Abstract
Initial attachment of the cariogenic Streptococcus mutans onto dental enamel is largely promoted by the adsorption of specific salivary proteins on enamel surface. Some phosphorylated salivary proteins were found to reduce S. mutans adhesion by competitively inhibiting the adsorption of S. mutans-binding salivary glycoproteins to hydroxyapatite (HA). The aim of this study was to develop antiadherence compounds for preventing dental biofilm development. We synthesized phosphorylated polyethylene glycol (PEG) derivatives and examined the possibility of surface pretreatment with them for preventing S. mutans adhesion in vitro and dental biofilm formation in vivo. Pretreatment of the HA surface with methacryloyloxydecyl phosphate (MDP)-PEG prior to saliva incubation hydrophilized the surface and thereby reduced salivary protein adsorption and saliva-promoted bacterial attachment to HA. However, when MDP-PEG was added to the saliva-pretreated HA (S-HA) surface, its inhibitory effect on bacterial binding was completely diminished. S. mutans adhesion onto S-HA was successfully reduced by treatment of the surface with pyrophosphate (PP), which desorbs salivary components from S-HA. Treatment of S-HA surfaces with MDP-PEG plus PP completely inhibited saliva-promoted S. mutans adhesion even when followed by additional saliva treatment. Finally, mouthwash with MDP-PEG plus PP prevented de novo biofilm development after thorough teeth cleaning in humans compared to either water or PP alone. We conclude that MDP-PEG plus PP has the potential for use as an antiadherence agent that prevents dental biofilm development.
Dental plaque, a complex microbial biofilm, is the primary etiologic factor in dental caries. Colonization of enamel surfaces by the cariogenic bacterium Streptococcus mutans is thought to be initiated by attachment to a saliva-derived conditioning film, the acquired enamel pellicle (9). The acquired enamel pellicle is formed largely by adsorption of heterogeneous salivary proteins (1, 18) onto dental enamel (∼98% [wt/wt] hydroxyapatite [HA]) and promotes the adhesion of S. mutans by specific (2, 17) and nonspecific (4) mechanisms.
Saliva contains a multitude of proteins that contribute to oral microbial ecology and biofilm formation (6, 16, 32). A variety of salivary proteins have been shown to modulate bacterial adhesion onto HA surfaces in vitro. Adsorption of specific salivary proteins, such as acidic proline-rich proteins (8) and agglutinin (3), promotes the adhesion of S. mutans onto HA surfaces by providing ligands for bacterial attachment. Many studies thus far have suggested that an initial bacterial adhesion promoted by salivary protein adsorption onto the enamel surface contributes to facilitate dental biofilm development.
Recently, we reported the clinical relevance of saliva-promoted S. mutans adhesion in both de novo dental biofilm development and caries experience (29). Dental biofilm formation after thorough teeth cleaning was positively correlated with both salivary glycoprotein content and S. mutans adhesion onto saliva-coated HA surfaces. A higher glycoprotein content forms an “adhesive” conditioning film (acquired pellicle) that promotes a higher rate of bacterial attachment onto HA surfaces in vitro (28).
In contrast to salivary glycoprotein, some small salivary proteins, statherin (26) and histatin 1 (20), have an inhibitory effect on saliva-mediated S. mutans adhesion in vitro (28). These non-S. mutans-binding phosphoproteins reduce S. mutans adhesion by competitively inhibiting the adsorption of S. mutans-binding salivary glycoproteins to HA surfaces. If competitive inhibition serves as a general mechanism for salivary protein adhesion to enamel surfaces, acquired enamel pellicle and dental biofilm formations may be controlled for prevention and therapy by the development of novel biomimetic compounds, e.g., statherin or histatin 1-like substances.
The aim of the present study was to develop antiadherence compounds for preventing de novo dental biofilm development. To design statherin or histatin 1-like compounds, we considered the following two factors. (i) Our previous study showed that the phosphate and carboxyl groups in the N-terminal domain of statherin and histatin 1, which are capable of binding to HA surfaces through charge interactions, were essential for their inhibitory effects on bacterial adhesion, as well as noncharged or weakly charged amino acid domains (28). (ii) The studies thus far have proved that a noncharged hydrophilic surface, which may result from treatment with, for example, polyethylene glycol (PEG) derivatives, prevent bacterial adhesion effectively (12, 21, 22). Taking these together, we investigated the possibility of HA surface pretreatment with phosphorylated PEG derivatives for preventing saliva-promoted S. mutans adhesion in vitro and de novo dental biofilm formation in vivo.
MATERIALS AND METHODS
Polymer.
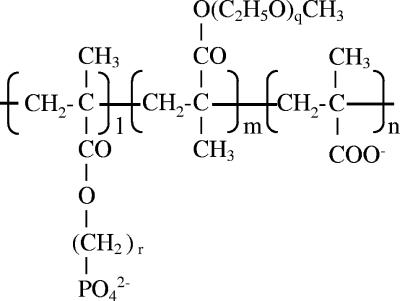
Fourteen compounds (Fig. 1 and Table 1) were synthesized in the present study. PEG methacrylate, methacrylic acid, and methacryloyloxydecyl phosphate (MDP) were polymerized in aqueous solution containing 2-mercaptoethanol, and ammonium persulfate at 80°C for 4 to 5 h under nitrogen bubbling. In some cases, MDP was substituted by methacryloyloxyethyl phosphate (MEP), and methacrylic acid was eliminated from the reaction. Polymerization was stopped by cooling, and sodium hydroxide solution was added to neutralize the reaction mixture. The mixture was dialyzed against distilled water (dW) for 3 days and freeze dried. The average molecular weight (MW) was estimated by gel permeation chromatography. The molar ratio of each polymer chain was estimated by using nuclear magnetic resonance and gel permeation chromatography.
FIG. 1.
Structure of phosphorylated PEG derivatives synthesized in the present study. l, m, and n, molar numbers of methacryloyloxyalkyl phosphate, PEG, and methacrylate groups, respectively, in the polymer. r, Number of methyl groups (linker length) between methacryloyl and phosphate groups; q, number of ethoxy groups of PEG.
TABLE 1.
Effects of pretreatment of sintered HA plates with polymers on saliva-promoted S. mutans adhesion
| Expt | Pretreatment | l/m/na | rb | qc | MW (104) | Concn (mg/ml) | Saliva treatment | Mean S. mutans adhesion (106 CFU) ± SEMd |
|---|---|---|---|---|---|---|---|---|
| 1 | dW | - | 5.0 ± 0.6‡ | |||||
| dW | + | 21.7 ± 0.9 | ||||||
| Polymer A | 31/16/53 | 10 | 120 | 1.6 | 1 | + | 12.3 ± 1.0† | |
| 5 | + | 5.9 ± 0.2‡ | ||||||
| 25 | + | 6.2 ± 0.9‡ | ||||||
| 2 | dW | - | 8.2 ± 4.5* | |||||
| dW | + | 26.0 ± 0.8 | ||||||
| Polymer B | 77/15/8 | 10 | 120 | 1.5 | 10 | + | 11.0 ± 2.2† | |
| Polymer C | 45/44/11 | 10 | 120 | 2 | 10 | + | 19.9 ± 0.2† | |
| Polymer D | 58/25/17 | 2 | 120 | 2.2 | 10 | + | 23.7 ± 0.6e | |
| 3 | dW | - | 3.5 ± 0.3‡ | |||||
| dW | + | 16.3 ± 0.8 | ||||||
| Polymer E | 87/13/0 | 10 | 120 | 10.9 | 10 | + | 4.8 ± 0.6‡ | |
| Polymer F | 42/58/0 | 10 | 90 | 10.3 | 10 | + | 8.5 ± 1.1† | |
| Polymer G | 39/61/0 | 10 | 90 | 3.5 | 10 | + | 6.8 ± 0.5‡ | |
| 4 | dW | - | 6.7 ± 0.5‡ | |||||
| dW | + | 31.6 ± 0.8 | ||||||
| Polymer H | 24/76/0 | 10 | 120 | 2.9 | 10 | + | 9.8 ± 1.0‡ | |
| Polymer I | 42/58/0 | 10 | 120 | 2.8 | 10 | + | 6.9 ± 0.6‡ | |
| Polymer J | 39/61/0 | 10 | 23 | 2.7 | 10 | + | 17.1 ± 1.7† | |
| Polymer K | 87/13/0 | 10 | 120 | 3.2 | 10 | + | 7.2 ± 2.3‡ | |
| Polymer L | 90/10/0 | 10 | 23 | 5.1 | 10 | + | 18.1 ± 3.9* |
l/m/n, Molar ratio of methacryloyloxyalkyl phosphate/PEG/methacrylate groups (see Fig. 1).
r, Number of methyl groups (linker length) between methacryloyl and phosphate groups.
q, Number of ethoxy groups of PEG.
Unpaired Student t tests after a preliminary F test of the homogeneity of within-group variance were conducted between dW-pretreated, saliva-treated, and saliva-untreated or polymer-pretreated groups. *, P < 0.05; †, P < 0.01; ‡, P < 0.001.
Not significant (P > 0.05).
Collection of stimulated human parotid salivary secretion.
A human parotid saliva sample was obtained on ice from a 32-year-old healthy male subject by using a modified Carlsson-Crittenden cup (30). Parotid salivary flow was stimulated by the application of lemon juice (Pocca Corp., Japan) to the tongue. The protocol was approved by the ethical committee of the Kao Tochigi Institute. The salivary sample was used immediately for bacterial adhesion studies. It was centrifuged at 2,000 × g for 20 min to remove any insoluble material or cell debris.
In vitro studies. (i) Bacterial adhesion assay.
Streptococcus mutans MT8148 (serotype c), Streptococcus sanguis (serotype H), Actinomyces viscosus TPR-12, and Porphyromonas gingivalis were obtained from the Japanese Collection of Microorganisms of the Riken Bioresource Center (Tsukuba, Japan). Stock cultures were stored in 50% glycerol at −80°C. Inoculum from stock culture was grown anaerobically in brain heart infusion broth (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) for S. mutans and S. sanguis, in tryptic soy (TS) broth (Nippon Becton Dickinson Company, Ltd., Tokyo, Japan) for A. viscosus, or in TS broth supplemented with 5 mg of hemin/ml and 0.5 mg of menadione/ml for P. gingivalis in a model ANX-1W anaerobic box (Hirasawa Works, Inc., Tokyo, Japan) filled with 80% N2, 10% H2, and 10% CO2 at 37°C. For adhesion studies, the organisms were radiolabeled by growth at 37°C in brain heart infusion broth supplemented with 0.2% sucrose (for S. mutans and S. sanguis), TS broth (for A. viscosus), or TS broth supplemented with 5 μg of hemin/ml and 0.5 μg of menadione/ml and methyl[3H]thymidine (10 μCi/ml; Amersham Biosciences Corp., Tokyo, Japan) for 16 h in anaerobic conditions. Early-stationary-phase cells were washed three times with buffered KCl (0.05 M KCl, 1 mM CaCl2, 1 mM potassium phosphate, 0.1 mM MgCl2 [pH 6.0]) by centrifugation. The bacterial pellet was dispersed into buffered KCl containing 5 mg of bovine serum albumin/ml at approximately 109 CFU per ml (optical density at 600 nm of 0.2) by forced passage through a needle (25 gauge by 1 in.; Terumo, Japan) (15).
Bacterial adhesion onto HA was measured in vitro according to the method of Kishimoto et al. (15) with minor modifications. Sintered HA plates (sHA; 10 by 10 by 1 mm, Ca/P = 1.57; Olympus, Japan) (27) were equilibrated in buffered KCl at room temperature overnight. The plates were then treated in triplicate with 1 ml of dW or test polymer solution for 1 h at room temperature. After being washed with 2 ml of buffered KCl, the plates were treated with 500 μl of buffered KCl (for saliva-untreated HA plates) or saliva overnight at 4°C. The plates were washed twice with buffered KCl, followed by the addition of 1 ml of buffered KCl containing BSA (5 mg/ml) to block any uncoated regions of sHA (7), and then incubated at 37°C for 1 h with 1 ml of radiolabeled bacteria in buffered KCl containing albumin (5 mg/ml). The plates were washed three times with buffered KCl, and the number of bacteria attached to the plates was determined by direct scintillation counting using a liquid scintillation counter (2550 TR/LL; Packard, Tokyo, Japan). We counted background scintillation by using untreated HA plates in each experiment. The background was subtracted from each scintillation measurement to obtain the radioactivity of attached bacteria. We also counted the specific radioactivity of labeled bacteria (dpm per 109 CFU). The number of attached bacteria (CFUs) was calculated by dividing the radioactivity of attached bacteria by the specific radioactivity of labeled bacteria.
(ii) Labeling of salivary proteins.
Salivary glycoproteins were radiolabeled by reductive methylation according to the method of Jentoft and Dearborn (14). One milligram of protein from parotid salivary secretion was incubated in a 1-ml reaction volume containing 4 mM [14C]formaldehyde (NEC-039H; Perkin-Elmer Japan Co., Ltd., Yokohama, Japan), 25 mM NaCNBH3, and 100 mM potassium phosphate buffer (pH 7.0). After incubation at 22°C for 95 min, 3 ml of 10% trichloroacetic acid was added. The precipitated protein was isolated by centrifugation, dissolved in buffered KCl, and dialyzed against buffered KCl (molecular cutoff, 10,000), and the protein concentration and radioactivity were measured with a liquid scintillation counter (2550 TR/LL; Packard).
(iii) Contact angle measurements.
The hydrophobicities of the polymer coatings and the conditioned surfaces of HA plates were determined by the measurement of water contact angles formed by droplets with the volume of 1 μl using a CA-D contact angle meter (Kyowa Interface Science Co., Ltd., Saitama, Japan). Values for contact angles are the means of 5 drops on two surfaces.
In vivo studies. (i) Dental biofilm development in rats.
Two-month-old male dental biofilm-susceptible ODU rats (13) were obtained from Kiyoshi Ohura (Osaka Dental University). We housed each two rats in a plastic cage in a room maintained at 23 ± 2.0°C, with a relative humidity at 55 × 10% and a daily photoperiod of 12 h. Rats had free access to a standard diet for the maintenance of rats (CE-2; CLEA Japan, Inc., Tokyo, Japan) and drinking water. All animal experiments were conducted in the Experimental Animal Facility of Kao Tochigi Institute. The Animal Care Committee of Kao Tochigi Institute approved the present study. All experiments strictly followed the guidelines of that committee. The handling of animals was monitored by officially qualified animal care personnel.
Rats were anesthetized with an intraperitoneal injection of Nembutal (25 mg/kg). Dental biofilm on the surface of lower incisors was disclosed prior to its removal by using a sterile scaler and cotton balls. The remaining interdental biofilm was removed by using dental floss. Complete plaque removal was confirmed by further disclosing.
Twenty rats were randomly divided into two groups. Control and experimental groups had free access to dW and the test solution, respectively. Both groups were fed on a powdered MF diet (Oriental Yeast Co., Ltd., Tokyo, Japan) containing 50% sucrose for 96 h. Both longitudinal and transverse distances of the disclosed area were measured on the buccal surface of each incisor by using a calibrated periodontal probe, to the nearest 0.5 mm (Thin Williams, Hu-Friedy, Chicago, IL). The area (in square millimeters) of dental biofilm deposition was calculated by multiplying the above measurement.
Each rat served as its own control, using a crossover experimental design in which half of the animals received test solution first and half received vehicle (dW) first. The drinking solution was replaced by the alternate treatment 5 days after the previous treatment had ceased.
(ii) Dental plaque development in humans.
Systemically healthy adult subjects from Kao Tochigi Institute participated. No subjects exhibited clinical evidence of salivary gland disorder. Prior to the experiment, all subjects gave informed consent to the protocol, which was approved by the Ethics Committee of Kao Tochigi Institute.
Ten male subjects (26 to 40 years old) were randomly assigned to control (four subjects), pyrophosphate (PP; three subjects), or PP+polymer (three subjects) groups. Tooth biofilm was removed mechanically by a single well-trained oral hygienist. Dental plaque was disclosed prior to its removal by using rubber cups with polishing paste. The remaining interdental biofilm was removed with dental floss and polishing via Eva chips with polishing paste. Complete biofilm removal was confirmed by further disclosing.
Immediately after teeth cleaning, the subjects were requested to rinse their teeth with 20 ml of dW (for control subjects) or PP or PP+polymer solution. The subjects were instructed to rinse their teeth with the supplied solution before sleep. They were requested not to clean their teeth and not to consume alcohol and high sugar-containing products (e.g., sweets or sweet beverages) during the 24-h test period.
Dental biofilm accumulation was measured 24 h after teeth cleaning according to our previous study (29). Briefly, a single well-trained oral hygienist disclosed and measured the dental plaque on all buccal and lingual surfaces according to the method of Suzuki et al. (31). The distance from the gingival margin to the edge of the disclosed area was measured to the nearest 0.5 mm at five sections on either the buccal or lingual surfaces of each tooth by using a calibrated periodontal probe. Plaque-free sections were designated as “0 mm.” A filled section of restored teeth was excluded from the measurement. To produce an unbiased estimate, the measurements on each tooth were averaged, and then the whole-mouth average distance (in millimeters) was calculated as the dental biofilm development.
The study had a single-blinded, crossover design. The mouth rinse solution was replaced by separate treatment at least 5 days after the previous treatment had ceased.
Statistical analysis.
For the in vitro bacterial adhesion study, Student t tests after a preliminary F test of the homogeneity of within-group variance were used when we compared values between the parametric data. When more than two groups were compared, statistical analysis was conducted by using analysis of variance (ANOVA) and subsequently using Fisher PLSD multiple comparison (STATVIEW for Windows, version 5.0; SAS Institute, Inc., Cary, NC). For in vivo studies on dental biofilm formation, we adopted the Wilcoxon matched-pair signed-rank test for comparisons between nonparametric data. Numerical data were expressed as means ± the standard error of the mean (SEM). Differences were considered significant when the error probability was smaller than 0.05.
RESULTS
Effect of phosphorylated PEGs on parotid saliva-promoted S. mutans adhesion onto HA surfaces in vitro.
The number of S. mutans organisms that adhered to sintered HA plates was significantly increased by parotid saliva treatment of the plates. However, pretreatment of HA plates with phosphorylated PEG derivatives (Fig. 1) followed by saliva treatment significantly reduced S. mutans adhesion on HA surfaces. Polymer A inhibited saliva-promoted S. mutans adhesion in a dose-dependent manner (Table 1, experiment 1). The maximum inhibition was observed at 5 mg/ml. There was no further inhibitory effect of the compound when used at a higher concentration (25 mg/ml).
Substitution of MDP (polymers B and C) by MEP (polymer D) completely diminished the inhibitory effect of phosphorylated PEG on saliva-promoted S. mutans adhesion (Table 1, experiment 2). The inhibitory effect of phosphorylated PEG on S. mutans adherence onto saliva-treated HA surfaces decreased significantly when PEG was changed from120 EO (ethoxy group) (polymers E, H, I, and K) to either 90 (F and G) or 23 EO (J and L) (Table 1, experiments 3 and 4). Elimination of the methacrylate group did not affect the inhibitory effect of phosphorylated PEG.
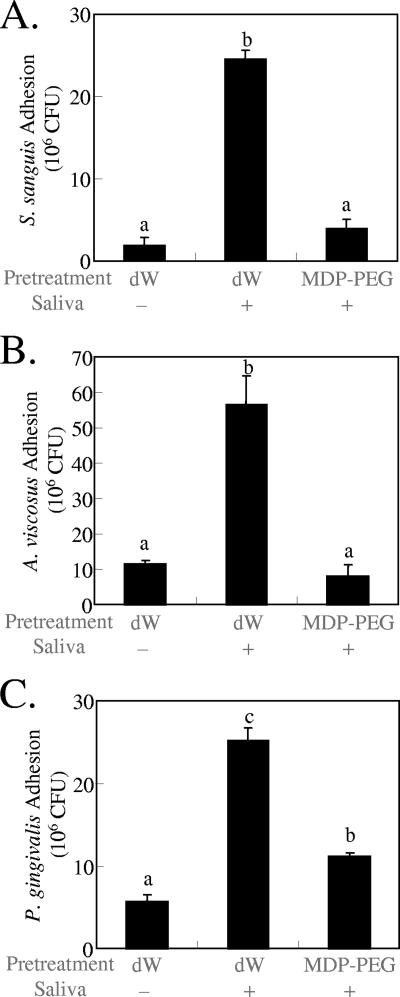
Effect of MDP-PEG on adhesion of oral bacteria.
The effect of MDP-PEG on the adhesion of other oral bacteria onto HA surfaces was examined. Adhesion of either S. sanguis (Fig. 2A), A. viscosus (Fig. 2B), or P. gingivalis (Fig. 2C) was promoted by the treatment of HA surfaces with parotid saliva. Pretreatment of HA plates with MDP-PEG, followed by saliva treatment, significantly reduced the adsorption of each bacterial type.
FIG. 2.
Inhibitory effect of MDP-PEG (10 mg/ml, l/m/n = 32/8/60, r = 10, q = 120, average MW = 35,000) on S. sanguis (A), A. viscosus (B), and P. gingivalis (C) adhesion onto sintered HA plates (means ± the SEM; n = 3). Statistical analysis was conducted by using ANOVA and then Fisher PLSD multiple comparison. Means not sharing a given letter (a, b, and c) differ significantly (P < 0.05).
Effect of MDP-PEG on hydrophobicity of HA surfaces.
The effect of polymer treatment on surface properties was examined by measuring the water contact angle. The water contact angle of the HA surface was significantly reduced when treated by active (antiadherent for S. mutans, see Table 1) polymers (polymers A to C), but not by inactive polymer (polymer D) (Table 2).
TABLE 2.
Water contact angles of polymer-treated HA surfaces
| Treatment | Concn (mg/ml) | Mean water contact angle (θw) ± SEMa |
|---|---|---|
| dW | 63.6 ± 3.3 | |
| Polymer A | 10 | 32.8 ± 1.3* |
| Polymer B | 10 | 36.5 ± 1.1* |
| Polymer C | 10 | 40.3 ± 2.0* |
| Polymer D | 10 | 68.3 ± 2.5 |
Unpaired Student t tests after a preliminary F test of the homogeneity of within-group variance were conducted between dW treatment and polymer treatment results. *, P < 0.001.
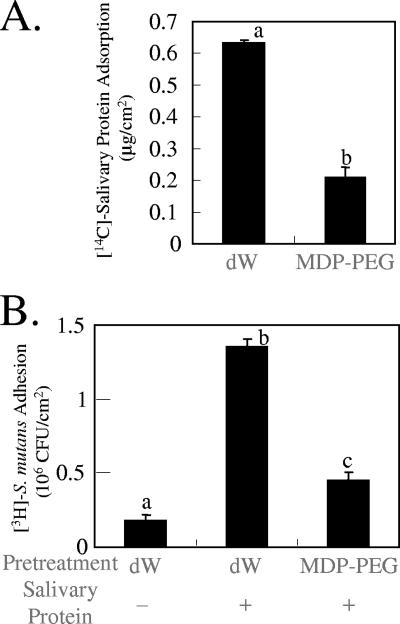
Effect of MDP-PEG on salivary protein adsorption onto HA surfaces.
To investigate the effect of MDP-PEG on salivary protein adsorption onto HA surfaces, an adsorption experiment was carried out with 14C-labeled salivary protein and 3H-labeled S. mutans. Pretreatment of HA plates with MDP-PEG, followed by salivary protein treatment significantly reduced the adsorption of 14C-labeled salivary protein (Fig. 3A) and concomitantly 3H-labeled S. mutans adherence (Fig. 3B) onto sHA plates.
FIG. 3.
Inhibition of salivary protein adsorption onto sHA by polymer. Sintered HA plates (7 by 5 by 1 mm) were treated with MDP-PEG (l/m/n = 50/10/40, r = 10, q = 120, average MW = 34,000), followed by 14C-labeled salivary protein. After being washing, the plates were treated with 3H-labeled S. mutans. 14C-labeled protein adsorption (μg/cm2) (A) and 3H-labeled S. mutans adhesion (CFU/cm2) (B) onto the sHA surfaces were determined by direct scintillation counting (means ± the SEM; n = 3). Means not sharing a given letter (a, b, and c) differ significantly (P < 0.05).
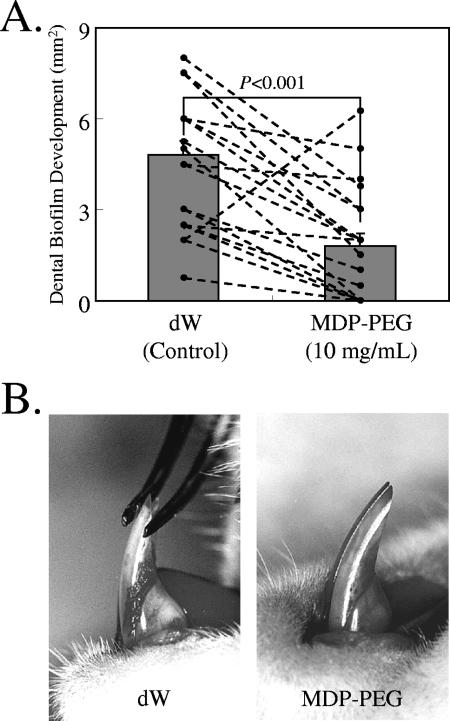
Effects of MDP-PEG on dental biofilm development in rats.
High sucrose diet-fed ODU rats developed dental plaque on the lower incisors. However, the rats administered MDP-PEG (10 mg/ml) via drinking water developed significantly less dental plaque than the control rats (Fig. 4). Water intake was significantly (P < 0.01) less in the experimental group (24.8 ± 3.1 ml/day) than in the control group (33.7 ± 7.6 ml/day). Average food intake (15.2 ± 2.2 and 15.0 ± 1.5 g/day/rat for the control and experimental groups, respectively) and body weight gain (6.9 ± 6.0 and 8.1 ± 3.3 g, respectively) were not significantly different between the groups.
FIG. 4.
Inhibitory effect of MDP-PEG (polymer A, Table 1) on dental biofilm formation in ODU rats at 96 h after feeding a high-sucrose powder diet. (A) Data represent the mean and standard error of 20 incisors (10 rats). A Wilcoxon matched-pair signed-rank test was conducted between dW (control) and polymer treatments. (B) Dental biofilm deposition on lower incisors of ODU rats administered dW (left) and MDP-PEG (10 mg/ml) (right) via drinking water.
Effects of MDP-PEG on parotid saliva-pretreated HA surfaces.
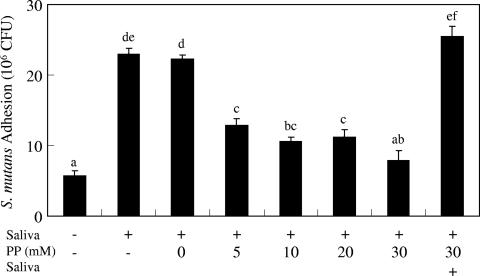
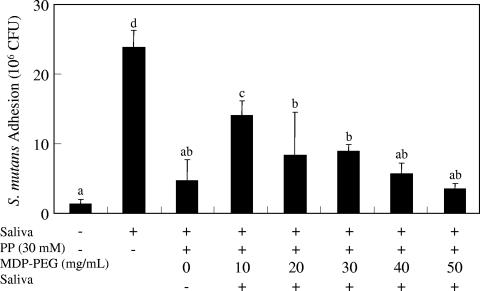
When MDP-PEG was applied prior to saliva, a reduction in bacterial adhesion on HA surfaces was observed. However, when saliva was added before MDP-PEG, no effect on HA was seen (data not shown). To overcome the ineffectiveness of MDP-PEG on saliva-pretreated HA surfaces, the efficacy of PP treatment, which has been proven to desorb proteins from HA surfaces (25), was investigated. PP treatment decreased S. mutans adhesion onto parotid saliva-pretreated HA plates in a dose-dependent manner (Fig. 5). Saliva-promoted S. mutans adhesion was inhibited completely by 30 mM PP treatment. However, the inhibitory effect of PP was completely diminished when the HA plates were treated further with parotid saliva after pyrophosphate treatment. Therefore, we investigated the combined effect of MDP-PEG and PP on bacterial adhesion onto saliva-pretreated HA surfaces. Treatment of saliva-pretreated HA surfaces by MDP-PEG plus PP (30 mM) reduced S. mutans adhesion depending upon the MDP-PEG concentration, even followed by additional saliva treatment (Fig. 6). The maximum inhibition by MDP-PEG was observed at 50 mg/ml.
FIG. 5.
Inhibitory effect of PP on S. mutans adhesion onto saliva-pretreated HA plates. Sintered HA plates were treated with parotid saliva followed by PP solution (0 to 30 mM). Three plates were treated further with parotid saliva after 30 mM PP treatment. After being washed, the plates were treated with 3H-labeled S. mutans. 3H-labeled S. mutans adhesion (CFU) onto the sHA surface was determined by direct scintillation counting (means ± the SEM; n = 3). Statistical analysis was conducted by using ANOVA and then a Fisher PLSD multiple comparison. Means not sharing a given letter (a, b, c, d, e, and f) differ significantly (P < 0.05).
FIG. 6.
Inhibition of S. mutans adhesion onto saliva-pretreated HA plates by MDP-PEG plus PP treatment. Sintered HA plates were treated with parotid saliva followed by PP (30 mM) or PP plus MDP-PEG (polymer E, Table 1, 10 to 50 mg/ml) solution. The plates were treated further with parotid saliva. After washing, the plates were treated with 3H-labeled S. mutans. 3H-labeled S. mutans adhesion (CFU) onto the sHA surface was determined by direct scintillation counting (means ± the SEM; n = 3). Statistical analysis was conducted by using ANOVA and then Fisher PLSD multiple comparison. Means not sharing a given letter (a, b, c, and d) differ significantly (P < 0.05).
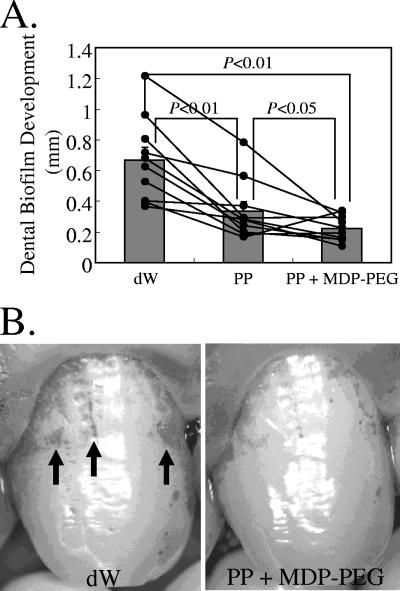
Effects of MDP-PEG plus PP on dental biofilm development in humans.
The subjects had developed significantly less dental biofilm at 24 h after professional teeth cleaning when administered PP solution via mouthwash compared to dW (control) (Fig. 7). In addition, mouth rinse with MDP-PEG plus PP solution further reduced dental biofilm development compared to PP alone (Fig. 7).
FIG. 7.
Inhibitory effect of MDP-PEG plus PP on dental biofilm formation in humans. (A) Inhibitory effect of MDP-PEG (polymer E, Table 1) plus PP on dental biofilm formation in humans. The black dots indicate the dental biofilm deposition of each subject. The gray bars show the mean and standard error of 10 subjects. A Wilcoxon matched-pair signed-rank test was conducted between the groups. (B) Dental biofilm deposition in humans at 24 h after professional teeth cleaning, followed by dW (left) or MDP-PEG plus PP (right) mouth rinse.
DISCUSSION
Hydrophobic interactions were shown to be important in bacterial adhesion onto tooth surfaces (23). Therefore, modification of the hydrophobicity of a surface could be used as an antiadherence strategy. In the present study, the surface hydrophilization with phosphorylated PEG derivatives was used for preventing saliva-promoted bacterial adhesion on hydroxyapatite. Pretreatment of the HA surface with MDP-PEG copolymers prior to saliva incubation hydrophilized the surface and thereby reduced salivary protein adsorption and saliva-promoted bacterial attachment to HA surfaces, mimicking statherin and histatin 1 (28).
It is well-known that phosphorylated biomolecules interact strongly with HA surfaces (10, 11). It has been suggested that statherin and histatin 1 bind quickly via their negatively charged N-terminal domains onto HA surfaces (5, 26) and compete with larger salivary glycoproteins for a similar binding site upon HA surfaces (8, 28). Our protein adsorption study clearly shows that 14C-labeled salivary protein adsorption is inhibited by pretreatment of the HA surface with MDP-PEG. This compound probably competes favorably with ionic groups, e.g., carboxyl groups, of S. mutans-binding salivary proteins for calcium ions on the HA surface. MDP-PEG reduced the HA attachment of not only S. mutans but also A. viscosus and P. gingivalis, of which adhesion was strongly promoted by salivary acidic proline-rich proteins (6). Thus, the inhibitory effect of MDP-PEG on the adhesion of these bacteria might be attributed to the reduced adsorption of salivary components, e.g., acidic proline-rich proteins that promote bacterial binding. Substitution of MDP (polymers B and C) by MEP (polymer D) completely diminished the hydrophilizing effect of MDP-PEG on the HA surface, as well as its inhibitory effect on saliva-promoted S. mutans adhesion. This implies that the alkyl spacer separating the phosphate group from the polymer chain may be critical for hydrophilization of the HA surface by this compound and thereby the prevention of salivary protein adsorption onto HA surfaces.
MDP-PEG, a novel antiadherent polymer, prevents biofilm development on lower incisors in ODU rats when applied immediately after teeth cleaning and then via drinking water. MDP-PEG did not show bactericidal activities in our preliminary study (data not shown), suggesting that the anti-dental biofilm activities of this compound in vivo are attributed to reduced initial bacterial attachment onto enamel surfaces.
In the human oral environment, the tooth surface is covered by salivary secretion immediately after teeth cleaning. When MDP-PEG was added to saliva-pretreated HA surfaces, its inhibitory effect on bacterial binding was completely diminished. This is consistent with the study of Olsson et al. (19), who showed that triphosphate of polyalkylene oxide glycerol did not reduce S. mutans adherence to saliva-coated HA surfaces, probably because the binding of this compound was effectively prevented by salivary components already bound to the HA. Thus, desorption of salivary components from the HA surface seemed essential for the antiadherence strategy using MDP-PEG in the human oral environment.
PP treatment of saliva-pretreated HA reduced S. mutans adherence successfully to the saliva-untreated HA level at a concentration of 30 mM. This is compatible with earlier studies which showed PP desorbed protein and acquired pellicles from HA surfaces (24, 25). However, reduced bacterial binding onto saliva-pretreated HA by PP was overshadowed by additional treatment of the HA surface with saliva. This may be due to the readsorption of salivary proteins on HA surfaces. Therefore, we combined PP and MDP-PEG to accomplish either the desorption of salivary components from HA surfaces or the prevention of protein and bacterial attachment onto HA surfaces. Treatment of saliva-pretreated HA surfaces with MDP-PEG plus PP could reduce S. mutans adhesion even when followed by additional saliva treatment. However, the inhibitory effect of MDP-PEG (10 mg/ml) on bacterial adhesion was greatly diminished by the addition of PP (30 mM), conceivably because PP may interfere with MDP-PEG adsorption onto HA surfaces. When combined with PP, a higher concentration (50 mg/ml) of MDP-PEG is necessary for the complete inhibition of saliva-promoted bacterial adherence.
In our clinical study, mouthwash with PP alone prevented de novo biofilm development after thorough teeth cleaning in humans, presumably because PP desorbs acquired pellicles and retards pellicle formation. It should be noted that MDP-PEG plus PP reduced biofilm formation more than PP alone. The additional prevention of biofilm development by MDP-PEG plus PP may be attributed to the prevention of protein and bacterial attachment onto the HA surface by MDP-PEG.
We previously found that de novo dental biofilm development in vivo was positively correlated with saliva-promoted S. mutans adhesion onto HA surface in vitro (29). This finding encouraged us to study whether dental biofilm formation could be reduced by an antiadherence strategy. Reduction of de novo dental biofilm development by MDP-PEG plus PP was consistent with our previous finding (29). Dental biofilm formation could likely be controlled by antiadherence MDP-PEG plus PP treatment. The results of this pilot study, while encouraging, should be considered preliminary and not conclusive. Further study using a larger number of human subjects is necessary to corroborate this preliminary finding.
Finally, we conclude MDP-PEG plus PP has the potential for use as an antiadherence agent in mouthwash and prevents dental biofilm formation after thorough teeth cleaning. The use of antimicrobial agents is probably the most common approach for oral hygiene in developed nations. An alternative approach is to prevent the initial attachment of bacteria to the tooth surface. Although the above antiadherence agents are effective, total reduction of dental biofilm deposition is unlikely. Dental biofilm should be preferably controlled accompanied by a skewed repopulation of the innocuous members of the commensal flora. Thus, further studies are still required to control dental biofilm, e.g., by directing a combined antiadherence/antimicrobial strategy at specific pathogenic threats.
Acknowledgments
We thank Naoki Yamamoto, Miyuki Okajima, and Hatsumi Souno for excellent technical assistance.
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Al-Hashimi, I., and M. J. Levine. 1989. Characterization of in vivo salivary-derived enamel pellicle. Arch. Oral Biol. 34:289-295. [DOI] [PubMed] [Google Scholar]
- 2.Bowden, G. H., and I. R. Hamilton. 1998. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 9:54-85. [DOI] [PubMed] [Google Scholar]
- 3.Brady, L. J., D. A. Piacentini, P. J. Crowley, P. C. Oyston, and A. S. Bleiweis. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 60:1008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busscher, H. J., M. M. Cowan, and H. C. van der Mei. 1992. On the relative importance of specific and nonspecific approaches to oral microbial adhesion. FEMS Microbiol. Rev. 8:199-209. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll, J., Y. Zuo, T. Xu, J. R. Choi, R. F. Troxler, and F. G. Oppenheim. 1995. Functional comparison of native and recombinant human salivary histatin 1. J. Dent. Res. 74:1837-1844. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons, R. J. 1989. Bacterial adhesion to oral tissues: a model for infectious diseases. J. Dent. Res. 68:750-760. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons, R. J., and I. Etherden. 1985. Albumin as a blocking agent in studies of streptococcal adsorption to experimental salivary pellicles. Infect. Immun. 50:592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons, R. J., and D. I. Hay. 1989. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J. Dent. Res. 68:1303-1307. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons, R. J., and J. V. Qureshi. 1976. Microbial aspects of dental caries. Information Retrieval, Washington, DC.
- 10.Hay, D. I. 1973. The isolation from human parotid saliva of a tyrosine-rich acidic peptide which exhibits high affinity for hydroxyapatite surfaces. Arch. Oral Biol. 18:1531-1541. [DOI] [PubMed] [Google Scholar]
- 11.Hay, D. I. 1975. Fractionation of human parotid salivary proteins and the isolation of a histidine-rich acidic peptide which shows high affinity for hydroxyapatite surfaces. Arch. Oral Biol. 20:553-558. [DOI] [PubMed] [Google Scholar]
- 12.Humphries, M., J. F. Jaworzyn, and J. B. Cantwell. 1986. The effect of a range of biological polymers and synthetic surfactants on the adhesion of a marine Pseudomonas sp. strain NCMB 2021 to hydrophilic and hydrophobic surfaces. FEMS Microbiol. Lett. 38:299-308. [Google Scholar]
- 13.Ito, N., M. Shinohara, Y. Azuma, and M. Mori. 1977. Experimental gingivitis in ODU plaque-susceptible rats. I. Changes of plaque formation and body weight. J. Periodontol. 48:201-208. [DOI] [PubMed] [Google Scholar]
- 14.Jentoft, N., and D. G. Dearborn. 1979. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J. Biol. Chem. 254:4359-4365. [PubMed] [Google Scholar]
- 15.Kishimoto, E., D. I. Hay, and R. J. Gibbons. 1991. Inhibition of adhesion-promoting activity of a human salivary protein which promotes adhesion of Streptococcus mutans JBP to hydroxyapatite. FEMS Microbiol. Lett. 69:19-22. [DOI] [PubMed] [Google Scholar]
- 16.Lamkin, M. S., and F. G. Oppenheim. 1993. Structural features of salivary function. Crit. Rev. Oral Biol. Med. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 17.Liljemark, W. F., and C. Bloomquist. 1996. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 7:180-198. [DOI] [PubMed] [Google Scholar]
- 18.Mogi, M., B. Y. Hiraoka, M. Harada, T. Kage, and T. Chino. 1986. Analysis and identification of human parotid salivary proteins by micro two-dimensional electrophoresis and Western blot techniques. Arch. Oral Biol. 31:337-339. [DOI] [PubMed] [Google Scholar]
- 19.Olsson, J., A. Carlen, and K. Holmberg. 1990. Inhibition of Streptococcus mutans adherence by means of surface hydrophilization. J. Dent. Res. 69:1586-1591. [DOI] [PubMed] [Google Scholar]
- 20.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion: isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 21.Paul, J. H., and W. H. Jeffrey. 1985. Evidence for separate adhesion mechanisms for hydrophilic and hydrophobic surfaces in Vibrio proteolytica. Appl. Environ. Microbiol. 50:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridgway, H. F., M. G. Rigby, and D. G. Argo. 1985. Bacterial adhesion and fouling of reverse osmosis membranes. J. Am. Water Works Assoc. 77:97-106. [Google Scholar]
- 23.Rosenberg, M., H. Judes, and E. Weiss. 1983. Cell surface hydrophobicity of dental plaque microorganisms in situ. Infect. Immun. 42:831-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rykke, M., and G. Rölla. 1990. Desorption of acquired enamel pellicle in vivo by pyrophosphate. Scand. J. Dent. Res. 98:211-214. [DOI] [PubMed] [Google Scholar]
- 25.Rykke, M., G. Rölla, and T. Sonju. 1988. Effect of pyrophosphate on protein adsorption to hydroxyapatite in vitro and on pellicle formation in vivo. Scand. J. Dent. Res. 96:517-522. [DOI] [PubMed] [Google Scholar]
- 26.Schlesinger, D. H., and D. I. Hay. 1977. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J. Biol. Chem. 252:1689-1695. [PubMed] [Google Scholar]
- 27.Shimomura, M. 1996. Surface characterizations of, and protein adsorption to, synthetic and sintered apatite plates. J. Osaka Odontol. Soc. 59:193-201. [Google Scholar]
- 28.Shimotoyodome, A., H. Kobayashi, I. Tokimitsu, T. Matsukubo, and Y. Takaesu. 2006. Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res. 40:403-411. [DOI] [PubMed] [Google Scholar]
- 29.Shimotoyodome, A., H. Kobayashi, I. Tokimitsu, T. Hase, T. Inoue, T. Matsukubo, and Y. Takaesu. 2007. Saliva-promoted adhesion of Streptococcus mutans MT8148 associates with dental plaque and caries experience. Caries Res. 41:212-218. [DOI] [PubMed] [Google Scholar]
- 30.Stephen, K. W., and C. F. Speirs. 1976. Methods for collecting individual components of mixed saliva: the relevance to clinical pharmacology. Br. J. Clin. Pharm. 3:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, K., T. Sueda, R. Asa, M. Toyoda, and S. Kinoshita. 1971. The effect of different types of toothbrushes on oral hygiene. J. Dent. Health 20:9-16. [DOI] [PubMed] [Google Scholar]
- 32.Whittaker, C. J., C. M. Klier, and P. E. Kolenbrander. 1996. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 50:513-552. [DOI] [PubMed] [Google Scholar]