between increasing mortality and increasing duration of inappropriate empirical therapy, the observation was not statistically significant. In contrast to our study, Osih et al. included patients who developed bacteremia within the first 2 days of hospital admission. Because these patients may be receiving antibiotic treatment later in their disease progression than patients who developed P. aeruginosa bacteremia while in the hospital, the inclusion of these patients may explain why a statistically significant association was not observed. Furthermore, in the study by Osih et al. 86% of patients received appropriate empirical therapy versus only 52% of patients who received appropriate therapy within 52 h in the present study. The difference in selection criteria may account for the discrepancy between the two studies.
We also evaluated risk factors for delayed therapy for >52 h and found that antibiotic resistance to multiple drug classes was the most important determinant of delayed therapy. This observation is not surprising because antibiotic resistance to multiple classes limits treatment options and our ability to deliver timely appropriate treatment. Knowledge of institution-specific risk factors for multidrug resistance should be considered when initiating empirical antimicrobial therapy. Patients with COPD were also found to be at an increased risk of receiving delayed therapy, and this is consistent with our previous evaluation of patients with methicillin-resistant Staphylococcus aureus bacteremia (13). These patients most likely harbor resistant pathogens and efforts should be made to ensure that such patients are evaluated thoroughly and receive timely and adequate empirical therapy.
There are several limitations to the present study that should be noted. First, the P. aeruginosa blood culture data were collected from a single site; institutional differences in prescribing patterns, antibiotic formularies, and patient populations may affect the applicability of these results to other institutions. Further larger-scale, multicenter studies are still needed. Second, our study excluded neutropenic patients, and thus these results cannot be generalized to this patient group. Third, institution-specific antibiotic practices may impact the likelihood of delayed treatment; such practices may benefit from review to maximize the likelihood of early appropriate treatment. Another limitation is the small population size in the bivariate comparison of 30-day mortality between treatment regimens. Although no difference in 30-day mortality was noted between combination therapy and monotherapy, further study is still needed to adequately address this issue.
In conclusion, we quantified here the adverse impact of antimicrobial treatment delayed for more than 52 h. Clinicians must ensure prompt administration of appropriate therapy with a spectrum of activity consistent with likely resistance patterns. When developing antibiotic restriction policies, the potential adverse outcomes resulting from delayed treatment must be balanced with the potential ecological benefit of limiting excessive antibiotic use.
Acknowledgments
This study greatly benefited from the thoughtful editing of Allison Krug.
This study was supported by a grant from the Merck & Co, Inc. T.P.L. was the principal investigator for this grant.
Note that Merck only provided support to complete the project and was not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation and review of the manuscript. No other conflicts of interest exist for any of the authors.
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Bodey, G. P., L. Jadeja, and L. Elting. 1985. Pseudomonas bacteremia: retrospective analysis of 410 episodes. Arch. Intern. Med. 145:1621-1629. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-M9, 9th ed. Clinical Laboratory Standards Institute, Wayne, PA.
- 3.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 4.Harbarth, S., K. Ferriere, S. Hugonnet, B. Ricou, P. Suter, and D. Pittet. 2002. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch. Surg. 137:1353-1359. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 6.Kang, C. I., S. H. Kim, H. B. Kim, S. W. Park, Y. J. Choe, M. D. Oh, E. C. Kim, and K. W. Choe. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37:745-751. [DOI] [PubMed] [Google Scholar]
- 7.Kang, C. I., S. H. Kim, W. B. Park, K. D. Lee, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 49:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 9.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 10.Lasko, T. A., J. G. Bhagwat, K. H. Zou, and L. Ohno-Machado. 2005. The use of receiver operating characteristic curves in biomedical informatics. J. Biomed. Inform. 38:404-415. [DOI] [PubMed] [Google Scholar]
- 11.Leibovici, L., I. Shraga, M. Drucker, H. Konigsberger, Z. Samra, and S. D. Pitlik. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379-386. [DOI] [PubMed] [Google Scholar]
- 12.Lodise, T. P., and P. S. McKinnon. 2005. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 52:113-122. [DOI] [PubMed] [Google Scholar]
- 13.Lodise, T. P., P. S. McKinnon, L. Swiderski, and M. J. Rybak. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418-1423. [DOI] [PubMed] [Google Scholar]
- 14.MacArthur, R. D., M. Miller, T. Albertson, E. Panacek, D. Johnson, L. Teoh, and W. Barchuk. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin. Infect. Dis. 38:284-288. [DOI] [PubMed] [Google Scholar]
- 15.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osih, R. B., J. C. McGregor, S. E. Rich, A. C. Moore, J. P. Furuno, E. N. Perencevich, and A. D. Harris. 2007. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 51:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal, F., J. Mensa, M. Almela, J. A. Martinez, F. Marco, C. Casals, J. M. Gatell, E. Soriano, and M. T. Jimenez de Anta. 1996. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment: analysis of 189 episodes. Arch. Intern. Med. 156:2121-2126. [PubMed] [Google Scholar]
- 18.Zhang, H., and B. Singer. 1999. Recursive partitioning in the health sciences. Springer-Verlag, New York, NY.