Abstract
Since there is a likelihood of coadministration of voriconazole and ritonavir, two studies were conducted to evaluate the potential of drug interaction. Study A was a randomized, placebo-controlled, two-period, parallel-group trial (n = 34). Study B had the same design without the placebo group (n = 17). In period 1, subjects received 200 mg voriconazole or placebo twice daily (BID) for 3 days (400 mg BID on day 1). In period 2, following a 7-day washout, subjects received ritonavir alone at 400 mg BID (study A) or 100 mg BID (study B) for 10 days (days 11 to 20), and then ritonavir was coadministered with 200 mg BID voriconazole or placebo for the next 10 days (days 21 to 30). Serial plasma samples were collected on days 3, 20, and 30, and safety data were collected throughout the study. High-dose (400 mg BID) ritonavir substantially reduced the steady-state mean voriconazole exposure (area under the concentration-time curve from 0 to 12 h [AUC0-12], −82%; maximum concentration [Cmax], −66%). However, the effect of low-dose (100 mg BID) ritonavir was less pronounced (AUC0-12, −39%; Cmax, −24%). The decrease in voriconazole exposure was probably due to the induction of CYP2C19 and CYP2C9 by ritonavir. It is interesting that one subject in each study exhibited the opposite effect of ritonavir on voriconazole exposure (a 2.5- to 3-fold increase), probably due to lack of CYP2C19. Voriconazole had no apparent effect on the exposure of high-dose ritonavir but slightly decreased the exposure of low-dose ritonavir (AUC0-12, −14%; Cmax, −24%). The safety profile of combination therapy was not notably different from that of voriconazole or ritonavir alone. Due to the significant effect of ritonavir on voriconazole exposure, coadministration of voriconazole with 400 mg BID ritonavir is contraindicated; coadministration with 100 mg BID ritonavir should be avoided, unless an assessment of the benefit/risk to the patient justifies the use.
Subjects most susceptible to serious fungal infections are typically immunocompromised, which includes patients infected with human immunodeficiency virus (HIV)/AIDS. For HIV/AIDS patients, protease inhibitors (PIs) such as ritonavir are part of standard combination antiretroviral therapy. It is likely that such patients who require antifungal treatment with voriconazole are already receiving ritonavir in combination with other antiviral agents.
Voriconazole is a broad-spectrum triazole antifungal agent approved for the primary treatment of acute invasive aspergillosis and as a salvage therapy for serious fungal infections caused by Scedosporium apiospermum and Fusarium species, as well as for candidemia in nonneutropenic patients. In common with other triazole antifungal agents, voriconazole inhibits fungal cytochrome P450 (CYP)-dependent 14-α-sterol demethylase, an essential enzyme in the synthesis of ergosterol (7, 8, 21). The results of in vitro and in vivo studies have shown that voriconazole is primarily metabolized by CYP2C19, and to a lesser extent by CYP2C9 and CYP3A, and it also inhibits the activities of CYP2C19, CYP2C9, and CYP3A, possibly through the saturation of active sites (11, 13, 23, 24, 28). It has been demonstrated that the genetic polymorphism of CYP2C19 accounts for a considerable proportion of the intersubject variability in voriconazole exposure (13, 14, 28). Studies conducted with healthy Caucasian and Japanese subjects have shown that poor metabolizers (PMs) have, on average, fourfold-higher voriconazole exposure (area under the concentration-time curve from 0 to 12 h [AUC0-12]) than their homozygous extensive-metabolizer (EM) counterparts; subjects who are heterozygous extensive metabolizers (HEMs) have, on average, twofold-higher voriconazole exposure than their EM counterparts (VFEND [voriconazole] package insert; Pfizer Inc. New York, NY). Nevertheless, no dose adjustment is recommended based on CYP2C19 status in clinical practice.
Ritonavir is a peptidomimetic inhibitor of HIV-1 protease, which disables the HIV protease process gag-pol polyprotein precursor and leads to production of noninfectious immature HIV particles. Based on in vitro and in vivo studies, ritonavir is primarily metabolized by CYP3A and to a lesser extent by CYP2D6, and it is a potent CYP3A inhibitor (9, 10, 12, 20). Ritonavir has dual effects of simultaneous CYP3A inhibition and induction, but the net pharmacokinetic outcome during chronic ritonavir therapy is inhibition of CYP3A activity. However, the magnitude of inhibition cannot be easily predicted, since the dose dependence of CYP3A induction by ritonavir has not been established (9, 10, 20). In addition, ritonavir appears to induce the activities of CYP1A2, CYP2C9, CYP2C19, and glucuronosyl transferase (12, 27). Because of the complexities associated with ritonavir metabolism, the magnitude of interaction between ritonavir and other drugs is difficult to predict, particularly for drugs that are metabolized by multiple enzymes.
Since voriconazole and ritonavir share at least one common hepatic metabolic pathway, CYP3A, and may also compete for CYP2C19 and CYP2C9, a potential pharmacokinetic interaction was expected. The recommended therapeutic dosing regimen of oral voriconazole (400-mg twice-daily [BID] loading doses on day 1, followed by 200-mg BID maintenance doses) was evaluated. Although the recommended maximum ritonavir dosing regimen is 600 mg BID, a 400-mg BID regimen was selected for evaluation in the first study (study A) due to concern about possible gastrointestinal tolerability issues in healthy subjects at the maximum ritonavir dose (15). In the second study (study B), 100 mg BID ritonavir was evaluated, since highly active antiretroviral therapy, using low-dose ritonavir (100 to 400 mg/day) in combination with other PIs, is increasingly common due to exposure enhancement of other PIs by ritonavir (2, 3, 6).
The primary objective of these two studies was to evaluate the effect of ritonavir on voriconazole pharmacokinetics at steady state in healthy male subjects and vice versa. The tolerability and safety of repeated doses of voriconazole coadministered with ritonavir were also evaluated.
(Some of the data in this article were presented as an abstract and poster presentation at the Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, Orlando, FL, March 2 to 6 2005. The abstract was published previously [Clin. Pharmacol. Ther. 77:40, 2005].)
MATERIALS AND METHODS
Study design.
Study A was a randomized, subject- and investigator-blind with respect to voriconazole, placebo-controlled (voriconazole only), two-period, parallel-group, multiple-dose, intragroup fixed-dose-sequence study of 34 healthy male subjects. Half of the enrolled subjects received voriconazole-matching placebo administered in the same fixed-dose sequence as active voriconazole. The subjects received the treatment displayed in Table 1. In period 1, the subjects received 200 mg BID voriconazole or placebo alone for 3 days (with 400-mg BID loading doses on day 1). The subjects were discharged from the Clinical Research Unit (CRU) of MDS Pharma Services (Phoenix, AZ) after the last pharmacokinetic sample was obtained on day 4 and returned to the CRU on day 10 with a minimum 7-day washout. In period 2, all subjects received 400 mg BID ritonavir alone for 10 days (days 11 to 20), and then ritonavir was coadministered with 200 mg BID voriconazole or placebo for the next 10 days (with 400-mg BID loading doses of voriconazole on day 21). The subjects were discharged from the CRU on day 31 after the last pharmacokinetic sample was obtained and returned for a follow-up visit 10 to 14 days after the last dose of study medication. The parallel placebo group was used as a control to rule out any potential changes in the pharmacokinetics of ritonavir given alone for 20 days.
TABLE 1.
Study A dosing schedule by treatment groupa
| Treatment group | Period 1 (days 1-3)b | Days 4-10 | Period 2
|
|
|---|---|---|---|---|
| Days 11-20 | Days 21-30b | |||
| 1 | Voriconazole alone (day 1, 400 mg BID; days 2 and 3, 200 mg BID) | Washout | Ritonavir alone (400 mg BID) | Coadministration (ritonavir 400 mg BID + voriconazole 200 mg BID)c |
| 2 | Matching placebo alone: (days 1 to 3, BID) | Washout | Ritonavir alone (400 mg BID) | Coadministration (ritonavir 400 mg BID + placebo BID)c |
Study A had both groups; study B had group 1 only, and the low-dose ritonavir (100 mg) was evaluated.
Only the morning dose of voriconazole/placebo was administered on days 3 and 30; only the morning dose of ritonavir was administered on day 30.
Two 400-mg loading doses of voriconazole/matching placebo were administered on day 21.
In study B, the same study design was used to evaluate the effect of low-dose ritonavir (100 mg BID) on voriconazole pharmacokinetics, except that the placebo group was dropped (Table 1) and the study was conducted at the CRU of Comprehensive NeuroScience (Ft. Lauderdale, FL).
The study protocols were approved by the local institutional review boards (study A, MDS Pharma Services Inc. Institutional Review Board, Lincoln, NE; study B, Independent Investigational Review Board, Plantation, FL). In both studies, subjects were enrolled after they signed the informed consent.
Study population.
The subjects enrolled in both studies were healthy nonsmoking males 18 to 55 years old with body mass indexes between 18 and 30 kg/m2 and weighing >50 kg. Their health was determined by a detailed medical history; a full physical examination, including vital signs; 12-lead electrocardiograms (ECGs); and clinical laboratory tests. Subjects were excluded if they had known hypersensitivity to azoles, a positive urine drug screen, or evidence of liver disease. The subjects were prohibited from taking medications known to be inhibitors, inducers, or substrates of the CYP3A enzyme or to interact with voriconazole. No consumption of grapefruit or grapefruit-containing products was allowed within 7 days before the first dose of voriconazole and throughout the study. The subjects abstained from alcohol and tobacco or nicotine-containing products for at least 14 days before the first dose and throughout the study.
Drug administration and sample collection.
Voriconazole (VFEND; Pfizer) and matching placebo tablets were supplied to the CRU by Pfizer (New York, NY). Ritonavir (NORVIR; Abbott Laboratories) capsules were obtained by the CRU from commercial sources. While confined to the CRU, subjects fasted for at least 4 h before any safety laboratory evaluations and for 8 h before the morning dose of voriconazole, and they continued without food for at least 1 h following dosing. For the evening dose of voriconazole, the subjects were not allowed to consume food for at least 1 h before and 1 h after dosing. Ritonavir was administered with breakfast or an evening snack, as recommended by the product label information, in order to improve the gastrointestinal tolerability. When coadministered with ritonavir, voriconazole or placebo was given under fasting conditions 1 hour prior to ritonavir administration. For both medications, each dose was administered with 240 ml water under the direct supervision of study personnel.
In both studies, blood samples (3 ml) to characterize voriconazole pharmacokinetics were collected on days 3 and 30 at 0.5, 1, 1.5, 2, 3, 5, 6, 8, 10, and 12 h postdose; blood samples were also collected prior to the morning dose of voriconazole on days 1 to 3, 21, and 27 to 30 for the measurement of trough concentrations (Cmin). Blood samples (7 ml) to characterize ritonavir pharmacokinetics were collected on days 20 and 30 at 0.5, 1, 2, 4, 5, 7, 9, 11, and 12 h postdose; blood samples were also collected prior to the morning dose of ritonavir on days 11, 17 to 20, 21, and 27 to 30 for the measurement of Cmin. All blood samples were centrifuged at 1,700 × g for about 10 min at approximately 4°C, and the plasma was obtained and stored at approximately −20°C within 1 h of collection until it was analyzed.
Analytical methods.
PPD Development (Richmond, VA) analyzed plasma samples for voriconazole using a previously validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) method (25). The plasma samples (0.100 ml) were extracted using a solid-phase extraction procedure, followed by LC/MS/MS separation and detection. The dynamic range of the assay for voriconazole was 10 to 2,500 ng/ml. The accuracy of the quality control samples used during sample analysis ranged from −1.9% to 5.2%, with a precision of ≤10.6% for voriconazole. PPD Development also analyzed plasma samples for ritonavir using a validated LC/MS/MS assay similar to a previously published method (5). The dynamic range of the assay for ritonavir was 10 to 10,000 ng/ml. The accuracy of the quality control samples used during sample analysis ranged from −11.8% to 9.4% with a precision of ≤10% for ritonavir. All the samples were analyzed within the established long-term stability period.
Pharmacokinetic analysis.
Pharmacokinetic analysis was performed with WinNonlin version 3.2 (Pharsight, Mountain View, CA) using standard noncompartmental methods. The maximum observed plasma concentration (Cmax), time to reach Cmax (Tmax), and Cmin for voriconazole and ritonavir were estimated directly from concentration-time data. The AUCs during the dosing interval (AUC0-12) for voriconazole and ritonavir were estimated using the linear/log trapezoidal approximation.
Safety assessment.
Assessments included repeated safety laboratory tests (hematology, chemistry, and urinalysis) on days 0, 4 (before discharge from the CRU), 10, 15, 21, 25, and 30; physical examinations on days 0, 4, 10, 15, 21, 25, and 31 (before discharge from the CRU); vital signs (supine heart rate and blood pressure) and 12-lead ECGs on days 1, 3, 4, 11, 15, 20, 21, 25, 30, and 31; and continuous adverse-event (AE) monitoring. These assessments were also measured at screening and the follow-up visit. On days 1, 3, 11, 15, 20, 21, 25, and 30, there were multiple measures of vital signs and single 12-lead ECGs at predose and 1, 3, and 8 h after the voriconazole morning dose. Prior to the voriconazole morning dosing on day 1 only, triplicate ECG measurements were collected, and the mean served as each subject's baseline value.
Statistical methods. (i) Sample size determination.
Assuming a dropout rate of approximately 30%, 34 subjects were randomized into study A to ensure that 24 subjects (12 subjects per treatment group) would complete the study. This sample size provided an 80% probability of calculating the 90% confidence intervals (CIs) and levels of precision we could expect to obtain for various possible relative bioavailability estimates for the AUC0-12 and Cmax of voriconazole in the presence of ritonavir (16). For instance, if the estimated ratio of AUC0-12 (day 30/day 3) was 0.9, the 90% CI would be no wider than 0.82 and 0.99. These calculations were based on the intrasubject coefficient of variation estimates for voriconazole AUC0-12 and Cmax of 0.108 and 0.178, respectively, from a previous bioequivalence study with crossover design evaluating three research tablet formulations, which were used in the clinical development program (phase 1 and 3 studies). The same calculation was also applied in study B, where the calculations were based on the intrasubject coefficient of variation estimates for voriconazole AUC0-12 and Cmax of 0.299 and 0.243, respectively, obtained from study A.
(ii) Statistical analysis of pharmacokinetic parameters.
For voriconazole and ritonavir, the AUC0-12 and Cmax are presented as arithmetic means with standard deviations (SD), and Tmax is presented as median and range. The natural log-transformed AUC0-12 and Cmax of voriconazole and ritonavir were analyzed using a mixed-effects analysis of variance model with SAS MIXED procedure using SAS version 8.2 (SAS Institute Inc., Cary, NC). Restricted maximum likelihood estimation was used. The treatment was specified as the fixed effect with a random effect for subjects within a group. For voriconazole, the point estimates of the adjusted mean treatment differences (day 30 − day 3) and their respective 90% CIs around the differences were calculated. These estimated treatment differences and their respective confidence limits were antilog (exponent) transformed to the ratios of the adjusted geometric means (day 30/day 3) and their respective 90% CIs around the ratios. The adjusted geometric mean ratios (day 30/day 20) for ritonavir and their respective 90% CIs around the ratios were calculated in the same manner.
(iii) Safety data.
For both studies, all the safety data were summarized descriptively. In addition, vital signs and ECG data (Bazett corrected QT [QTcB] and Fridericia corrected QT [QTcF] were qualitatively described and categorized relative to the change from the baseline value (the mean of triplicate values prior to dosing on day 1). Additionally, for study A only, a linear mixed-effects model for repeated measures (vital signs and ECGs) was used to model the change from baseline data for all nominal time points obtained on days 3, 20, and 30, respectively, for each treatment regimen with SAS version 8.2. This model had the treatment as the fixed effect and the baseline as a covariate. The within-treatment group differences between the voriconazole group and the placebo group were also compared. Appropriate linear contrasts were used to obtain point estimates of mean differences of interest, and their 95% CIs of the mean differences were constructed. No adjustments were made for multiple comparisons.
RESULTS
Subject disposition.
In study A, 34 adult male subjects were enrolled and 29 completed dosing. Among the five discontinuations, three were due to treatment-related AEs: one with mild nausea and vomiting (voriconazole alone), one with a mild laboratory abnormality (placebo alone), and one displaying an altered mental status (placebo plus ritonavir). The other two were not treatment related: one had a viral respiratory tract infection (RTI) (voriconazole plus ritonavir), and one withdrew voluntarily (voriconazole plus ritonavir). Two additional subjects were lost to follow-up but completed the dosing periods and were included in the pharmacokinetic analysis. The two treatment groups (voriconazole versus placebo) had similar demographics (Table 2). In study B, 17 adult male subjects entered and completed the study, and their demographics are presented in Table 2.
TABLE 2.
Summary of demographic characteristics of subjects
| Study | No. of subjects enrolled | Agea (yr) | Wta (kg) | Body mass indexa (kg/m2) | No. C/B/H/Ob | No. of subjects evaluable for pharmacokinetics/for safety |
|---|---|---|---|---|---|---|
| Study A | ||||||
| Voriconazole/ritonavir (400 mg) | 17 | 31 (19-52) | 77.6 (62.0-88.0) | 25.4 (19.6-29.4) | 5/0/11/1 | 14/17 |
| Placebo/ritonavir (400 mg) | 17 | 31 (19-52) | 76.1 (60.0-90.0) | 25.8 (21.3-29.4) | 4/1/12/0 | 15/17 |
| Study B | ||||||
| Voriconazole/ritonavir (100 mg) | 17 | 40 (26-54) | 79.9 (64.9-94.8) | 26.6 (23.0-29.1) | 1/0/16/0 | 17/17 |
Mean (range).
C, Caucasian; B, black; H, Hispanic; O, other.
In each study, there was one subject in whom ritonavir had an opposite effect on voriconazole exposure (a 2.5- to 3-fold increase) compared to the other subjects. These two subjects were not included in the statistical analysis of voriconazole exposure (i.e., calculation of mean pharmacokinetic parameters). The exposure and safety data from these two subjects are described separately.
Effect of ritonavir on steady-state voriconazole pharmacokinetics.
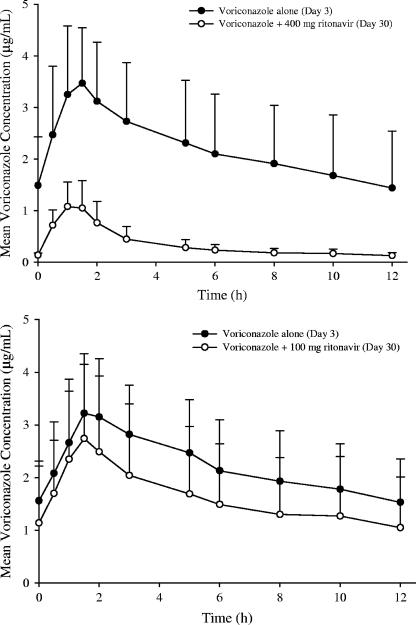
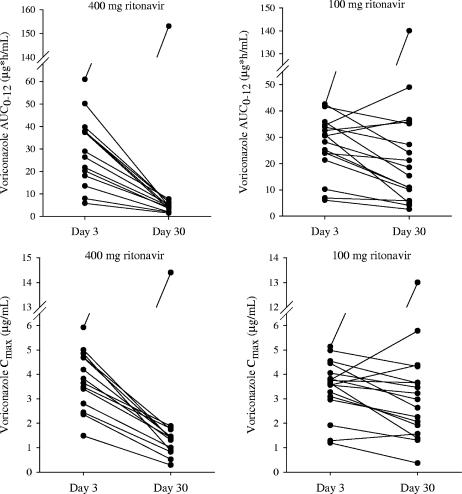
The steady-state mean voriconazole concentrations decreased significantly when the drug was coadministered with 400 mg BID ritonavir (Fig. 1, top). The effect of 100 mg BID ritonavir on the steady-state mean voriconazole concentrations was substantially lower compared to that of 400 mg BID ritonavir (Fig. 1, bottom). As shown in Fig. 2 (left), there was a consistent decrease in the individual steady-state exposure parameters (AUC0-12 and Cmax) of voriconazole during coadministration with 400 mg BID ritonavir, except for one subject. The magnitude of the decrease was substantial (AUC0-12, 73% to 91%; Cmax, 48% to 82%). The decreases in mean voriconazole AUC0-12 and Cmax were 83% and 68% during coadministration with 400 mg BID ritonavir (Table 3).
FIG. 1.
Mean steady-state voriconazole plasma concentration-time profiles following 200 mg BID voriconazole alone (day 3) and coadministration with 400 mg BID or 100 mg BID ritonavir (day 30). One subject in each study who had very high voriconazole exposure on day 30 was excluded from the calculation of the mean voriconazole concentration-time profile. The error bars indicate (SD).
FIG. 2.
Individual steady-state voriconazole AUC0-12 and Cmax following 200 mg BID voriconazole alone (day 3) and coadministration with 400 mg BID or 100 mg BID ritonavir (day 30).
TABLE 3.
Summary of statistical analysis of voriconazole pharmacokinetic parameters following administration of 200 mg BID voriconazole in the absence (day 3) or presence (day 30) of 400 mg or 100 mg BID ritonavir (studies A and B)a
| Pharmacokinetic parameter | Arithmetic mean (SD)
|
Geometric mean ratio (day 30/day 3 [%])c (90% CI) | ||
|---|---|---|---|---|
| 200 mg BID voriconazole alone | With 400 mg BID ritonavir | With 100 mg BID ritonavir | ||
| Study A (n = 13) | ||||
| AUC0-12 (μg · h/ml) | 26.5 (13.5) | 4.25 (1.88) | 16.9 (14.2, 20.2) | |
| Cmax (μg/ml) | 3.60 (1.09) | 1.22 (0.489) | 31.9 (27.1, 37.6) | |
| Tmax (h)b | 1.5 (0.5-2) | 1 (0.5-1.5) | ||
| Study B (n = 16) | ||||
| AUC0-12 (μg · h/ml) | 26.8 (11.2) | 19.5 (14.0) | 61.1 (48.0, 77.6) | |
| Cmax (μg/ml) | 3.36 (1.09) | 2.81 (1.39) | 76.2 (63.6, 91.3) | |
| Tmax (h)b | 1.5 (0.5-3) | 1.5 (1-5) | ||
One subject in each study who had very high voriconazole exposure on day 30 was excluded from the calculation.
Median (range).
Day 3, voriconazole alone; day 30, voriconazole plus ritonavir.
The subject who had an opposite effect of ritonavir on voriconazole exposure was a 38-year-old Hispanic male. His voriconazole exposure on day 3 was the highest among all the subjects (AUC0-12, 60.9 μg · h/ml; Cmax, 5.9 μg/ml) and increased approximately 2.5-fold on day 30 when the drug was coadministered with 400 mg BID ritonavir (AUC0-12, 153 μg · h/ml; Cmax, 14.4 μg/ml). In addition, the steady state of voriconazole was not achieved on day 30 in this subject, since the Cmin continued to increase from day 27 to day 30.
When coadministered with 100 mg BID ritonavir, the effect on the steady-state voriconazole exposure parameters was inconsistent and lower than that of 400 mg BID ritonavir (Fig. 2, right). Four subjects exhibited increases in voriconazole exposure: three subjects had slight increases in voriconazole exposure (i.e., 10% to 42%), and one subject, who was a 40-year-old Hispanic male, showed approximately threefold-higher voriconazole exposure on day 30 (AUC0-12, 42.1 versus 140 μg · h/ml; Cmax, 5.1 versus 13.0 μg/ml) in the presence of 100 mg BID ritonavir. With exclusion of this subject, the decreases in mean voriconazole AUC0-12 and Cmax were 39% and 24% when the drug was coadministered with 100 mg BID ritonavir (Table 3).
The steady state of voriconazole was achieved on day 2 following 400-mg BID loading doses on day 1, as indicated by similar Cmin values on days 2 and 3. Following coadministration with either 400 mg or 100 mg BID ritonavir, the steady state of voriconazole was achieved within 7 days, as indicated by similar Cmin values on days 27 to 30, except for the subject in study A who had the opposite effect of ritonavir (resulting in high voriconazole exposure on day 30).
Effect of voriconazole on steady-state ritonavir pharmacokinetics.
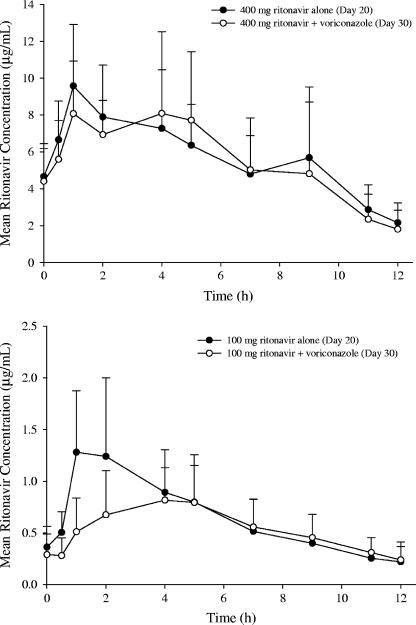
The steady-state mean ritonavir concentrations following 400 mg BID ritonavir alone were similar to those following coadministration with 200 mg BID voriconazole (Fig. 3, top). However, compared to low-dose ritonavir alone (100 mg BID), the steady-state mean ritonavir concentrations following coadministration with 200 mg BID voriconazole were lower during the absorption phase (Fig. 3, bottom).
FIG. 3.
Mean steady-state ritonavir plasma concentration-time profiles following 400 mg BID or 100 mg BID ritonavir alone (day 20) and coadministration with 200 mg BID voriconazole (day 30). The error bars indicate SD.
There was no consistent trend in individual AUC0-12 and Cmax of high-dose ritonavir (400 mg BID) in the presence of voriconazole, and the day 30/day 20 ratio of ritonavir AUC0-12 ranged from 0.49 to 2.32. In the placebo group (study A only), the day 30/day 20 ratios of ritonavir AUC0-12 ranged from 0.55 to 1.33. Only two subjects in the voriconazole group showed ritonavir ratios (1.68 and 2.32) higher than the maximum in the placebo group. There was no special finding on ritonavir exposure in the subject who had unusually high voriconazole exposure on day 30. In the voriconazole group, the 90% CIs for AUC0-12 and Cmax ratios of ritonavir included 100% (Table 4), indicating no statistically significant effect of voriconazole on the high-dose ritonavir exposure. In the placebo group, the steady-state mean ritonavir exposure parameters on day 20 and day 30 were similar (Table 4), indicating that 10-day ritonavir 400-mg BID dosing was sufficient for maximizing enzymatic induction. The similar steady-state ritonavir exposures in the voriconazole and placebo groups confirmed that the pharmacokinetics of high-dose ritonavir was not affected by coadministration with 200 mg BID voriconazole.
TABLE 4.
Summary of statistical analysis of ritonavir pharmacokinetic parameters following administration of 400 mg or 100 mg BID ritonavir in the absence (Day 20) or presence (Day 30) of 200 mg BID voriconazole or placebo (Studies A and B)
| Pharmacokinetic parameter | Arithmetic mean (SD)
|
Geometric mean ratio (day 30/day 20 [%])b (90% CI) | |||
|---|---|---|---|---|---|
| 400 mg BID ritonavir alone | 100 mg BID ritonavir alone | With placebo | With 200 mg BID voriconazole | ||
| Study A | |||||
| Group 1 (n = 14) | |||||
| AUC0-12 (μg · h/ml) | 68.0 (21.0) | 65.9 (28.2) | 94.7 (76.9, 116.8) | ||
| Cmax (μg/ml) | 10.7 (3.19) | 10.4 (3.84) | 97.1 (81.9, 115.0) | ||
| Cmin (μg/ml)a | 4.7 (2.2-7.8) | 4.3 (0.92-9.3) | |||
| Tmax (h)a | 1.5 (1-9) | 1 (1-5) | |||
| Group 2 (n = 15) | |||||
| AUC0-12 (μg · h/ml) | 65.3 (18.4) | 58.4 (17.2) | 89.2 (80.3, 99.2) | ||
| Cmax (μg/ml) | 10.5 (3.43) | 10.2 (3.95) | 95.7 (84.9, 107.8) | ||
| Tmax (h)a | 1 (1-5) | 1 (1-4) | |||
| Study B (n = 17) | |||||
| AUC0-12 (μg · h/ml) | 7.81 (3.87) | 6.43 (2.41) | 86.2 (73.8, 100.7) | ||
| Cmax (μg/ml) | 1.41 (0.721) | 0.992 (0.299) | 75.7 (61.2, 93.7) | ||
| Cmin (μg/ml)a | 0.32 (0.02-0.81) | 0.25 (0.04-0.67) | |||
| Tmax (h)a | 1 (1-5) | 3 (1-5) | |||
Median (range).
Day 20, ritonavir alone; day 30, ritonavir plus voriconazole/placebo.
There was also no consistent trend in individual AUC0-12 and Cmax of low-dose ritonavir (100 mg BID) in the presence of voriconazole. The day 30/day 20 ratio of the ritonavir AUC0-12 ranged from 0.48 to 1.69, while the ratio of Cmax ranged from 0.31 to 2.07. There was no special finding on ritonavir exposure in the subject who had unusually high voriconazole exposure on day 30. The adjusted geometric mean ratios (day 30/day 20) of 100 mg ritonavir AUC0-12 and Cmax were 86.2% (90% CI, 73.8%, 100.7%) and 75.7% (90% CI, 61.2%, 93.7%), respectively (Table 4). The 90% CI for AUC0-12 ratio included 100% marginally, but the upper bound of the 90% CI for the Cmax ratio fell slightly below 100%, indicating an effect of voriconazole on the Cmax of low-dose ritonavir.
In both studies, the steady state of ritonavir was achieved within 7 days of repeated dosing of ritonavir alone or coadministered with voriconazole, and the steady-state Cmin values remained generally unchanged following coadministration, as indicated by similar Cmins on days 17 to 20 and 27 to 30 (data not shown).
Safety.
There were no deaths, serious AEs, or severe AEs reported in the two studies. There were no dose reductions due to AEs, but there were three discontinuations due to AEs in study A, as described above.
(i) Study A (high-dose ritonavir).
In the voriconazole group (n = 17), AEs were reported by 9 subjects receiving voriconazole alone (16 events), 15 subjects receiving ritonavir alone (63 events), and 14 subjects receiving coadministration (78 events) (Table 5). In the placebo group (n = 17), AEs were reported by 10 subjects receiving placebo (10 events), 13 subjects receiving ritonavir alone (59 events), and 12 subjects receiving coadministration (50 events). Most AEs (97%) were mild in intensity. There were three moderate AEs: headache (ritonavir plus placebo), photophobia (voriconazole plus ritonavir), and viral RTI (voriconazole plus ritonavir). The incidents of headache and photophobia resolved without intervention, and the subject with viral RTI was discontinued from the study. Most of the AEs (86%) were considered treatment related. When ritonavir was coadministered with voriconazole, there was a slight increase in the total number of AEs, but the occurrence of the most frequently reported treatment-related AEs was not notably increased, with the exception of headache and hot flashes (Table 5). In the voriconazole group, abnormal vision was reported in three subjects receiving voriconazole alone and photophobia was reported in two subjects during coadministration. In the placebo group, abnormal vision was reported in one subject receiving placebo with ritonavir. Visual disturbances (abnormal vision and photophobia) are known side effects of voriconazole and are reported on the current product label. Dry eyes and hematuria were two AEs that had not been identified in the labeling for either voriconazole or ritonavir; all these incidents were mild and resolved without intervention. There were no clinically significant changes in vital signs and ECGs (QTcB and QTcF) when mean changes from baseline over time were compared in each treatment regimen in the voriconazole group. In addition, it did not appear to be a difference in QTcB or QTcF that was responsible for the change from baseline over time when the voriconazole group was compared to the placebo group.
TABLE 5.
Summary of all-causality AEs and incidence of frequently reported treatment-related AEs in studies A and B
| Parameter | Value
|
|||||
|---|---|---|---|---|---|---|
| Study Aa (high-dose ritonavir)
|
Study B (low-dose ritonavir)
|
|||||
| Vorib | Ritoc | Vori + Ritod | Vorib | Ritoc | Vori + Ritod | |
| No. of subjects exposed | 17 | 16 | 15 | 17 | 17 | 17 |
| No. of subjects with AEs | 9 | 15 | 14 | 10 | 6 | 17 |
| No. of all-causality AEs | 16 | 63 | 78 | 14 | 11 | 47 |
| No. of treatment-related AEs | 12 | 55 | 57 | 14 | 7 | 37 |
| No. of subjects with treatment-related AEs | ||||||
| Abdominal pain | 1 | 5 | 5 | 0 | 0 | 0 |
| Headache | 0 | 1 | 5 | 3 | 1 | 5 |
| Hot flashes | 0 | 2 | 4 | 0 | 0 | 0 |
| Diarrhea | 0 | 7 | 7 | 0 | 0 | 0 |
| Nausea | 1 | 8 | 4 | 0 | 0 | 1 |
| ALT ↑e | 0 | 4 | 5 | 0 | 0 | 1 |
| AST ↑e | 0 | 3 | 1 | 0 | 0 | 0 |
| Hypesthesia | 0 | 7 | 1 | 0 | 0 | 0 |
| Dizziness | 0 | 1 | 2 | 1 | 0 | 2 |
| Insomnia | 0 | 2 | 1 | 0 | 0 | 10 |
| Dry eyes | 1 | 1 | 3 | 0 | 0 | 0 |
| Photophobia | 0 | 0 | 2 | 10 | 0 | 3 |
| Abnormal vision | 3 | 0 | 0 | 0 | 0 | 5 |
| Dry skin | 0 | 0 | 0 | 0 | 1 | 7 |
| Hematuria | 1 | 2 | 2 | 0 | 0 | 0 |
The AEs in the placebo group are not presented.
Three-day voriconazole alone plus seven-day washout.
Ten-day ritonavir alone.
Ten-day ritonavir and voriconazole plus seven-days after last dose.
↑, increased.
A total of 17 subjects had at least one abnormal laboratory value, but no values exceeded the criteria for potential clinical concern, except for one subject. This subject had significantly elevated hepatic enzymes and was the one with very high voriconazole exposure in the presence of ritonavir. He had no laboratory abnormalities up to day 25, but his day 30 laboratory results showed increases in alkaline phosphatase (∼1.5 times the upper limit of normal [ULN]), alanine aminotransferase (ALT) (>3 times the ULN), and aspartate aminotransferase (AST) (>7 times the ULN). He was subsequently asked to return to the CRU, and his alkaline phosphatase, ALT, AST, and gamma-glutamyl transferase (GGT) were monitored frequently until all of the parameters returned to normal. GGT was not evaluated originally but was added on follow-up by the principal investigator and was found to be >26 times the ULN 3 days after discharge from the CRU. The magnitude of the elevated GGT is suggestive of alcohol intoxication, but the subject denied having imbibed alcoholic beverages after being discharged from the CRU. While other enzymes returned to the normal range by 10 to 32 days postdischarge from the CRU, GGT was the last to return to normal at 57 days postdischarge from the CRU.
(ii) Study B (low-dose ritonavir).
All AEs were mild in intensity, and most AEs (80%) were considered to be treatment related (Table 5). Coadministration of voriconazole and ritonavir was associated with a higher overall incidence of treatment-emergent AEs than with administration of ritonavir alone. Three treatment-related AEs observed during coadministration that had higher rates than in the single-agent treatment were insomnia (10 subjects), abnormal vision (5 subjects), and dry skin (7 subjects). Skin reactions and visual abnormalities are known side effects of voriconazole and are reported on the current product label. Similarly, there were no clinically significant changes in vital signs and ECGs when mean changes from baseline over time were compared in each treatment regimen.
Although a total of five subjects had at least one abnormal laboratory value, no values exceeded the criteria for potential clinical concern, except for one subject. This subject had elevated ALT (>3 times the ULN) on day 30, which was not present when either voriconazole or ritonavir was being administered alone. This subject was the one who had very high voriconazole exposure in the presence of ritonavir. He was subsequently asked to return to the CRU and was monitored frequently until the ALT returned to the normal range at 15 days postdischarge from the CRU.
DISCUSSION
It is apparent that the extent of the ritonavir effect on voriconazole pharmacokinetics was dependent on the ritonavir dose. High-dose ritonavir (400 mg BID) produced a clinically significant decrease in steady-state voriconazole exposure. The effect of low-dose ritonavir (100 mg BID) was less pronounced and inconsistent. It is interesting that there was one subject in each study who had a substantial increase in voriconazole exposure in the presence of ritonavir (2.5- to 3-fold) (Fig. 2). Although these two subjects were different from other subjects with respect to voriconazole exposure when the drug was administered alone and with ritonavir, their ritonavir exposures were similar to those of other subjects. Except for these two subjects, the net effect of ritonavir on voriconazole metabolism was induction, probably due to CYP2C19 and CYP2C9 enzyme induction. Although the net effect of ritonavir on CYP3A was inhibitory, it appears that CYP3A inhibition was offset by CYP2C19 and CYP2C9 induction, since CYP3A is not a major pathway for voriconazole metabolism. In addition, the results suggest that the induction of CYP2C19 and CYP2C9 by ritonavir is dose dependent. In the presence of low-dose ritonavir (100 mg BID), a slight increase in voriconazole exposure on day 30 was observed in 3 out of 17 subjects (i.e., 10% to 42%), suggesting the net effect of ritonavir on voriconazole metabolism was inhibition in these subjects.
In one subject in each study, an opposite effect of ritonavir on voriconazole was observed. This net inhibitory effect of ritonavir on voriconazole metabolism could be due to the lack of CYP2C19 enzyme in these two subjects. This hypothesis is supported by the fact that voriconazole exposures in these two subjects on day 3 were significantly higher than in others and comparable to the historical control in PMs (14, 18, 22, 28). The opposite effect of ritonavir could be explained by the absence of CYP2C19 enzymes in these PMs, so that alternative metabolic pathways via CYP3A became the primary pathway for voriconazole in these subjects, and therefore the inhibitory effect of ritonavir on CYP3A resulted in the increase in voriconazole exposure during coadministration. Although genotyping was not done in this study, the percentage of PMs projected in both studies (1/17) is consistent with the prevalence (3 to 5%) of CYP2C19 PMs in the study population (Caucasians, Hispanics, and blacks) (17, 29).
It was reported in a recently published clinical study that the short-term (2-day) administration of ritonavir resulted in a significant increase in voriconazole exposure regardless of the CYP2C19 genotyping status (18). This increase in voriconazole exposure when the drug was administered with acute ritonavir therapy (300 mg BID for 2 days) was probably due to CYP3A inhibition by ritonavir (18). However, during long-term therapy with ritonavir, voriconazole exposures are expected to be decreased significantly for CYP2C19 EMs and HEMs, and the extent of the decrease depends on the dose of ritonavir, due to inductive effect on CYP2C19 and CYP2C9. In CYP2C19 PMs, an increase in voriconazole exposure would be expected during coadministration with ritonavir regardless of the ritonavir doses and dosing duration.
On the other hand, voriconazole had no apparent effect on steady-state high-dose ritonavir exposure, although voriconazole is also a substrate and a weak inhibitor of CYP3A. These findings are consistent with previously published clinical studies of ritonavir with other CYP3A inhibitors, such as fluconazole and clarithromycin (4, 19), which could be explained by the higher affinity of ritonavir for CYP3A than for other inhibitors, including voriconazole. However, the mean Cmax of low-dose ritonavir was slightly decreased when the drug was coadministrated with voriconazole (Cmax, −24%). The mechanism of this effect is not clear. Although Cmax of 100 mg ritonavir was slightly decreased in the presence of voriconazole, the Cmin remained generally unchanged, which was important for PIs, since keeping the Cmin above certain threshold values is the key determinant to achieve and maintain adequate antiviral efficacy (1, 12).
Overall, the safety profile of coadministration of voriconazole with high-dose or low-dose ritonavir was not notably different from that of administration of voriconazole or ritonavir alone. Although all visual AEs were assessed by the investigator as treatment related, all but one were mild in nature (the exception was moderate photophobia). All the visual disturbances were transient and fully reversible without any intervention, which was consistent with what had been reported in the phase 3 clinical trials (26). There were no clinically significant trends in postdose clinical laboratory assessments suggesting a relationship to concurrent use of voriconazole and ritonavir, except for two subjects with very high voriconazole exposure on day 30. Elevated hepatic transaminases in these two subjects were not unexpected, since positive associations between plasma voriconazole concentrations and rates of both liver function test abnormalities and visual disturbances have been identified (26). The net inhibitory effect of ritonavir on voriconazole exposure in CYP2C19 PMs, resulting in significant accumulation of voriconazole, could raise a potential safety concern.
In summary, chronic ritonavir therapy significantly decreased steady-state voriconazole exposure. Due to the significant effect of ritonavir on voriconazole exposure, coadministration of voriconazole with 400 mg BID ritonavir is contraindicated; coadministration with 100 mg BID ritonavir should be avoided, unless an assessment of the benefit/risk to the patient justifies the use.
Acknowledgments
We sincerely thank all the clinicians and the staff of MDS Pharma Services (Phoenix, AZ) and Comprehensive NeuroScience, Inc. (Ft Lauderdale, FL) who were involved in these studies. We thank our assay specialist, Penelope Crownover, and PPD Development (Richmond, VA) for analytical assay support.
All the authors are employees of Pfizer, except M. J. Allison and M. J. Gutierrez, who were the principal clinical investigators for these two studies.
These studies were sponsored by Pfizer Inc.
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Acosta, E. P., T. N. Kakuda, R. C. Brundage, P. L. Anderson, and C. V. Fletcher. 2000. Pharmacodynamics of human immunodeficiency virus type 1 protease inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S151-S159. [DOI] [PubMed] [Google Scholar]
- 2.Acosta, E. P., H. Wu, S. M. Hammer, S. Yu, D. R. Kuritzkes, A. Walawander, J. J. Eron, C. J. Fichtenbaum, C. Pettinelli, D. Neath, E. Ferguson, A. J. Saah, and J. G. Gerber. 2004. Comparison of two indinavir/ritonavir regimens in the treatment of HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 37:1358-1366. [DOI] [PubMed] [Google Scholar]
- 3.Boffito, M., D. Maitland, Y. Samarasinghe, and A. Pozniak. 2005. The pharmacokinetics of HIV protease inhibitor combinations. Curr. Opin. Infect. Dis. 18:1-7. [DOI] [PubMed] [Google Scholar]
- 4.Cato, A., III, G. Cao, A. Hsu, J. Cavanaugh, J. Leonard, and R. Granneman. 1997. Evaluation of the effect of fluconazole on the pharmacokinetics of ritonavir. Drug Metab. Dispos. 25:1104-1106. [PubMed] [Google Scholar]
- 5.Chi, J., A. L. Jayewardene, J. A. Stone, T. Motoya, and F. T. Aweeka. 2002. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J. Pharm. Biomed Anal. 30:675-684. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, C. L., R. P. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 36:1585-1592. [DOI] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., J. L. Rodriguez-Tudela, E. Mellado, J. V. Martinez-Suarez, and A. Monzon. 1998. Comparison of the in-vitro activity of voriconazole (UK-109,496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J. Antimicrob Chemother. 42:531-533. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gass, R. J., J. Gal, P. W. Fogle, D. Detmar-Hanna, and J. G. Gerber. 1998. Neither dapsone hydroxylation nor cortisol 6β-hydroxylation detects the inhibition of CYP3A4 by HIV-1 protease inhibitors. Eur. J. Clin. Pharmacol. 54:741-747. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt, D. J., L. L. von Moltke, J. S. Harmatz, A. L. Durol, J. P. Daily, J. A. Graf, P. Mertzanis, J. L. Hoffman, and R. I. Shader. 2000. Alprazolam-ritonavir interaction: implications for product labeling. Clin. Pharmacol. Ther. 67:335-341. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, H. L., and R. C. Rathbun. 2002. Review of the safety and efficacy of voriconazole. Exp. Opin. Investig. Drugs 11:409-429. [DOI] [PubMed] [Google Scholar]
- 12.Hsu, A., G. R. Granneman, and R. J. Bertz. 1998. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 35:275-291. [DOI] [PubMed] [Google Scholar]
- 13.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, Y., K. Umemura, K. Kondo, K. Sekiguchi, S. Miyoshi, and M. Nakashima. 2004. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin. Pharmacol. Ther. 75:587-588. [DOI] [PubMed] [Google Scholar]
- 15.Kilby, J. M., A. Hill, and N. Buss. 2002. The effect of ritonavir on saquinavir plasma concentration is independent of ritonavir dosage: combined analysis of pharmacokinetic data from 97 subjects. HIV Med. 3:97-104. [DOI] [PubMed] [Google Scholar]
- 16.Kupper, L., and K. Hafner. 1989. How appropriate are popular sample size formulas? Am. Statist. 43:101-105. [Google Scholar]
- 17.Luo, H. R., R. E. Poland, K. M. Lin, and Y. J. Wan. 2006. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: a cross-ethnic comparative study. Clin. Pharmacol. Ther. 80:33-40. [DOI] [PubMed] [Google Scholar]
- 18.Mikus, G., V. Schowel, M. Drzewinska, J. Rengelshausen, R. Ding, K. D. Riedel, J. Burhenne, J. Weiss, T. Thomsen, and W. E. Haefeli. 2006. Potent cytochrome P450 2C19 genotype-related interaction between voriconazole and the cytochrome P450 3A4 inhibitor ritonavir. Clin. Pharmacol. Ther. 80:126-135. [DOI] [PubMed] [Google Scholar]
- 19.Ouellet, D., A. Hsu, G. R. Granneman, G. Carlson, J. Cavanaugh, H. Guenther, and J. M. Leonard. 1998. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin. Pharmacol. Ther. 64:355-362. [DOI] [PubMed] [Google Scholar]
- 20.Ouellet, D., A. Hsu, J. Qian, C. S. Locke, C. J. Eason, J. H. Cavanaugh, J. M. Leonard, and G. R. Granneman. 1998. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br. J. Clin. Pharmacol. 46:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., J. Zhang, S. A. Messer, M. E. Brandt, R. A. Hajjeh, C. J. Jessup, M. Tumberland, E. K. Mbidde, and M. A. Ghannoum. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob. Agents Chemother. 43:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purkins, L., N. Wood, P. Ghahramani, D. Kleinermans, G. Layton, and D. Nichols. 2003. No clinically significant effect of erythromycin or azithromycin on the pharmacokinetics of voriconazole in healthy male volunteers. Br. J. Clin. Pharmacol. 56(Suppl. 1):30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purkins, L., N. Wood, P. Ghahramani, E. R. Love, M. D. Eve, and A. Fielding. 2003. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br. J. Clin. Pharmacol. 56(Suppl. 1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purkins, L., N. Wood, D. Kleinermans, and D. Nichols. 2003. Voriconazole potentiates warfarin-induced prothrombin time prolongation. Br. J. Clin. Pharmacol. 56(Suppl. 1):24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stopher, D. A., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B 691:441-448. [DOI] [PubMed] [Google Scholar]
- 26.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 27.von Moltke, L. L., D. J. Greenblatt, J. M. Grassi, B. W. Granda, S. X. Duan, S. M. Fogelman, J. P. Daily, J. S. Harmatz, and R. I. Shader. 1998. Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavir. J. Clin. Pharmacol. 38:106-111. [DOI] [PubMed] [Google Scholar]
- 28.Wood, N., K. Tan, L. Purkins, G. Layton, J. Hamlin, D. Kleinermans, and D. Nichols. 2003. Effect of omeprazole on the steady-state pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56(Suppl. 1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie, H. G., C. M. Stein, R. B. Kim, G. R. Wilkinson, D. A. Flockhart, and A. J. Wood. 1999. Allelic, genotypic and phenotypic distributions of S-mephenytoin 4′-hydroxylase (CYP2C19) in healthy Caucasian populations of European descent throughout the world. Pharmacogenetics 9:539-549. [PubMed] [Google Scholar]