Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is an emerging pathogen that primarily manifests as uncomplicated skin and soft tissue infections. We conducted a cluster randomized, double-blind, placebo-controlled trial to determine whether targeted intranasal mupirocin therapy in CA-MRSA-colonized soldiers could prevent infection in the treated individual and prevent new colonization and infection within their study groups. We screened 3,447 soldiers comprising 14 training classes for CA-MRSA colonization from January to December 2005. Each training class was randomized to either the mupirocin or placebo study group, and the participants identified as CA-MRSA colonized were treated with either mupirocin or placebo. All participants underwent repeat screening after 8 to 10 weeks and were monitored for 16 weeks for development of infection. Of 3,447 participants screened, 134 (3.9%) were initially colonized with CA-MRSA. Five of 65 (7.7%; 95% confidence interval [95% CI], 4.0% to 11.4%) placebo-treated participants and 7 of 66 (10.6%; 95% CI, 7.9% to 13.3%) mupirocin-treated participants developed infections; the difference in the infection rate of the placebo- and mupirocin-treated groups was −2.9% (95% CI, −7.5% to 1.7%). Of those not initially colonized with CA-MRSA, 63 of 1,459 (4.3%; 95% CI, 2.7% to 5.9%) of the placebo group and 56 of 1,607 (3.5%; 95% CI, 2.6% to 5.2%) of the mupirocin group developed infections; the difference in the infection rate of the placebo and mupirocin groups was 0.8% (95% CI, −1.0% to 2.7%). Of 3,447 participants, 3,066 (89%) were available for the second sampling and completed follow-up. New CA-MRSA colonization occurred in 24 of 1,459 (1.6%; 95% CI, 0.05% to 2.8%) of the placebo group participants and 23 of 1,607 (1.4%; 95% CI, 0.05% to 2.3%) of the mupirocin group participants; the difference in the infection rate of the placebo and mupirocin groups was 0.2% (95% CI, −1.3% to 1.7%). Despite CA-MRSA eradication in colonized participants, this study showed no decrease in infections in either the mupirocin-treated individuals or within their study group. Furthermore, CA-MRSA eradication did not prevent new colonization within the study group.
Once considered to be an unusual occurrence, infections with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) have become increasingly common, emerging as a growing public health concern (13, 20). The majority of CA-MRSA infections manifest as uncomplicated skin and soft tissue infections; however, severe infections resulting in considerable morbidity and mortality have been reported (4, 19, 20, 22, 34, 36). The scope of the problem with CA-MRSA continues to expand with infections occurring across a broad epidemiological spectrum that includes neonates (24) and professional athletes (26). Indeed, some groups, among whom soldiers are included, appear to be at increased risk (1, 3, 5, 6, 23, 33, 40, 53).
With mounting concern over CA-MRSA infections, many authors have displayed considerable interest in determining the best strategy to prevent CA-MRSA colonization and infection. Many have posited that the best method may be to identify and decolonize nasal carriers of CA-MRSA who appear at highest risk for infection (7, 10, 30, 32, 46, 47). Although other anatomical sites may be colonized, the anterior nares are the principal reservoir for S. aureus, and nasal colonization is associated with an increased risk of subsequent infection with the endogenous strain (12, 28, 45, 47, 49, 51, 52). Similarly, in a prospective observational study, we found that nasal colonization with CA-MRSA strains was associated with a significantly increased risk of subsequent soft tissue infections (15).
Mupirocin is a topical antimicrobial agent that decolonizes S. aureus from the anterior nares and reduces colonization at extranasal sites (14, 21, 42). The eradication of S. aureus colonization with mupirocin in order to prevent infections has been studied in various populations and has been shown to be effective in some groups (32, 43). The efficacy of intranasal mupirocin targeted at CA-MRSA-colonized individuals to prevent subsequent infections has yet to be determined.
Topical mupirocin avoids the potential adverse effects of systemic antimicrobial agents and can be easily administered in a directly observed manner. The widespread use of mupirocin in a community is not without consequence, as its indiscriminate use may result in the development of mupirocin-resistant strains, especially among MRSA (9, 29, 37). Additionally, in our recent natural history study of CA-MRSA colonization, we noted no new colonization in participants who were already colonized with methicillin-susceptible Staphylococcus aureus (MSSA), suggesting that occupying the ecological niche with a less virulent strain may be salutary (15, 28). For these reasons, a strategy of blanket administration to at-risk populations to prevent infections may be unwise; instead, a targeted approach aimed at only CA-MRSA carriers who are at higher risk for infection may be the most prudent strategy. Reducing the number of CA-MRSA-colonized people in a population also carries the potential benefit of reducing new colonization and infection in those not currently colonized.
We conducted this cluster randomized, double-blind, placebo-controlled trial to determine whether targeted and rapidly administered intranasal mupirocin in CA-MRSA-colonized soldiers would reduce the risk of infection in the treated individual, prevent new colonization within the larger, noncolonized population with whom they have direct contact (their study group), and reduce the risk of infection within the study group.
MATERIALS AND METHODS
Study design and participants.
This was a cluster randomized, double-blind, placebo-controlled trial approved by the Brooke Army Medical Center Institutional Review Board (C.2004.163) designed to determine whether selective CA-MRSA decolonization with mupirocin reduces infection in treated participants and prevents new colonization and infection within the treated individual's study group. U.S. Army personnel enrolled in the Health Care Specialist Course from 10 January 2005 to 16 December 2005 were eligible for the trial. This course is a 16-week program that trains soldiers (both women and men) to become combat medics, with the first 14 weeks of training conducted entirely outside of a health care setting. The study period included 14 sequential classes of soldiers. Soldiers in each class dined, trained, and were housed with each other in a highly regimented fashion. Although the training classes overlap at the same location (a new class every 3 weeks), classes are housed in separate barracks, conduct training separately, and have minimal to no direct or indirect interaction with other classes. With few exceptions, soldiers remain in the same class throughout the 16-week course.
Screening and assignment to study groups.
After written informed consent was obtained, soldiers were given a demographic questionnaire and screened for CA-MRSA nasal colonization on the first day of training. We prospectively defined CA-MRSA colonization as occurring in any study participant who was without known risk factors for whom MRSA was recovered on anterior nares screening culture. The pharmacy randomized classes with block randomization (by blocks of two) in an attempt to minimize the effect of possible seasonal variation of CA-MRSA prevalence. Study participants whose screening culture revealed CA-MRSA were eligible to receive either placebo or mupirocin treatment. After a second written informed consent was obtained, these CA-MRSA-colonized participants received either 2% nasal mupirocin calcium ointment (GlaxoSmithKline, Research Triangle Park, NC) or an identical-appearing placebo ointment in identical-appearing containers based on class randomization. All treated CA-MRSA-colonized participants within a class received the same study medication (placebo or mupirocin). Investigators used cotton-tipped applicators to administer all of the doses (0.5 gram), given in each nasal vestibule twice daily for 5 days. In the event of a missed dose, an additional day was added to ensure that a total of 10 doses were administered. No unsupervised doses were given. During the consent process, CA-MRSA-colonized participants were informed of their culture status, but investigators and participants remained blinded to the administered treatment throughout the study.
Using the same technique, investigators performed a second anterior nares culture and administered a second questionnaire to all study participants 8 to 10 weeks after the initial screening culture and prior to any health care exposure in the training program. A 2-week window was allotted to account for variations in the participants' military training schedule. Throughout the 16-week training course, all participants were monitored prospectively to assess for clinical skin and soft tissue infections. For any illness that occurred during this time, participants were seen in the same health care system (outpatient clinics, emergency department, and hospital, all served by the same microbiology laboratory), thus allowing complete capture of all clinical infections. Skin and soft tissue infections were identified by daily review of clinic records and administrative codes from the International Classification of Diseases, 9th revision (23a). Health care providers were blinded to anterior nares culture results obtained in the study. Anterior nares cultures to assess colonization status were not done on participants if they presented with an infection.
Specimen collection, identification, and analysis.
Investigators obtained anterior nares cultures using CultureSwabs with Stuart's transport medium (BD, BBL, Sparks, MD). Within 4 h of collection, technicians plated these specimens onto two special selective and differential culture media specifically aimed at recovery and identification of all S. aureus and MRSA. BBL CHROMagar MRSA and BBL CHROMagar Staph aureus media (BD Diagnostics, Sparks, MD) were inoculated with each specimen. BBL CHROMagar MRSA plates were incubated and read at 20 and 48 h, and BBL CHROMagar Staph aureus plates were incubated and read at 24 h according to the manufacturer's specifications. Definitive bacterial colony morphology on the two media precluded the need for any further identification tests (17). Wound culture isolates from abscesses were processed by the Brooke Army Medical Center clinical microbiology laboratory according to their standard protocols. All CA-MRSA isolates from the anterior nares screening and from clinical infection isolates obtained during the follow-up period were tested for antimicrobial susceptibility using the Clinical Laboratory Standards Institute (CLSI) disk diffusion method (8). Mupirocin susceptibility was also assessed by disk diffusion using previously published criteria (16). Additionally, all CA-MRSA isolates underwent molecular typing by pulsed-field gel electrophoresis following SmaI digestion. Pulsed-field gel electrophoresis findings were resolved and analyzed using Molecular Analyst software (Bio-Rad, Hercules, CA). Investigators interpreted results using established criteria (2, 44), and grouped them into pulsed-field types (PFTs) by the method of McDougal et al. (35).
Statistical analysis and sample size.
This was a modified intent-to-treat analysis of all participants who completed follow-up. The null hypotheses were that eradication of CA-MRSA colonization will not reduce soft tissue infections in mupirocin-treated participants and will not prevent new colonization or infection within their study group. Based on the natural history study conducted in this population, we anticipated a CA-MRSA prevalence of 3.0% and an infection rate of 38% in colonized participants (15). We calculated that 42 CA-MRSA-colonized participants were needed in each group to detect a reduction in infection rate from 38% to 10%. According to this estimate, at least 1,400 participants per group (2,800 total) would be needed to detect this decrease with a level of confidence of 95% and a power of 80% assuming an intraclass correlation coefficient of 0.0. The intraclass correlation coefficient describes the correlation among subjects within a training class (18). We estimated an overall infection rate within the entire study sample of 3.57% (15). According to this estimate, we calculated that at least 1,094 participants per group (2,188 total) would be needed to detect a 2% decrease (to 1.57%) with a level of confidence of 95% and a power of 80%. We estimated a new colonization rate within the entire study population of 1.6% (15); based on this estimate, we calculated that at least 1,529 participants per group (3,058 total) would be needed to detect a decrease to 0.005% with a significance level of 0.05 and a power of 80%. Based on an anticipated average class size of 250 soldiers, we estimated that 14 classes would be needed for the investigation. Proportions (culture results and infection rates) were compared, accounting for correlation within classes, following the method given by Fleiss et al. (18). We used SPSS statistical software, version 14.0 (SPSS, Chicago, IL) and SAS software (SAS Institute, Cary, NC) for our analyses.
RESULTS
Population characteristics.
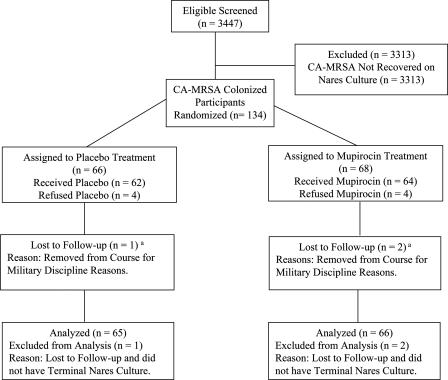
Of the 4,003 soldiers eligible for the study, 3,447 (86.1%) volunteered to participate and 556 declined. Seven classes (1,669 participants) were randomized to the placebo-treated group, and seven classes (1,778 participants) were randomized to the mupirocin-treated group (Fig. 1). Overall, at the initial anterior nares culture, 134 participants (3.9%) were colonized with CA-MRSA, 1,316 participants (38.2%) were colonized with MSSA, and 1,997 participants (57.9%) did not have S. aureus recovered on screening culture. Initial colonization with CA-MRSA was noted in 66 of 1,669 participants (4.0%) in the placebo group and 68 of 1,778 participants (3.8%) in the mupirocin group. Of the 134 CA-MRSA-colonized participants initially identified, 62 were treated with placebo and 64 were treated with mupirocin (Fig. 2). These treated CA-MRSA-colonized participants are hereafter referred to as placebo-treated or mupirocin-treated participants. Four CA-MRSA-colonized participants (three from the same class and one from another class) from the placebo group declined treatment, and four CA-MRSA-colonized participants (three from the same class and one from another class) from the mupirocin group declined treatment. All of those who declined were men.
FIG. 1.
Overall study profile. A total of 1,459 participants in the placebo-treated group and 1,607 participants in the mupirocin-treated group completed follow-up and were included in the modified intent-to-treat analyses for infection and colonization. The a superscript indicates that no participants left the study due to CA-MRSA infection.
FIG. 2.
Treatment profile for CA-MRSA-colonized participants. A total of 65 placebo-treated participants and 66 mupirocin-treated participants completed follow-up and were included in the modified intent-to-treat analyses for infection and colonization. One of the 66 CA-MRSA-colonized participants in the placebo group and 2 of the 68 in the mupirocin group left training prematurely and had declined treatment. The a superscript indicates that no participants left the study due to CA-MRSA infection.
Demographic data for the two study groups (Table 1) and for the placebo-treated and the mupirocin-treated CA-MRSA-colonized participants are shown in the tables (Table 2). Placebo-treated and mupirocin-treated participants received their first dose on average 42 h after the initial anterior nares culture (range, 33 to 81 h). In the placebo-treated participants, 99.5% of possible doses were given, and in the mupirocin-treated participants, 99.2% possible doses were administered. One placebo-treated participant missed two doses, and two mupirocin-treated participants missed two doses. Medication doses were missed due to intervening military training requirements. All treated CA-MRSA-colonized participants completed the 16-week follow-up.
TABLE 1.
Characteristics of the 3,447 study participants by assigned study groupa
| Characteristic | Value for group
|
||
|---|---|---|---|
| Both (overall) (n = 3,447) | Placebo (n = 1,669) | Mupirocin (n = 1,778) | |
| No. of study classes | 14 | 7 | 7 |
| Mean class size (range) | 246 (124-373) | 238 | 254 |
| Mean age (yr) (SD) | 22.6 (5) | 23.0 (5) | 22.3 (5) |
| Gender | |||
| No. of males (%) | 2,493 (72.3) | 1,262 (75.6) | 1,231 (69.2) |
| No. of females (%) | 954 (27.7) | 407 (24.4) | 547 (30.8) |
The percentages may not total 100 due to rounding. See Materials and Methods for the differences noted between the study groups.
TABLE 2.
Characteristics of the treated participants
| Characteristic | Value for groupa
|
|
|---|---|---|
| Placebo (n = 62) | Mupirocin (n = 64) | |
| Mean age (yr) (SD) | 21.3 (3.3) | 21.6 (4.2) |
| Gender | ||
| No. of males (%) | 45 (72.6) | 39 (60.9) |
| No. of females (%) | 17 (27.4) | 25 (39.1) |
| First drug administration (h) (range) | 42 (33-81) | 42 (33-81) |
| Possible no. of doses delivered (%)b | 617/620 (99.5) | 635/640 (99.2) |
Data for the 62 of 65 placebo-treated participants and 64 of 66 mupirocin-treated participants included in the analysis who actually received treatment.
Ten possible doses of study medication per participant. See Materials and Methods for the dosing regimen.
Infections.
During the 16-week follow-up, 5 of 65 (7.7%; 95% confidence interval [95% CI], 4.0% to 11.4%) placebo-treated and 7 of 66 (10.6%; 95% CI, 7.9% to 13.3%) mupirocin-treated participants developed infections (Table 3). The estimated intraclass correlation coefficients for placebo- and mupirocin-treated groups were −0.034 and −0.057, respectively. The difference in the infection rate for the placebo and mupirocin groups was −2.9% (95% CI, −7.5% to 1.7%). None of the eight CA-MRSA-colonized participants who declined treatment with either placebo or mupirocin developed an infection. Overall, 119 of 3,066 (3.9%; 95% CI, 3.2% to 4.6%) study participants developed infections during the 16-week follow-up period: 63 of 1,459 (4.3%; 95% CI, 2.7% to 5.9%) in the placebo group and 56 of 1,607 (3.5%; 95% CI, 2.6% to 5.2%) in the mupirocin group (Table 4). The estimated intraclass correlation coefficients for the placebo and mupirocin groups were 0.006 and 0.0001, respectively. The difference in infection rate for the placebo and mupirocin groups was 0.8% (95% CI, −1.0% to 2.7%). Based on initial nares culture results, 22 of 548 (4.0%; 95% CI, 2.8% to 5.3%) MSSA-colonized participants in the placebo group and 20 of 645 (3.1%; 95% CI, 1.7% to 4.5%) in the mupirocin group developed skin and soft tissue infections; the difference in the infection rate of placebo and mupirocin group was 0.9% (95% CI, −0.9% to 2.8%). The estimated intraclass correlation coefficients were −0.0052 and 0.0009 for the placebo group and mupirocin group, respectively. In the participants for whom no S. aureus was recovered, 36 of 846 (4.3%; 95% CI, 2.3% to 6.2%) in the placebo group and 29 of 896 (3.2%; 95% CI, 2.6% to 3.9%) in the mupirocin group developed skin and soft tissue infections; the difference in the infection rate of the placebo and mupirocin groups was 1.1% (95% CI, −1.0% to 3.1%). The estimated intraclass correlation coefficients were 0.0081 and −0.005 for the placebo group and mupirocin group, respectively. There were no bacteremias detected in either group.
TABLE 3.
Infections in treated participants
| Characteristic | Value for group
|
|
|---|---|---|
| Placebo | Mupirocin | |
| No. of participants completing follow-upa | 65 | 66 |
| No. of infections/no. of participants (%)b | 5/65 (7.7) | 7/66 (10.6) |
| Total abscesses | 4 | 3 |
| CA-MRSA abscess | 2 | 3 |
| MSSA abscess | 0 | 0 |
| Abscess not culturedc | 2 | 0 |
| Folliculitis | 0 | 1 |
| Cellulitis | 1 | 3 |
| No. of hospital admissions | 0 | 0 |
Participants who were assigned to the placebo-treated or mupirocin-treated group who completed follow-up and were included in the analysis.
Data based on infections identified during the 16-week follow-up period. The difference in the number of infections for the placebo and mupirocin groups was −2.9% (95% CI, −7.5% to 1.7%).
Describes an abscess that was incised and drained but no specimen was sent for bacterial culture.
TABLE 4.
Infections in all participants by assigned study group
| Characteristic | Value for group
|
|
|---|---|---|
| Placebo | Mupirocin | |
| No. of participants completing follow-upa | 1,459 | 1,607 |
| No. of clinical infections/no. of participants (%)b | 63/1,459 (4.3) | 56/1,607 (3.5) |
| Total abscesses | 45 | 38 |
| CA-MRSA abscess | 20 | 19 |
| MSSA abscess | 4 | 6 |
| Abscess not culturedc | 21 | 13 |
| Folliculitis | 1 | 2 |
| Cellulitis | 17 | 16 |
| No. of hospital admissions | 2 | 5 |
Participants who were assigned to the placebo or mupirocin group who completed follow-up and were included in the analysis.
Clinical infections occurring in the 3,066 participants during the 16-week follow-up period. The difference in the number of infections for the placebo and mupirocin groups was 0.8% (95% CI, −1.0% to 2.7%).
Describes an abscess that was incised and drained but no specimen was sent for bacterial culture.
Persistence of CA-MRSA colonization.
Overall, 3,066 (89%) participants were available for the second anterior nares culture and completed the 16-week study period and were included in the colonization analysis: 1,459 (87.4%) in the placebo-treated group and 1,607 (90.4%) in the mupirocin-treated group. Reasons why the participants did not complete the study included the following: academic failure, disciplinary action, military reassignment, and personal emergencies. Those who did not complete the study were excluded from the analysis. The initial colonization results and demographic characteristics (age and gender) of those who completed the study and those who did not complete the study did not differ. Three CA-MRSA-colonized participants, one in the placebo group and two in the mupirocin group, left the course prematurely and did not have a final nares culture. Thus, for the colonization analysis, data from 131 (65 placebo-treated and 66 mupirocin-treated) participants of the 134 (97.8%) initially CA-MRSA-colonized participants were available and included in the analysis. At the 8- to 10-week follow-up anterior nares culture, CA-MRSA colonization decreased from 4.0% (95% CI, 1.1% to 6.9%) to 3.2% (95% CI, 1.0% to 5.5%) in the placebo group; the estimated intraclass correlation coefficients in the placebo and mupirocin groups were 0.035 and 0.032, respectively (Table 5). In the mupirocin group, CA-MRSA colonization decreased from 3.8% (95% CI, 1.9% to 5.7%) to 1.9% (95% CI, 1.1% to 2.8%); the estimated intraclass correlation coefficients in the placebo and mupirocin groups were 0.012 and 0.002, respectively. In CA-MRSA-colonized participants in the placebo group, CA-MRSA colonization was eliminated in 42 of 65 (64.6%; 95% CI, 52.5% to 75.1%). In CA-MRSA-colonized participants in the mupirocin group, colonization was eliminated in 58 of 66 participants (87.9%; 95% CI, 77.9% to 93.7%).
TABLE 5.
Initial sampling results versus second sampling resultsa
| Initial screening culture result (no.) | Second screening culture result (no. [%])
|
||
|---|---|---|---|
| CA-MRSA-colonized | MSSA-colonized | No S. aureus | |
| Placebo group | |||
| CA-MRSA-colonized (65) | 23 (35.4) | 6 (9.2) | 36 (55.4) |
| MSSA-colonized (548) | 8 (1.5)b | 344 (62.8) | 196 (35.8) |
| No S. aureus (846) | 16 (1.9)b | 84 (9.9) | 746 (88.2) |
| Mupirocin group | |||
| CA-MRSA-colonized (66) | 8 (12.1) | 10 (15.2) | 48 (72.7) |
| MSSA-colonized (645) | 7 (1.1)b | 405 (62.8) | 233 (36.1) |
| No S. aureus (896) | 16 (1.8)b | 78 (8.7) | 802 (89.5) |
Data are tabulated from the 3,066 participants (131 of 134 initially CA-MRSA-colonized participants) with both initial and second culture results. A total of 210 participants in the placebo group and 171 in the mupirocin group were excluded from this analysis (see text).
Difference in the new CA-MRSA colonization for the placebo group (24/1,459 [1.6%; 95% CI, 0.5% to 2.8%]) and mupirocin group (23/1,607 [1.4%; 95% CI, 1.3 to 2.3%]) is 0.2% (95% CI, −1.3% to 1.7%).
In the placebo-treated group, new colonization was noted in 24 of 1,459 participants (1.6%; 95% CI, 0.05% to 2.8%). The estimated intraclass correlation coefficient was 0.01. Among these 24 participants, 8 had previously been colonized with MSSA and 16 had previously been colonized but S. aureus had not been recovered. In the mupirocin-treated group, new colonization was detected in 23 of 1,607 (1.4%; 95% CI, 0.05% to 2.3%). The estimated intraclass correlation coefficient was 0.006. Among the 23 participants who were newly colonized with CA-MRSA, 7 had previously been colonized with MSSA and 16 had previously been colonized but S. aureus had not been recovered. The difference in new CA-MRSA colonization rate for the placebo and mupirocin groups was 0.2% (95% CI, −1.3% to 1.7%). At the follow-up anterior nares culture, MSSA colonization in the entire study population (placebo and mupirocin groups combined) decreased from 38.2% to 30.2%.
During the study period, a total of 13 CA-MRSA-colonized participants also received trimethoprim-sulfamethoxazole (TMP-SMX), a drug that was active against all colonizing strains. Seven placebo-treated participants received TMP-SMX for soft tissue infections (four participants) or urinary tract infections (UTIs) (three participants). Only one of these seven had a positive CA-MRSA screening culture at the second sampling. Likewise, six mupirocin-treated participants received TMP-SMX for soft tissue infections (three participants) or UTIs (three participants), all of whom had a negative CA-MRSA screening culture at the second sampling.
Molecular analysis and mupirocin resistance.
The predominant colonizing PFT in our study was USA 300 (54% of CA-MRSA colonizing isolates [34 of 66 in the placebo-treated group and 38 of 68 in the mupirocin-treated group]), and the second most common was USA 800 (40% of CA-MRSA colonizing isolates). Only one of the 134 colonizing isolates was PFT USA 100. Of the 31 study participants who were CA-MRSA colonized at both the initial and terminal culture, all but 2 participants remained colonized with the identical strain. The two participants colonized with different strains had received no antimicrobial therapy and had had no hospital exposure. All CA-MRSA-colonized participants who developed soft tissue abscesses were infected with their original colonizing strain.
No mupirocin resistance was detected by disk diffusion in the 199 CA-MRSA isolates tested (165 colonizing isolates and 34 abscess isolates).
Of the 62 placebo-treated participants, 20 (32.3%) noted at least one adverse effect, and of the 64 mupirocin-treated participants, 9 (14.1%) noted at least one adverse effect. The primary adverse effect was rhinorrhea. No adverse effects that were deemed significant were noted in either treated group.
DISCUSSION
In this cluster randomized, double-blind, placebo-controlled trial of mupirocin-based intranasal decolonization of CA-MRSA-colonized soldiers, we found that despite intranasal decolonization, there was no decrease in the incidence of soft tissue infection in either the mupirocin-treated participants or within the mupirocin-treated study group. Additionally, treatment with mupirocin did not reduce new CA-MRSA colonization within the mupirocin study group.
Our study is unique because it is the first mupirocin-based eradication effort aimed at affecting CA-MRSA colonization and infection in a general population. The majority of mupirocin-based intervention studies have focused on preventing nosocomial infections or infections in patients undergoing dialysis (32, 43). Previous studies of intranasal mupirocin have shown benefit only in the subset of patients with S. aureus colonization (7, 39, 42). For example, Perl et al. showed a decrease from 7.7% to 4% in postoperative wound infections in patients who were colonized with S. aureus (42). Our investigation did not show a similar benefit in individual participants colonized with CA-MRSA, and there did not appear to be an impact on the study group. Several recently conducted trials demonstrated similar results, showing no benefit for either orthopedic patients (25) or nonsurgical patients (50).
There were significantly fewer infections in the placebo-treated group than we had anticipated despite the fact that CA-MRSA prevalence was similar to our previous study conducted in this population (15). The infection rate of 7.7% in the placebo-treated participants was considerably lower than the rate of 38% noted in our natural history study (15). This is the study's principal limitation. There are several possible reasons why we observed fewer infections. First, during the consent process, CA-MRSA-colonized participants were informed of their colonization status and educated about their possible increased risk for skin and soft tissue infections. Despite being blind to their treatment, it is possible that with their awareness of CA-MRSA colonization and the education provided by investigating physicians that participants may have altered personal hygiene practices in a manner that affected both colonization and subsequent infections. Additionally, the study group consisted of combat medic trainees, a group of individuals whose training includes the basic management of skin infections. It is possible that they used the information given in the study introduction and their training to institute hygiene changes or other interventions on their own that altered the measured outcomes. Another possibility is that the overall virulence of the colonizing strains in this study was somehow different. Evidence of this has been demonstrated in recent investigations that showed that PFT USA 300 is the principal cause of suppurative soft tissue infections in some urban areas and university-affiliated emergency departments (27, 38). In our study, PFT USA 300 represented only 54% of the initial isolates colonizing the anterior nares, although there was no difference in the number of PFT USA 300-colonized participants between both treatment groups. Further investigation using molecular techniques assessing virulence factors may further clarify reasons for this observation.
The point prevalence of CA-MRSA colonization at the initial anterior nares culture was 3.9%, similar to the 3.0% in our previous investigation in this population (15). This finding is considerably higher than a recent report that noted a national MRSA prevalence of 0.8% for the years 2001 and 2002; however, the authors of that study suggest that their work might underestimate the current MRSA prevalence (30). At the 8- to 10-week follow-up anterior nares culture, CA-MRSA colonization in the mupirocin-treated group decreased from 3.8% to 1.9%. In the placebo-treated group, CA-MRSA colonization decreased from 4.0% to 3.2% over the study period. Interestingly, in the initially CA-MRSA-colonized participants in the placebo group, only 23 of the 65 remained colonized at the terminal sampling. The seven CA-MRSA-colonized participants who received TMP-SMX (four for soft tissue infection and three for UTI) during the study period do not account for this reduction. There are several possible explanations for this decrease. The subjects' awareness of their CA-MRSA colonization status may have encouraged other hygiene interventions. It may be that overall personal hygiene improved after the earlier basic training environment and affected colonization overall (28). This notion is supported by the fact that during this investigation we observed a decrease in MSSA colonization from 38.2% to 30.2%, mirroring the decrease in CA-MRSA colonization. Likewise, in our prior investigation in this study population, we noted a similar decrease in MSSA colonization from 28% to 20% (15). Additionally, less crowding and fewer skin abrasions than probably occurred during the earlier basic training experience may also have had some impact (28, 31, 53). Some of the CA-MRSA-colonized participants we identified may have only been intermittent carriers, as S. aureus carriage is itself a dynamic process (28, 51). Weekly cultures of the anterior nares may have helped to describe the natural history of the carrier state in our study population; however, we were limited by military training requirements to the number of cultures that could be done (41). Likewise, the intermittent carrier state could account for the similar rates of new CA-MRSA-colonized participants in both study groups, as these too might have been intermittent carriers. The remarkably similar rates of new CA-MRSA colonization in each study group (1.6% versus 1.4%) suggests a consistent epidemiological dynamic which we were unable to assess with only two samplings.
Although known to compete for the same ecological niche (11, 47), new CA-MRSA colonization detected at the second sampling was not prevented by MSSA colonization at the initial anterior nares culture. Those who had been colonized with MSSA at the outset of the study developed CA-MRSA colonization at a rate that was not different from those who had had no S. aureus recovered on the initial culture.
This investigation benefits from several strengths. First, because we used a selective culture medium, we were able to rapidly identify CA-MRSA-colonized participants and begin eradication therapy within 2 days of the soldiers arriving for their training. This minimized the time available for noncolonized participants to become colonized. Second, our study population is comprised of healthy soldiers drawn from all over the country and who are also free of the confounder of prior health care exposure. Third, the level of compliance and the number of participants who were available to be followed rivals that of an inpatient interventional study. We were able to directly administer the study medication to these participants in an outpatient setting achieving a greater than 99% compliance with therapy. A full 89% of the study participants were monitored throughout the study period. Those who did not complete follow-up were physically unavailable, and they did not differ from those who completed the study; it is unlikely that these participants altered the study outcomes. Last, all of the study participants received care from a single medical institution, Brooke Army Medical Center, to include outpatient clinic, urgent care, emergency room, and inpatient encounters, thereby maximizing the capture of all pertinent clinical and microbiological data.
Our study has other limitations. First, additional anterior nares cultures would have helped to better describe the persistent colonization state and the epidemiological dynamic of CA-MRSA within our study population, but this was not possible for the reasons indicated above. Second, we were unable to assess carriage at extranasal sites. Such sites, especially the hands, pharynx, and perineum, can harbor S. aureus, and intranasal mupirocin has been shown to have sometimes failed in decolonizing these anatomic sites (47, 48). Intranasal colonization may sometimes be absent, even in outbreak settings (26). Last, we did not determine the colonization status of the training cadre who interact daily with the participants, and we did not determine whether these cadre developed infections. These people outside of our study may have served as a reservoir for new CA-MRSA colonization and somehow impacted our results.
We detected no mupirocin resistance in any colonizing or clinical CA-MRSA isolate. This finding is consistent with those of other short-term mupirocin intervention trials, suggesting that limited application does not select for resistance (42, 50).
In conclusion, this study showed no benefit of targeted, mupirocin-based CA-MRSA eradication in colonized participants or in their larger group. Despite intranasal eradication in CA-MRSA-colonized participants, there was no decrease in infections in the mupirocin-treated individuals or in their study group. CA-MRSA eradication did not prevent new colonization within the study group. Our data suggest that effective prevention strategies against CA-MRSA in at-risk populations may require broader and more elaborate interventions than simple 5-day intranasal mupirocin.
Acknowledgments
We thank BD Diagnostic Systems for their support with research materials. We thank GlaxoSmithKline for providing mupirocin and funding. Primary funding was provided by the Department of Clinical Investigations, Brooke Army Medical Center. We have no conflicts of interest.
Most importantly, we thank the soldiers of the 232nd Medical Battalion, Fort Sam Houston, TX; to whom we are indebted. Without their spirit of volunteerism, this work would not have been done. We thank Walter Mika, W. Tom Henderson, Kathy Johnson, and Johnny White for their efforts in obtaining screening samples; Cindy Kelly, who contributed to the molecular analysis; Harold Sano and Irene Lo, who provided research pharmacy support; and Michelle Wheeler who aided in data processing.
Footnotes
Published ahead of print on 6 August 2007.
REFERENCES
- 1.Baggett, H. C., T. W. Hennessy, K. Rudolph, D. Bruden, A. Reasonover, A. Parkinson, R. Sparks, R. M. Donlan, P. Martinez, K. Mongkolrattanothai, and J. C. Butler. 2004. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska, 1996-1998. J. Infect. Dis. 189:1565-1573. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman, T. L., G. A. Hancock, F. C. Tenover, and J. M. Miller. 1995. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J. Clin. Microbiol. 33:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckingham, S. C., L. K. McDougal, L. D. Cathey, K. Comeaux, A. S. Craig, S. K. Fridkin, and F. C. Tenover. 2004. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee children's hospital. Pediatr. Infect. Dis. J. 23:619-624. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997-1999. Morb. Mortal. Wkly. Rep. 48:707-710. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants- Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000-2003. Morb. Mortal. Wkly. Rep. 52:793-795. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections-Los Angeles County, California, 2002-2003. Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 7.Chambers, H. F. 2004. Mupirocin prophylaxis misses by a nose. Ann. Intern. Med. 140:484-485. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. Supplement M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Cookson, B. D. 1998. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J. Antimicrob. Chemother. 41:11-18. [DOI] [PubMed] [Google Scholar]
- 10.Creech, C. B., T. R. Talbot, and W. Schaffner. 2006. Community-associated methicillin-resistant Staphylococcus aureus: the way to the wound is through the nose. J. Infect. Dis. 193:169-171. [DOI] [PubMed] [Google Scholar]
- 11.Dall'Antonia, M., P. G. Coen, M. Wilks, A. Whiley, and M. Millar. 2005. Competition between methicillin-sensitive and methicillin-resistant Staphylococcus aureus in the anterior nares. J. Hosp. Infect. 61:62-67. [DOI] [PubMed] [Google Scholar]
- 12.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 13.Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin. Infect. Dis. 40:562-573. [DOI] [PubMed] [Google Scholar]
- 14.Doebbeling, B. N., D. R. Reagan, M. A. Pfaller, A. K. Houston, R. J. Hollis, and R. P. Wenzel. 1994. Long-term efficacy of intranasal mupirocin ointment: a prospective cohort study of Staphylococcus aureus carriage. Arch. Intern. Med. 154:1505-1508. [PubMed] [Google Scholar]
- 15.Ellis, M. W., D. R. Hospenthal, D. P. Dooley, P. G. Gray, and C. K. Murray. 2004. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:971-979. [DOI] [PubMed] [Google Scholar]
- 16.Finlay, J. E., L. A. Miller, and J. A. Poupard. 1997. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob. Agents Chemother. 41:1137-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flayhart, D., J. F. Hindler, D. A. Bruckner, G. Hall, R. K. Shrestha, S. A. Vogel, S. S. Richter, W. Howard, R. Walther, and K. C. Carroll. 2005. Multicenter evaluation of BBL CHROMagar MRSA medium for direct detection of methicillin-resistant Staphylococcus aureus from surveillance cultures of the anterior nares. J. Clin. Microbiol. 43:5536-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiss, J. L., B. Levin, and M. C. Paik. 2003. Statistical methods for rates and proportions, 3rd ed. John Wiley and Sons, New York, NY.
- 19.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 20.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 21.Fuller, A. T., G. Mellows, M. Woolford, G. T. Banks, K. D. Barrow, and E. B. Chain. 1971. Pseudomonic acid: an antibiotic produced by Pseudomonas fluorescens. Nature 234:416-417. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez, B. E., G. Martinez-Aguilar, K. G. Hulten, W. A. Hammerman, J. Coss-Bu, A. Avalos-Mishaan, E. O. Mason, and S. L. Kaplan. 2005. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115:642-648. [DOI] [PubMed] [Google Scholar]
- 23.Groom, A. V., D. H. Wolsey, T. S. Naimi, K. Smith, S. Johnson, D. Boxrud, K. A. Moore, and J. E. Cheek. 2001. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201-1205. [DOI] [PubMed] [Google Scholar]
- 23a.Hart, A. C., and C. A. Hopkins (ed.). 2001. ICD-9-CM: International Classification of Diseases, 9th revision, clinical modification, 6th ed. St. Anthony Publishing, Reston, VA.
- 24.Healy, C. M., K. G. Hulten, D. L. Palazzi, J. R. Campbell, and C. J. Baker. 2004. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin. Infect. Dis. 39:1460-1466. [DOI] [PubMed] [Google Scholar]
- 25.Kalmeijer, M. D., H. Coertjens, P. M. van Nieuwland-Bollen, D. Bogaers-Hofman, G. A. de Baere, A. Stuurman, A. van Belkum, and J. A. Kluytmans. 2002. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin. Infect. Dis. 35:353-358. [DOI] [PubMed] [Google Scholar]
- 26.Kazakova, S. V., J. C. Hageman, M. Matava, A. Srinivasan, L. Phelan, B. Garfinkel, T. Boo, S. McAllister, J. Anderson, B. Jensen, D. Dodson, D. Lonsway, L. K. McDougal, M. Arduino, V. J. Fraser, G. Killgore, F. C. Tenover, S. Cody, and D. B. Jernigan. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 27.King, M. D., B. J. Humphrey, Y. F. Wang, E. V. Kourbatova, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309-317. [DOI] [PubMed] [Google Scholar]
- 28.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kresken, M., D. Hafner, F. Schmitz, and T. A. Wichelhaus. 2004. Prevalence of mupirocin resistance in clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis: results of the Antimicrobial Resistance Surveillance Study of Paul-Ehrlich-Society for Chemotherapy, 2001. Int. J. Antimicrob. Agents 23:577-581. [DOI] [PubMed] [Google Scholar]
- 30.Kuehnert, M. J., D. Kruszon-Moran, H. A. Hill, G. McQuillan, S. K. McAllister, G. Fosheim, L. K. McDougal, J. Chaitram, B. Jensen, S. K. Fridkin, G. Killgore, and F. C. Tenover. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172-179. [DOI] [PubMed] [Google Scholar]
- 31.LaMar, J., R. Carr, C. Zinderman, and K. McDonald. 2003. Sentinel case of community-acquired methicillin-resistant Staphylococcus aureus onboard a naval ship. Mil. Med. 168:135-138. [PubMed] [Google Scholar]
- 32.Laupland, K. B., and J. M. Conly. 2003. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin. Infect. Dis. 37:933-938. [DOI] [PubMed] [Google Scholar]
- 33.Lindenmayer, J. M., S. Schoenfeld, R. O'Grady, and J. K. Carney. 1998. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch. Intern. Med. 158:895-899. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Aguilar, G., A. Avalos-Mishaan, K. Hulten, W. Hammerman, E. O. Mason, and S. L. Kaplan. 2004. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr. Infect. Dis. J. 23:701-706. [DOI] [PubMed] [Google Scholar]
- 35.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, L. G., F. Perdreau-Remington, G. Rieg, M. Sheherbano, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 37.Miller, M. A., A. Dascal, J. Portnoy, and J. Mendelson. 1996. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect. Control. Hosp. Epidemiol. 17:811-813. [DOI] [PubMed] [Google Scholar]
- 38.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, and D. A. Talan. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 39.The Mupirocin Study Group. 1996. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. J. Am. Soc. Nephrol. 7:2403-2408. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen, D. M., L. Mascola, and E. Bancroft. 2005. Recurring methicillin-resistant Staphylococcus aureus in a football team. Emerg. Infect. Dis. 11:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nouwen, J. L., A. Ott, M. F. Kluytmans-Vandenbergh, H. A. Boelens, A. Hofman, A. van Belkum, and H. A. Verbrugh. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin. Infect. Dis. 39:806-811. [DOI] [PubMed] [Google Scholar]
- 42.Perl, T. M., J. J. Cullen, R. P. Wenzel, M. B. Zimmerman, M. A. Pfaller, D. Sheppard, J. Twombley, P. P. French, L. A. Herwaldt, and the Mupirocin and the Risk of Staphylococcus aureus Study Team. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections in patients. N. Engl. J. Med. 346:1871-1877. [DOI] [PubMed] [Google Scholar]
- 43.Tacconelli, E., Y. Carmeli, A. Aizer, G. Ferreira, M. G. Foreman, and E. M. C. D'Agata. 2003. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin. Infect. Dis. 37:1629-1638. [DOI] [PubMed] [Google Scholar]
- 44.Tenover, F. C., R. D. Abreit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Weber, J. T. 2005. Community-associated methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 41(Suppl.):S269-S272. [DOI] [PubMed] [Google Scholar]
- 47.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 48.Wertheim, H. F., J. Verveer, H. A. Boelens, A. van Belkum, H. A. Verbrugh, and M. C. Vos. 2005. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob. Agents Chemother. 49:1465-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wertheim, H. F., M. C. Vos, A. Ott, A. van Belkum, A. Vos, J. A. Kluytmans, P. H. van Keulen, C. M. Vandenbroucke-Grauls, and H. A. Verbrugh. 2004. Risk and outcome of nosocomial Staphylococcus aureus in nasal carriers versus non-carriers. Lancet 364:703-705. [DOI] [PubMed] [Google Scholar]
- 50.Wertheim, H. F., M. C. Vos, A. Ott, A. Voss, J. A. Kluytmans, C. M. Vandenbroucke-Grauls, M. H. Meester, P. H. van Keulen, and H. A. Verbrugh. 2004. Mupirocin prophylaxis against nosocomial Staphylococcus aureus infections in nonsurgical patients. Ann. Intern. Med. 140:419-425. [DOI] [PubMed] [Google Scholar]
- 51.Williams, R. E. O. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27:56-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 315:91-96. [DOI] [PubMed] [Google Scholar]
- 53.Zinderman, C. E., B. Conner, M. A. Malakooti, J. E. LaMar, A. Armstrong, and B. K. Bohnker. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]