Abstract
A multiplex asymmetric PCR (MAPCR)-based microarray method was developed for the detection of 10 known extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamase genes in gram-negative bacteria and for the typing of six important point mutations (amino acid positions 35, 43, 130, 179, 238, and 240) in the blaSHV gene. The MAPCR is based on a two-round reaction to promote the accumulation of the single-stranded amplicons amenable for microarray hybridization by employing multiple universal unrelated sequence-tagged primers and elevating the annealing temperature at the second round of amplification. A strategy to improve the discrimination efficiency of the microarray was constituted by introducing an artificial mismatch into some of the allele-specific oligonucleotide probes. The microarray assay correctly identified the resistance genes in both the reference strains and some 111 clinical isolates, and these results were also confirmed for some isolates by direct DNA sequence analysis. The resistance genotypes determined by the microarray correlated closely with phenotypic MIC susceptibility testing. This fast MAPCR-based microarray method should prove useful for undertaking important epidemiological studies concerning ESBLs and plasmid-mediated AmpC enzymes and could also prove invaluable as a preliminary screen to supplement phenotypic testing for clinical diagnostics.
β-Lactam antibacterial agents are the most commonly used antibiotics (2, 9, 11, 18). However, resistance to β-lactam antibiotics has become increasingly serious and is mediated mainly by transferable β-lactamase enzymes, which can specifically and efficiently hydrolyze the β-lactam loop. Class A β-lactamases, especially extended-spectrum β-lactamases (ESBLs) and class C cephalosporinase (AmpC) are the two major β-lactamases prevalent in gram-negative bacteria, especially in Enterobacteriaceae (3, 13, 27). These enzymes are typically associated with multiple antibiotic resistances, leaving few treatment choices (4, 20, 38). Clinically, accurate and rapid antibiotic susceptibility information is crucial for clinicians to make appropriate therapy decisions (8, 26). Differentiation of organisms harboring ESBLs from organisms harboring plasmid-mediated AmpC β-lactamases is necessary in order to address therapeutics and epidemiology as well as hospital infection control issues associated with these resistance mechanisms (37). Traditional culture-based antimicrobial susceptibility tests such as the disk diffusion method or MIC determinations provide only phenotypic susceptibilities and may at times be discordant with in vivo susceptibility (16, 24, 35). Many clinical laboratories have problems with the accurate detection of ESBLs and AmpC β-lactamases. However, failure to detect and distinguish between these enzymes has contributed to their uncontrolled spread and sometimes resulted in severe therapeutic failures (14, 26, 35).
Assays employing PCR and, subsequently, real-time quantitative PCR have grown rapidly, and some are currently used for in vitro diagnostics in the fields of infectious diseases and hereditary diseases, particularly in academic medical centers and large referral laboratories. However, the throughput of these methods is generally low, and only a limited number of targets can be detected and differentiated in each assay. Multiplex PCR, first developed and reported in 1988 (5), provides the simultaneous amplification of many targets of interest in one reaction, thus increasing the assay throughput and allowing more efficient use of each DNA sample. This technology could become a rapid and convenient screening procedure, as it has been successfully applied in mutation and polymorphism analyses (21) and the identification of bacterial species (7) and bacterial drug resistance genes (28).
Microarray technology, which allows the simultaneous analysis of a large amount of genetic information in a single assay and avoids the need for gel electrophoresis for fragment sizing analysis to identify gene variants (39), has recently been developed for the identification of viruses (41) and for the detection of a few microbial antibiotic resistance determinants (40). In the present study, we developed a multiplex asymmetric PCR (MAPCR)-based microarray procedure for the detection of 10 ESBLs and plasmid-mediated AmpC β-lactamase genes and also for the typing of six important point mutations in the blaSHV gene.
MATERIALS AND METHODS
Bacterial strains. (i) Reference strains.
Reference bacterial strains are listed in Table 1. Sixteen highly characterized reference strains, including six sequenced clinical strains, BJ-7 (Klebsiella pneumoniae) (blaSHV-2), TT-12 (K. pneumoniae) (blaCTX-M-3, blaSHV-5, blaDHA-1, and blaTEM-1), TT-13 (Escherichia coli) (blaCMY-2, blaCTX-M-9, and blaTEM-1), TT-18 (K. pneumoniae) (blaCTX-M-9, blaSHV-1, blaDHA-1, and blaTEM-1), TT-30 (Enterobacter cloacae) (blaSHV-12, chromosomal ampC, and blaTEM-1), and TR3104 (E. cloacae) (blaCTX-M-3, chromosomal ampC, and blaTEM-1); two E. coli J53 transformants, J53/pMG233 (blaMIR-1 and blaTEM-1) and J53/pMG251 (blaACT-1 and blaTEM-1), both kindly provided by G. A. Jacoby; and eight E. coli DH5α transformants, DH5α/pFOX-5 (blaFOX-5) (kindly provided by A. M. Queenan), DH5α/T-mox, DH5α/T-acc, TSV-6, TSV-8, TSV-24, TSV-29a, and TSV-130-1, with blaMOX-1, blaACC-1, blaSHV-6, blaSHV-8, blaSHV-24, blaSHV-29, and blaSHV-10 genes, respectively, on the T-easy vectors (Promega, Madison, WI), constructed in this study were used as standards to develop and optimize the MAPCR-based microarray method.
TABLE 1.
Well-characterized bacterial strains used in this study
| Straina | Relevant characteristic(s)b | Reference or source |
|---|---|---|
| E. coli DH5α/pFOX-5 | blaFOX-5-producing isolate | 31 |
| E. coli TT-13 | blaCMY-2-, blaCTX-M-9-, and blaTEM-1-producing isolate | This study |
| K. pneumoniae TT-18 | blaCTX-M-9-, blaSHV-1-, blaDHA-1-, and blaTEM-1-producing isolate | This study |
| E. cloacae TR3104 | blaCTX-M-3-, cs-ampC-, and blaTEM-1-producing isolate | This study |
| K. pneumoniae BJ-7 | blaSHV-2-producing isolate | This study |
| K. pneumoniae TT-12 | blaCTX-M-3-, blaSHV-5-, blaDHA-1-, and blaTEM-1-producing isolate | This study |
| E. cloacae TT-30 | blaSHV-12-, cs-ampC-, and blaTEM-1-producing isolate | This study |
| E. coli J53/pMG233 | blaMIR-1- and blaTEM-1-producing isolate | 25 |
| E. coli J53/pMG251 | blaACT-1- and blaTEM-1-producing isolate | 1 |
| E. coli DH5α/T-mox | blaMOX-1- and blaTEM-1-producing isolate | This study |
| E. coli DH5α/T-acc | blaACC-1- and blaTEM-1-producing isolate | This study |
| TSV-6 | blaSHV-6-producing isolate | This study |
| TSV-8 | blaSHV-8-producing isolate | This study |
| TSV-24 | blaSHV-24-producing isolate | This study |
| TSV-29a | blaSHV-29-producing isolate | This study |
| TSV-130-1 | blaSHV-10-producing isolate | This study |
DH5α/T-mox and DH5α/T-acc, with the blaMOX-1 and blaACC-1 genes, respectively, on the T-easy vectors (Promega), were constructed in this study because strains harboring the blaMOX-1 or blaACC-1 gene were unavailable. TSV-6, TSV-8, TSV-24, TSV-29a, and TSV-130-1 were E. coli DH5α transformants that contained the blaSHV-6, blaSHV-8, blaSHV-24, blaSHV-29, and blaSHV-10 genes (constructed by site-directed mutagenesis in this study), respectively, and were used for the validation of probes of the mutant type for positions 179, 43, 238Ala, and 130; other reference strains were clinical isolates which had been characterized fully.
cs-ampC, chromosomal ampC gene.
(ii) Clinical isolates.
A total of 111 gram-negative clinical isolates, including 46 E. coli, 46 K. pneumoniae, and 19 E. cloacae isolates obtained from Beijing Hospital and Beijing Tiantan Hospital, were identified for their β-lactamase genotypes. Among the isolates, 68 were from sputum, 19 were from blood, 18 were from urine, and 6 were from other samples. All isolates were identified to the species level by the Vitek GNI system (bioMérieux, France) or by the MicroScan Autoscan-4 system (Dade Behring, Inc., West Sacramento, CA). Isolates were screened for ESBL production by the CLSI (formerly NCCLS) phenotypic confirmatory method using disks containing 30 μg of cefotaxime and 30 μg of ceftazidime alone and in combination with 10 μg of clavulanate (23). AmpC β-lactamase activity was examined by the modified three-dimensional extraction method (17). The MICs of several β-lactam antibiotics, including cefoxitin, imipenem, cefotaxime, and ceftazidime alone or in association with clavulanate (4 μg/ml) (22), were determined for all isolates by the agar dilution method with Müeller-Hinton agar (Tiantan biotechnology Co., Ltd., Beijing, People's Republic of China) with an inoculum of 104 CFU per spot.
Primers and probes.
Fluorescence-labeled universal unrelated sequence-tagged primers (UT primers), sequence-specific primers (Table 2), and hybridization oligonucleotide probes (Table 3) were designed for the amplification and detection of the 10 known ESBLs and plasmid-mediated AmpC β-lactamase genes in gram-negative bacteria. The primers and probes were obtained from BioAsia (Shanghai, People's Republic of China). The UT primers were designed to be tagged with an unrelated universal sequence at their 5′ ends. A fluorescent dye, 6-carboxytetramethylrhodamine (TAMRA), labeled the 5′ end of the UT primer for simultaneously incorporation into the PCR products for subsequent hybridization. The melting temperature (Tm) of the primers was calculated according to the nearest-neighbor formula by using the MELTING program (15) (available at http://bioweb.pasteur.fr/seqanal/interfaces/melting.html). Oligonucleotide probe sequences were designed based on multiple-sequence alignment analysis with the DNAMAN (version 4.0) program. The probes for fabrication of the microarray were amino modified, and a poly(T)12 spacer was used between the amino group and the 5′ end of the probe.
TABLE 2.
Primers used for the MAPCR procedure
| Primer | Target(s) | Sequence (5′-3′)a | Concn(s) (nM) | Tm(s) (°C)b |
|---|---|---|---|---|
| Mox-uf | blaMOX-1, blaCMY-1, and blaCMY-8 | Uni-CTGCTCAAGGAGCACAGGAT | 200 | 71.9 |
| Mox-r to blaCMY-11 | CACATTGACATAGGTGTGGTGC | 200/40/20/10 | 58.3/56.3/55.4/54.5 | |
| cit-uf | blaLAT-1 to blaLAT-4, and blaCMY-2 to blaCMY-7 | Uni-TGGCCAGAACTGACAGGC | 200 | 72.1 |
| cit-r | TTTCTCCTGAACGTGGCTGGC | 200/40/20/10 | 61.7/59.6/58.7/57.8 | |
| dha-uf | blaDHA-1 and blaDHA-2 | Uni-AACTTTCACAGGTGTGCTGGGT | 100 | 71.3 |
| dha-r | CCGTACGCATACTGGCTTTGC | 100/20/10/5 | 60.0/57.9/57.0/56.1 | |
| acc-uf | blaACC-1 and blaACC-2 | Uni-AACAGCCTCAGCAGCCGGTTA | 100 | 72.4 |
| acc-r | TTCGCCGCAATCATCCCTAGC | 100/20/10/5 | 60.8/58.7/57.8/56.9 | |
| ebc-uf | blaMIR-1 and blaACT-1 | Uni-CAGGCCATTCCGGGTATGG | 100 | 71.8 |
| ebc-r | CTTCCACTGCGGCTGCCAGTT | 100/20/10/5 | 63.4/61.3/60.4/59.5 | |
| fox-uf | blaFOX-1 to blaFOX-6 | Uni-AACATGGGGTATCAGGGAGATG | 100 | 69.6 |
| fox-r | CAAAGCGCGTAACCGGATTGG | 100/20/10/5 | 60.5/58.4/57.5/56.6 | |
| ctx-m-3-uf | blaCTX-M-3 type | Uni-GTTGTTAGGAAGTGTGCCGCTG | 200 | 72.4 |
| ctx-m-3-r | CCTTAGGTTGAGGCTGGGTGAAGT | 200/40/20/10 | 62.3/60.3/59.5/58.7 | |
| ctx-m-9-uf | blaCTX-M-9 type | Uni-TGCAACGGATGATGTTCGCGG | 200 | 73.0 |
| ctx-m-9-r | CCTTTGAGCCACGTCACCAAC | 200/40/20/10 | 60.6/58.5/57.6/56.8 | |
| shv-f | blaSHV type | CCCTCACTCAAGGATGTATTGTGG | 400/80/40/20 | 59.1/57.2/56.4/55.6 |
| shv-ur | Uni-TTAGCGTTGCCAGTGCTCG | 400 | 72.7 | |
| tem-f | blaTEM type | CGCCCTTATTCCCTTTTTTGCGG | 40/8/4/2 | 59.4/57.5/56.8/55.9 |
| tem-ur | Uni-TCAGTGAGGCACCTATCTCAGCG | 40 | 71.3 | |
| Uni | TAMRA-GGTTTCGGATGTTACAGCGT |
The primer sequences of six groups of plasmid-mediated AmpC genes were obtained as described previously in reference 28, with minor modifications, and the primer sequences of other genes (blaTEM, blaSHV, blaCTX-M-3, and blaCTX-M-9) were designed in this study. Uni, universal.
The concentration-adjusted Tm was calculated according to the nearest-neighbor formula by using the MELTING program (available at http://bioweb.pasteur.fr/seqanal/interfaces/melting.html).
TABLE 3.
Oligonucleotide capture probes used in this study
| Oligonucleotide probe | Sequence (5′-3′)a | GenBank accession no. (ESBL or reference) |
|---|---|---|
| tem-U | NH2-T12-CGACGAGCGTGACACCACG | AB194682 |
| ctx-m-9-U | NH2-T12-GGAATGGCGGTATTCAGCGTA | AJ416341 |
| ctx-m-3-U | NH2-T12-TTCGTCTCCCAGCTGTCGG | AJ416342 |
| mox-U | NH2-T12-CGCCTTGTCATCCAGCTGCA | D13304 |
| cmy-U | NH2-T12-GCTTTATCCCTAACGTCATCGGG | X78117 |
| dha-U | NH2-T12-TGTGATCCCCTTCCACT | Y16410 |
| acc-U | NH2-T12-TACTCAGCGAACCCACTTCA | AJ133121 |
| ampc-U | NH2-T12-AGGGAGGCGTTATCCGT | AJ278995 |
| mir-U | NH2-T12-TAGAGCCCAGCTCAAACAG | M37839 |
| act-U | NH2-T12-CAAGGTTTGTGGAGTGACAG | U58495 |
| fox-U | NH2-T12-CGGTGTGGGTCAGCGCGATC | X77455 |
| shv-U | NH2-T12-GGCTGGTTTATCGCCGATA | X98099 (SHV-1) |
| 238Gly | NH2-T12-CGGAGCTGGCcAGCGGGGTb | X98099 (SHV-1) |
| 238Ser | NH2-T12-CGGAGCTAGCcAGCGGGGTb | AF148851 (SHV-2) |
| 238Ala | NH2-T12-CGGAGCTGCCcAGCGGGGTb | AF301532 (SHV-29) |
| 240Glu | NH2-T12-CGGAGCTcGCGAGCGGGGTb | X98099 (SHV-1) |
| 240Lys | NH2-T12-CGGAGCTcGCAAGCGGGGTb | AF117747 (SHV-5) |
| 35Leu | NH2-T12-AATTAAACTAAGCGAAAGCC | X98099 (SHV-1) |
| 35Gln | NH2-T12-AATTAAACAAAGCGAAAGCC | X53817 (SHV-2a) |
| 43Arg | NH2-T12-TGTCGGGCCGCcTAGGCATb | X98099 (SHV-1) |
| 43Ser | NH2-T12-TGTCGGGCAGCcTAGGCATb | AF301532 (SHV-29) |
| 130Ser | NH2-T12-CATTACCATGAGCGcTAACAGc | X98099 (SHV-1) |
| 130Gly | NH2-T12-CATTACCATGGGCGcTAACAGc | SHV-10 (30) |
| 17Asp | NH2-T12-GACGCCCGCGACcCCACTAc | X98099 (SHV-1) |
| 179Ala | NH2-T12-GACGCCCGCGCCcCCACTAc | Y11069 (SHV-6) |
| 179Asn | NH2-T12-GACGCCCGCAACcCCACTAc | U92041 (SHV-8) |
| 179Gly | NH2-T12-GACGCCCGCGGCcCCACTAc | AB023477 (SHV-24) |
| Position control | NH2-TCACTTGCTTCCGTTGAGG-HEX | AC007661 |
| Positive control | NH2-T12-CCTCAACGGAAGCAAGTGAT | AC007661 |
| Target of positive control | TAMRA-ATCACTTGCTTCCGTTGAGG | AC007661 |
| Negative control | NH2-T12-CAAGCAGCCACGCCAGTAC | BC112171 |
All probe sequences were designed for this study. Underlining indicates true point mutations, and lowercase type indicates the nucleotides introduced to form the artificial mismatches. HEX, hexachloro-6-carboxyfluorescein; T12, 12 consecutive thymidines.
The artificial mismatch introduced into the probe sequence is a C/C mismatch.
The artificial mismatch introduced into the probe sequence is a C/T mismatch.
MAPCR.
MAPCR was carried out in a single tube, which involved two distinct rounds of PCR to simultaneously amplify 10 β-lactamase genes prevalent in gram-negative bacteria. Before the optimization of the reaction of multiplex asymmetric amplification, individual primer pairs were evaluated by using template DNA from the reference strains to ensure that one primer pair amplified only one expected amplicon. Using standard PCR conditions (an initial denaturation step at 94°C for 3 min followed by 25 cycles of DNA denaturation at 94°C for 10 s, primer annealing at 55°C for 30 s, and primer extension at 72°C for 60 s), only one amplicon of the predicted size was observed for each template-primer pair tested, and amplicon sizes ranged from 190 to 885 bp (data not shown).
Based on thermal asymmetric PCR (19), the UT primers were designed with a universal unrelated sequence at their 5′ ends in order to obtain a Tm variance of at least 10°C compared with the sequence-specific primers. The procedure was based on a two-round reaction. In the first-round reaction, double-stranded products were produced under a lower annealing temperature (e.g., 55°C). In the second-round amplification, single-stranded DNA (ssDNA) was generated using a higher annealing temperature (e.g., 72°C) to ensure that only the UT primers annealed to the targets. Each PCR mixture (25 μl) contained 250 μM of each deoxynucleoside triphosphate, 1 U of Taq DNA polymerase (Tianwei Times Technology Co., Ltd., Beijing, People's Republic of China), 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 1 μl of lysate supernatant as a template. The template was prepared by boiling either a single colony or a few colonies suspended in 100 μl of 1× Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.5]). The final concentrations of the UT primers were 0.04 μM (for blaTEM), 0.1 μM (for blaDHA, blaACC, blaACT-1, and blaFOX-5), 0.2 μM (for blaMOX-1, blaCMY-2, blaCTX-M-3, and blaCTX-M-9), and 0.4 μM (for blaSHV). The final concentrations of the sequence-specific primers were adjusted according the concentration ratios of the sequence-specific primers to the UT primers (1:1, 1:5, 1:10, and 1:20) by fixing the concentrations of the UT primers. PCR was carried out in a Peltier PTC225 thermal cycler (MJ Research Inc., Watertown, MA) using two rounds of amplification, with an initial denaturation step at 94°C for 3 min, followed by the first round of exponential amplification of 15 cycles of 94°C for 10 s, 55°C for 30 s, and 72°C for 60 s; the second round of linear amplification of 25 cycles of 94°C for 10 s and 72°C for 120 s; and a final extension step at 72°C for 5 min. PCR products (2 μl) were analyzed by electrophoresis in 2.0% agarose in 1× Tris-borate-EDTA buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA [pH 8.0]) for 45 min at 100 V, stained with ethidium bromide, and photographed under a UV transilluminator (UVP Inc., Upland, CA).
Microarray preparation.
Microarrays were processed and spotted using a SmartArray-48 microarrayer (CapitalBio Co., Ltd., Beijing, People's Republic of China). The oligonucleotide probes were applied to the aldehyde-activated slides (CapitalBio) at a concentration of 10 μM in DNA Spotting solution (CapitalBio) and covalently immobilized on the slides by the mediation of an amino group at their 5′ ends.
Microarray hybridization and data analysis.
The initial studies used a microarray with the layout shown in Fig. 1A to validate the efficiency and accuracy of the MAPCR-based microarray assay. The hybridization mixture was made up of 8 μl of the fluorescence-labeled PCR products and 10 μl of hybridization buffer (4× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.2% sodium dodecyl sulfate, 5× Denhardt's solution, and 10% dextran sulfate) plus 0.1 pmol TAMRA-labeled positive control target. The mixtures were heated for 5 min at 95°C, cooled down rapidly on ice, and then applied to the gridded reaction chamber formed by the polyester frame covering the surface of the microarray. Hybridization was performed for 2 h at 55°C in a humidified chamber. After hybridization, the slides were washed once at room temperature for 5 min in 2× SSC-0.1% sodium dodecyl sulfate and twice for an additional 1 min in distilled water. Finally, the slides were dried by spinning at 250 × g for 2 min. Microarrays were analyzed using a confocal LuxScan-10K scanner (CapitalBio). Laser power was fixed at 90%, and the photomultiplier tube was set at 70%. Fluorescent intensities were quantified by using SpotData Pro 2.1 (CapitalBio). The mean of the fluorescence signals for the repeated spots was corrected by subtraction of the mean of the fluorescence signals of the negative control. The signal intensity of a perfect match (PM) (higher intensity among two allele-specific oligonucleotide probes for one single-nucleotide polymorphism) and the ratio of the intensities of PM to mismatch (MM) (i.e., ratio of the mean intensity of the PM to the mean intensity of MM) were used for blaSHV typing.
FIG. 1.
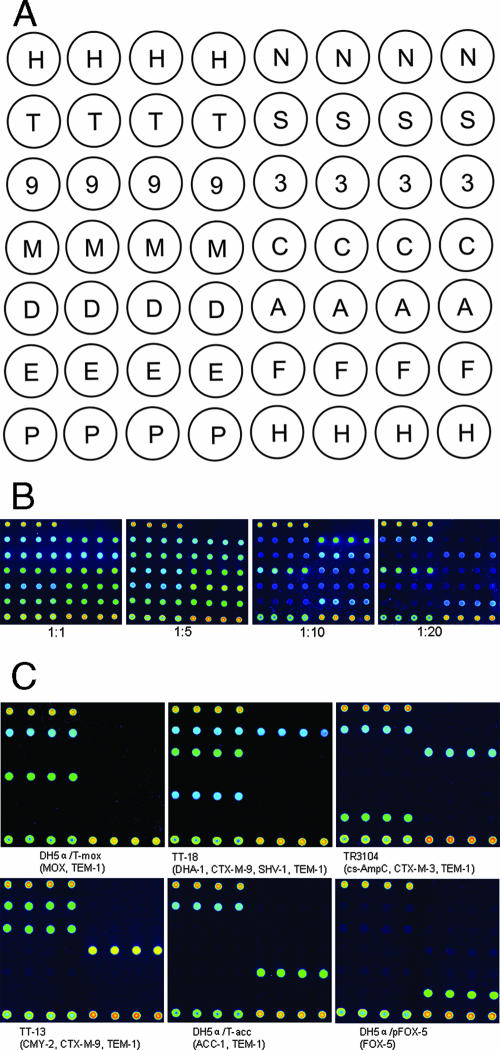
Multiplex detection on an oligonucleotide microarray in the initial study. (A) Oligonucleotide pattern printed on the array surface. H, position control; N, negative control; T, blaTEM; S, blaSHV; 9, blaCTX-M-9; 3, blaCTX-M-3; M, blaMOX; C, blaCMY; D, blaDHA; A, blaACC; E, E. cloacae chromosomal ampC; F, blaFOX; P, positive control. (B) Microarray hybridization using the PCR products from MAPCR for 10-plex detection. The concentration ratios of the sequence-specific primers to the UT primers in MAPCR were 1:1, 1:5, 1:10, and 1:20. (C) Specificity of the MAPCR-based microarray method.
RESULTS
Multiplex amplification and detection of β-lactamase genes by the MAPCR-based microarray method.
The MAPCR was evaluated in a 10-plex amplification of 10 groups of β-lactamase genes using a combination of the six templates (DH5α/pFOX-5, TT-13, TT-18, TR3104, DH5α/T-mox, and DH5α/T-acc) (Table 1). These genes could be simultaneously detected in a single reaction (Fig. 1B) in which concentration ratios of 1:1 and 1:5 of the sequence-specific primers to the UT primers both gave higher strength signals than a ratio of 1:10 did. A 1:20 ratio of primers produced the weakest and most unbalanced hybridization signals.
To further investigate the multiplex asymmetric amplification efficiency of the MAPCR, microarray hybridization was performed without denaturing the hybridization mixture. The results indicated that the signal intensities of the 10 β-lactamase genes were slightly reduced, with a decrease of about 10 to 30% compared with the signal intensities produced following denaturation (data not shown). MAPCR efficiently produces a large number of single-stranded products from multiple targets and also indicates that the denaturation step before hybridization could be eliminated to simplify the assay and to save time. We also examined the feasibility of further reducing the length of the assay by limiting the hybridization incubation to 30 min using the reference strains. Probe signals were detected with a decrease of the signal intensity of between 20 and 40% compared with the standard 2-h hybridization (data not shown), indicating that a truncated hybridization procedure is feasible, which could be useful for clinical diagnostics.
Specificity and sensitivity of the MAPCR-based microarray method.
Each of the six reference strains (DH5α/pFOX-5, TT-13, TT-18, TR3104, DH5α/T-mox, and DH5α/T-acc) was subjected to MAPCR amplification and hybridization to the microarray to assess specificity. Figure 1C shows that the different β-lactamase genes in each of the reference strains were correctly and unambiguously identified, regardless of how many genes the strain contained (ranging from one gene in DH5α/pFOX-5 to four genes in TT-18). Each of the expected PCR products was detected by the specific probes on the microarray and did not cross-hybridize with other probes. In contrast, not all of the expected amplification fragments of the MAPCR were visualized and differentiated clearly by electrophoresis (data not shown), illustrating the high sensitivity and specificity of the MAPCR-based microarray assay compared with traditional multiplex PCR sizing analyses.
To determine the detection limits (i.e., minimal number of bacterial cells that can be detected) of the MAPCR-based microarray assay, serial 10-fold dilutions of template (from 105 cells to 100 cells) from three of the reference strains, E. coli TT-13, K. pneumoniae TT-18, and E. cloacae TR3104, were tested. The MAPCR-based microarray assay had a detection limit of 102 to 103 cells for the three different species (data not shown). This result indicates that the MAPCR-based microarray assay can be used reliably and directly to detect antibiotic resistance genes from a single colony, which contains about 107 to 108 cells (32).
MAPCR-based microarray for detection of β-lactamase genes and typing of SHV-type ESBLs.
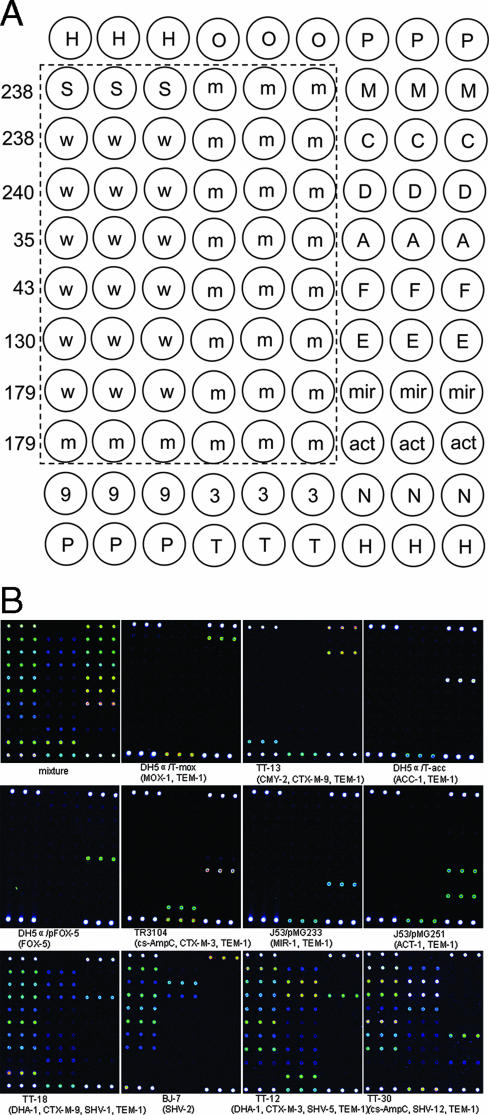
As shown in Fig. 2B, all gene-specific probes, except the blaSHV and blaACT-1 genes, hybridized specifically to the corresponding targets of each of the β-lactamase genotypes in the reference strains, and no cross-hybridization with other genes was observed. The blaACT-1 gene also gave a positive signal on the probe of the E. cloacae chromosomal ampC gene due to the sequence similarity. For the blaSHV gene, both the probes of a PM and those of an MM gave positive signals. Nonetheless, assignment to a genotype of the blaSHV gene was not affected. The signal intensities of the PM were significantly higher than those of the MM (P < 0.05 by t test), and the discrimination ratios (ratio of the mean intensity of the PM to the mean intensity of the MM) were more than 2 for all the reference strains tested.
FIG. 2.
Analysis of genotypes of the drug resistance genes by the MAPCR-based microarray method. (A) Oligonucleotide pattern printed on the array surface. H, position control; O, dimethyl sulfoxide; P, positive control; S, blaSHV (universal probe); w, wild-type blaSHV; m, mutant-type blaSHV; 9, blaCTX-M-9; 3, blaCTX-M-3; T, blaTEM; M, blaMOX; C, blaCMY; D, blaDHA; A, blaACC; F, blaFOX; E, E. cloacae chromosomal ampC; mir, blaMIR-1; act, blaACT-1; N, negative control. The probes in the rectangular area were used for blaSHV typing, and the numbers on the left indicate the amino acid positions. (B) Microarray hybridization results for 11 different reference isolates. Mixture is the positive control for monitoring the amplification and hybridization processes using a combination of the six templates (DH5α/pFOX-5, TT-13, TT-18, TR3104, DH5α/T-mox, and DH5α/T-acc) (Table 1).
Genotyping of clinical isolates by using MAPCR-based microarray hybridization.
The accuracy and reproducibility of this MAPCR-based microarray assay to detect the β-lactamase genotypes was tested using 111 clinical isolates of Enterobacteriaceae where the drug resistance had been previously characterized by microbiological methods and by DNA sequencing (for all the genes of DHA-1, CMY-2, and the SHV type and some of the genes of the CTX-M-3 type [five isolates], the CTX-M-9 type [five isolates], the E. cloacae chromosomal ampC [five isolates], and the TEM type [six isolates]). A total of 34 different resistance genotypes were detected in the collection of 111 clinical isolates by the MAPCR-based microarray assay, and these results were in complete agreement with complementary sequencing results (data not shown). As reported previously (6), CTX-M-9 (43 isolates), CTX-M-3 (19 isolates), and SHV-12 (11 isolates) were the major ESBL genotypes identified. In addition, DHA-1 (13 isolates) was the main plasmid-mediated AmpC enzyme. The results for the detection of six point mutations in the blaSHV gene obtained by the microarray assay were in 100% accordance with those obtained by DNA sequencing. Furthermore, a good correlation between the microarray results and MIC susceptibility testing of all the clinical isolates of K. pneumoniae and E. coli was found. Exceptions were four K. pneumoniae strains in which two isolates both harbored SHV-2a and TEM-type genotypes and another two isolates harbored CTX-M-3 and TEM-type and DHA-1 and SHV-1a genotypes, respectively, which were susceptible to all the antibiotics tested. E. cloacae isolates with ESBLs or plasmid-mediated AmpC enzymes were resistant to cefotaxime or ceftazidime. However, the phenotypic results for the E. cloacae isolates with the chromosomal ampC gene alone did not correlate well with the microarray assay.
DISCUSSION
In the absence of self-annealing, the hybridization of ssDNA targets to probes should be more efficient than the hybridization of double-stranded DNA targets, even if the double-stranded DNAs were denatured by boiling or alkali treatment before hybridization (10). Asymmetric PCR, first described by Gyllensten and Erlich (12), is a useful tool for generating ssDNA. Traditional asymmetric PCR uses conventional PCR primers at unequal concentrations to generate ssDNA. Unfortunately, this method is often inefficient and difficult to optimize and tends to promote nonspecific amplification. In addition, if the concentration of a primer designed for symmetric PCR is simply reduced, creating a limiting primer, the efficiency of the resulting symmetric reaction also decreases. These effects are compounded if a multiplex amplification is performed in the same reaction vessel, and appropriate conditions are even more difficult to optimize, particularly if asymmetric PCR is also involved. We performed the traditional asymmetric PCR for a 10-plex amplification and found that the amplification was both inefficient and variable (data not shown). The linear-after-the-exponential PCR (LATE-PCR) method has been recently shown to be an advantageous method for producing large amounts of ssDNA amplicons by a new primer design strategy and has been used successfully for real-time PCR analysis (29, 34), for pyrosequencing (33), and for universal microarray hybridization (36). Despite its obvious utility, the development of a multiplex LATE-PCR-based assay has not been reported. We attempted to use these stability considerations for the design of a set of PCR primers; however, they were not effective for multiplex asymmetric amplification (data not shown). In contrast, the present study reports the successful constitution of MAPCR using UT primers for the generation of a large number of ssDNA products from multiple targets for microarray hybridization. We also developed a diagnostic application of the MAPCR-based microarray assay for the identification of 10 groups of β-lactamase genes responsible for most of the drug-resistant Enterobacteriaceae encountered in clinics.
We have compared this microarray assay for the detection of antibiotic resistance genes with traditional phenotypic methods for the determination of antibiotic susceptibility. Overall, we found that correlations were more than 95% for the detection of phenotypic resistance for K. pneumoniae and E. coli isolates. The data presented here demonstrate several powerful features of the MAPCR-based microarray assay. First, the universal unrelated sequence in the 5′ end of the UT primer provides a higher annealing temperature so that only the UT primer anneals to the target for linear amplification of the ssDNAs, independent of the exhaustion of the limiting primer. This linear amplification at a higher annealing temperature performed well at an equal or equivalent primer concentrations, which should help maintain more uniform amplification of different targets and might permit a further increase in the level of achievable multiplexing. Second, the universal unrelated sequence at the 5′ end of the UT primers would allow multiplex PCR to be easily optimized if new genes are added. Third, differentiation efficiency during hybridization can be readily increased by the introduction of an artificial mismatch into allele-specific oligonucleotide probes to provide better differentiation between genotypes; such discrimination is less easily achieved with traditional methods. This general MAPCR-based microarray strategy has also been successfully applied to the detection of different drug resistance genes in 415 different clinical staphylococcal isolates (data not shown).
In conclusion, we have developed a MAPCR protocol to efficiently generate single-stranded products in a linear manner after the exponential phase. Combined with microarray technology, we simultaneously detected 10 prevalent β-lactamase genes accurately in clinically relevant gram-negative bacteria by starting with raw bacterial growth on primary isolation medium. Its ease, speed, and reliability make this MAPCR-based microarray assay a powerful tool for important epidemiological studies concerning plasmid-mediated AmpC and ESBL enzymes and suggest that it may be a useful tool to complement phenotypic susceptibility testing in clinical laboratories.
Acknowledgments
This work was funded by a grant of the National Hi-Tech Program of China (grant 2006AA020701) from the Department of Science and Technology, People's Republic of China.
We declare no conflict of interest.
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Alvarez, M., J. H. Tran, N. Chow, and G. A. Jacoby. 2004. Epidemiology of conjugative plasmid-mediated AmpC β-lactamases in the United States. Antimicrob. Agents Chemother. 48:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari, F. 2001. Use of systemic anti-infective agents in Iran during 1997-1998. Eur. J. Clin. Pharmacol. 57:547-551. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain, J. S., R. A. Gibbs, J. E. Ranier, P. N. Nguyen, and C. T. Caskey. 1988. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 16:11141-11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanawong, A., F. H. M'Zali, J. Heritage, J. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depardieu, F., B. Perichon, and P. Courvalin. 2004. Detection of the van alphabet and identification of enterococci and staphylococci at the species level by multiplex PCR. J. Clin. Microbiol. 42:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essack, S. Y. 2000. Treatment options for extended-spectrum beta-lactamase-producers. FEMS Microbiol. Lett. 190:181-184. [DOI] [PubMed] [Google Scholar]
- 9.Filius, P. M. G., T. B. Y. Liem, P. D. van der Linden, R. Janknegt, S. Natsch, A. G. Vulto, and H. A. Verbrugh. 2005. An additional measure for quantifying antibiotic use in hospitals. J. Antimicrob. Chemother. 55:805-808. [DOI] [PubMed] [Google Scholar]
- 10.Gao, H., S. Tao, D. Wang, C. Zhang, X. Ma, J. Cheng, and Y. Zhou. 2003. Comparison of different methods for preparing single stranded DNA for oligonucleotide microarray. Anal. Lett. 36:2845-2859. [Google Scholar]
- 11.Goossens, H., M. Ferech, R. Vander Stichele, M. Elseviers, D. L. Monnet, and the ESAC Project Group. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 12.Gyllensten, U. B., and H. A. Erlich. 1988. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc. Natl. Acad. Sci. USA 85:7652-7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new β-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 14.Karas, J. A., D. G. Pillay, D. Muckart, and A. W. Sturm. 1996. Treatment failure due to extended spectrum β-lactamase. J. Antimicrob. Chemother. 37:203-204. [DOI] [PubMed] [Google Scholar]
- 15.Le Nove're, N. 2001. MELTING, computing the melting temperature of nucleic acid duplex. Bioinformatics 17:1226-1227. [DOI] [PubMed] [Google Scholar]
- 16.Livermore, D. M., and M. Yuan. 1996. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J. Antimicrob. Chemother. 38:409-424. [DOI] [PubMed] [Google Scholar]
- 17.Manchanda, V., and N. P. Singh. 2003. Occurrence and detection of AmpC beta-lactamases among gram-negative clinical isolates using a modified three-dimensional test at Guru Tegh Bahadur Hospital, Delhi, India. J. Antimicrob. Chemother. 51:415-418. [DOI] [PubMed] [Google Scholar]
- 18.Matuz, M., R. Benko, P. Doro, E. Hajdu, G. Nagy, E. Nagy, D. L. Monnet, and G. Soos. 2006. Regional variations in community consumption of antibiotics in Hungary, 1996-2003. Br. J. Clin. Pharmacol. 61:96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazars, G. R., C. Moyret, P. Jeanteur, and C. G. Theillet. 1991. Direct sequencing by thermal asymmetric PCR. Nucleic Acids Res. 19:4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melano, R., A. Corso, A. Petroni, D. Centron, B. Orman, A. Pereyra, N. Moreno, and M. Galas. 2003. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum β-lactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 52:36-42. [DOI] [PubMed] [Google Scholar]
- 21.Moutou, C., N. Gardes, and S. Viville. 2002. Multiplex PCR combining deltaF508 mutation and intragenic microsatellites of the CFTR gene for pre-implantation genetic diagnosis (PGD) of cystic fibrosis. Eur. J. Hum. Genet. 10:231-238. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. NCCLS, Wayne, PA.
- 23.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests, 8th ed. Approved standard M2-A8. NCCLS, Wayne, PA.
- 24.Pai, H., C. I. Kang, J. H. Byeon, K. D. Lee, W. B. Park, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2004. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:3720-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papanicolaou, G. A., A. A. Medeiros, and G. A. Jacoby. 1990. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and α-methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 34:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson, D. L., W.-C. Ko, A. Von Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugman, R. A. Bonomo, L. B. Rice, J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implications for the clinical laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Pérez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce, K. E., J. A. Sanchez, J. E. Rice, and L. J. Wangh. 2005. Linear-after-the-exponential (LATE)-PCR: primer design criteria for high yields of specific single-stranded DNA and improved real-time detection. Proc. Natl. Acad. Sci. USA 102:8609-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinarakis, E. E., V. Miriagou, E. Tzelepi, M. Gazouli, and L. S. Tzouvelekis. 1997. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob. Agents Chemother. 41:838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Queenan, A. M., S. Jenkins, and K. Bush. 2001. Cloning and biochemical characterization of FOX-5, an AmpC-type plasmid-encoded β-lactamase from a New York City Klebsiella pneumoniae clinical isolate. Antimicrob. Agents Chemother. 45:3189-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramakrishnan, R., W. Buckingham, M. Domanus, L. Gieser, K. Klein, G. Kunkel, A. Prokhorova, and P. V. Riccelli. 2004. Sensitive assay for identification of methicillin-resistant Staphylococcus aureus, based on direct detection of genomic DNA by use of gold nanoparticle probes. Clin. Chem. 50:1949-1952. [DOI] [PubMed] [Google Scholar]
- 33.Salk, J. J., J. A. Sanchez, K. E. Pierce, J. E. Rice, K. C. Soares, and L. J. Wangh. 2006. Direct amplification of single-stranded DNA for pyrosequencing using linear-after-the-exponential (LATE)-PCR. Anal. Biochem. 353:124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez, J. A., K. E. Pierce, J. E. Rice, and L. J. Wangh. 2004. Linear-after-the-exponential (LATE)-PCR: an advanced method of asymmetric PCR and its uses in quantitative real-time analysis. Proc. Natl. Acad. Sci. USA 101:1933-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song, W., E. S. Moland, N. D. Hanson, J. S. Lewis, J. H. Jorgensen, and K. S. Thomson. 2005. Failure of cefepime therapy in treatment of Klebsiella pneumoniae bacteremia. J. Clin. Microbiol. 43:4891-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szemes, M., P. Bonants, M. de Weerdt, J. Baner, U. Landegren, and C. D. Schoen. 2005. Diagnostic application of padlock probes—multiplex detection of plant pathogens using universal microarrays. Nucleic Acids Res. 33:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson, K. S. 2001. Controversies about extended-spectrum and AmpC beta-lactamases. Emerg. Infect. Dis. 7:333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa, L., C. Pezzela, F. Tosini, P. Visca, A. Petrucca, and A. Carattoli. 2000. Multiple-antibiotic resistance mediated by structurally related IncL/M plasmids carrying an extended-spectrum β-lactamase gene and a class 1 integron. Antimicrob. Agents Chemother. 44:2911-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yershov, G., V. Barsky, A. Belgovskiy, E. Kirillov, E. Kreindlin, I. Ivanov, S. Parinov, D. Guschin, A. Drobishev, S. Dubiley, and A. Mirzabekov. 1996. DNA analysis and diagnostics on oligonucleotide microchips. Proc. Natl. Acad. Sci. USA 93:4913-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu, X., M. Susa, C. Knabbe, R. D. Schmid, and T. T. Bachmann. 2004. Development and validation of a diagnostic DNA microarray to detect quinolone-resistant Escherichia coli among clinical isolates. J. Clin. Microbiol. 42:4083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, Y., R. Yang, S. Tao, Z. Li, Q. Zhang, H. Gao, Z. Zhang, J. Du, P. Zhu, L. Ren, L. Zhang, D. Wang, L. Guo, Y. Wang, Y. Guo, Y. Zhang, C. Zhao, C. Wang, D. Jiang, Y. Liu, H. Yang, L. Rong, Y. Zhao, S. An, Z. Li, X. Fan, J. Wang, Y. Cheng, O. Liu, Z. Zheng, H. Zuo, Q. Shan, L. Ruan, Z. Lv, T. Hong, and J. Cheng. 2005. The design and application of DNA chips for early detection of SARS-CoV from clinical samples. J. Clin. Virol. 33:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]