Abstract
9-R-[2-(Phosphonomethoxy)propyl]-adenine (tenofovir) is an acyclic nucleoside phosphonate with antiviral activity against human immunodeficiency virus type 1 (HIV-1) and hepatitis B virus (HBV). Tenofovir is not orally bioavailable but becomes orally active against HIV-1 infection as the disoproxil ester (tenofovir disoproxil fumarate [Viread]). We have developed an alternative strategy for promoting the oral availability of nucleoside phosphonate analogs which involves esterification with a lipid to form a lysolecithin mimic. This mimic can utilize natural lysolecithin uptake pathways in the gut, resulting in high oral availability. Since the mimic is not subject to cleavage in the plasma by nonspecific esterases, it remains intact in the circulation and facilitates uptake by target cells. Significant drops in apparent antiviral 50% effective concentrations (EC50s) of up to 3 logs have been observed in comparison with non-lipid-conjugated parent compounds in target cells. We have applied this technology to tenofovir with the goal of increasing oral availability, decreasing the apparent EC50, and decreasing the potential for nephrotoxicity by reducing the exposure of the kidney to the free dianionic tenofovir. Here we report that, in vitro, the hexadecyloxypropyl ester of tenofovir, CMX157, is 267-fold more active than tenofovir against HIV-1 and 4.5-fold more active against HBV. CMX157 is orally available and has no apparent toxicity when given orally to rats for 7 days at doses of 10, 30, or 100 mg/kg/day. Consequently, CMX157 represents a second-generation tenofovir analog which may have an improved clinical profile.
Acyclic nucleoside phosphonates are an important class of nucleotide analogs that are in clinical use as antiviral agents for the treatment of cytomegalovirus (e.g., cidofovir [CDV]), human immunodeficiency virus (HIV) (e.g., tenofovir disoproxil fumarate), and hepatitis B virus (HBV) (e.g., adefovir dipivoxil) infections (11, 14). Acyclic nucleoside phosphonates have phosphonomethoxyethyl moieties and consequently do not require the first anabolic phosphorylation step in their conversion to the active antivirals (13). However, the phosphonate moiety imparts several limitations to the molecule, including poor oral bioavailability arising from being highly charged and nephrotoxicity resulting from rapid elimination and accumulation in the proximal tubules of the kidney (10). In addition, cellular uptake of nucleoside phosphonates, as exemplified by CDV, is reduced because the phosphonate is a dianion and must be transported by fluid-phase endocytosis, an active, energy-dependent process (1, 9). Once in the cell, nucleoside phosphonates require two subsequent anabolic phosphorylations for conversion to the active diphosphate (13).
The limited oral bioavailability of the phosphonates has been addressed in marketed compounds such as adefovir and tenofovir by conversion to their respective dipivoxil and disoproxil fumarate prodrugs. After absorption in the gut, both tenofovir disoproxil fumarate and adefovir dipivoxil are deesterified in plasma by nonspecific esterases. As a result, tenofovir and adefovir can accumulate in the kidney proximal tubules through the action of an organic anion transporter. In the case of CDV and adefovir, this accumulation results in dose-limiting nephrotoxicity.
We have developed an alternate strategy to increase the oral and cellular uptake of acyclic nucleoside phosphonates by converting the compounds to mimics of natural lipids which are designed to utilize natural lipid absorption pathways. For example, converting CDV to its hexadecyloxypropyl (HDP) ester (HDP-CDV) (CMX001) results in increased cellular uptake (1) and an increase of several log units in antiviral activity (2, 17, 24). In addition, CMX001 is active when administered orally to mice against lethal infections from poxvirus (6, 22), human cytomegalovirus (in the SCID-hu mouse model), and murine cytomegalovirus (16). Unlike the case for CDV, there is no evidence of nephrotoxicity in animals given CMX001, apparently because exposure of the kidney to CDV following parenteral administration of CDV is much greater than that occurring after oral administration of CMX001 (8, 21).
These results led us to apply the lipid conjugation strategy to tenofovir in an effort to improve antiviral activity, promote high oral bioavailability, and minimize the potential for nephrotoxicity. Here we report on the synthesis, antiviral activity (against HIV type 1 [HIV-1] and HBV), plasma levels achieved after oral dosing, and toxicology/toxicokinetics of HDP-tenofovir (CMX157) in rats.
(Portions of this paper were presented in abstract form at the HIV DART conference, Cancun, Mexico, 10 to 14 December 2006.)
MATERIALS AND METHODS
Synthesis of HDP-(R)-PMPA (CMX157).
CMX157 was synthesized using methods analogous to those previously described for 9-S-[3-hydroxy-2-(phosphonomethoxy)propyl]-adenine [(S)-HPMPA] derivatives (3). 1H and 31P nuclear magnetic resonance (NMR) spectra were recorded on a Varian HG-400 NMR spectrophotometer with tetramethylsilane (internal) and 85% D3PO4 in D2O (external) as reference standards for 1H and 31P (0.00 ppm), respectively. Elemental microanalyses were performed by Quantitative Technologies, Inc., Whitehouse, NJ. Analytical thin-layer chromatography was performed on Analtech 250-μm silica gel GF Uniplates visualized under UV light, with phospray (Supelco, Bellafonte, PA), and by charring at 400°C. Chromatographic purification was done by the flash method using Merck silica gel (240-400 mesh).
Synthesis of HDP-(S)-HPMPA.
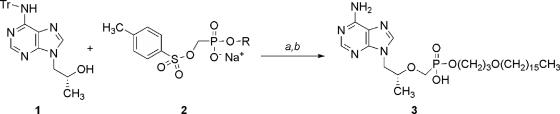
The synthetic route for HDP-(S)-HPMPA is shown in Fig. 1. (R)-9-(2-Hydroxypropyl)-N6-trityladenine (9.0 g, 20.6 mmol) (3) was added to a suspension of sodium hydride (0.72 g, 30 mmol) in dry N,N-dimethylformamide (60 ml) and stirred for 15 min. HDP-p-toluene-sulfonyloxymethylphosphonate sodium salt (17.0 g, 30 mmol), was prepared as previously described (3) and added, and the mixture was heated at 50°C for 4 h, poured into ice-water, and extracted with CH2Cl2 (3 time, 50 ml each). The combined organic extracts were dried (with MgSO4) and concentrated in vacuo. The crude residue was purified by flash chromatography on silica gel (eluant, 15% ethanol-CH2Cl2) to give 7.9 g (46% yield) of the tritylated intermediate as a glassy solid. To deprotect, the compound was taken up in 80% aqueous acetic acid and heated for 2 h at 60°C. After cooling to an internal temperature of 4°C, the mixture was filtered and the filtrate was concentrated to dryness in vacuo. The residue was purified by flash chromatography on silica gel (eluant, 30% methanol-CH2Cl2) to provide CMX157. The free acid was obtained by suspending the product in water and adding 1 N HCl to a pH of approximately 1. The precipitate was collected by filtration and dried in vacuo to provide 4.8 g CMX157 as a white solid (89% yield).
FIG. 1.
Synthesis of CMX157. Synthesis of CMX157 was carried out as described previously for the alkoxyalkyl esters of (S)-HPMPA (3). Reagents: a, NaH and N,N-dimethylformamide, 50°C; b, 80% aqueous CH3COOH. Abbreviations: Tr, trityl, R, HDP.
1H NMR (CD3OD) δ 8.35 (s, 1H), 8.25 (s, 1H), 4.42 (dd, 1H), 4.23 (dd, 1H), 3.91 to 3.97 (m, 3H), 3.77 (dd, 1H), 3.55 (dd, 1H), 3.47 (t, 2H), 3.37 (t, 2H), 1.83 (quintet, 2H), 1.50 to 1.54 (m, 2H), 1.27 (br s, 26H), 1.18 (d, 3H), 0.89 (t, 3H); 31P NMR δ 15.10; mass spectrometry (MS) (electrospray ionization) m/z 570.32 (M + H)+, 592.28 (M + Na)+. Calculated for C28H52N5O5P · 0.5H2O: %C, 58.11; %H, 9.23; %N 12.10. Found: %C, 57.39; %H, 9.30; %N 11.17.
Antiviral assays. (i) HIV-1 assays in MT-2 cells by p24 reduction.
MT-2 cells (AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (JRH Biosciences, Lenexa, KS), 10 mM HEPES buffer, 50 IU of penicillin/ml, and 50 μg of streptomycin/ml. HIV-1LAI was obtained from the AIDS Research and Reference Reagent Program. The antiviral activity of each compound was determined by inoculation of MT-2 cells with HIV-1LAI at a multiplicity of infection of 0.001 50% tissue culture infective doses/cell followed by incubation in the presence of threefold serial drug dilutions (three wells per dilution) as previously described (12). The antiviral activity of each compound is expressed as the 50% effective concentration (EC50), which is the concentration required to inhibit p24 antigen production by 50%. The 50% cytotoxic concentrations (CC50s) were also calculated from cell counts and viability as previously described (12).
(ii) HIV-1 assays in fresh human PBMCs by reverse transcriptase reduction.
Fresh human peripheral blood mononuclear cells (PBMCs) obtained from a commercial source (Biological Specialty Corp., Colmar, PA), seronegative for HIV and HBV, were prepared and used as previously described (5) to determine the EC50s of test drugs against HIV-1 (clade B strain HT/92/599). Cytotoxicity was assessed by XTT {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium hydroxide} staining. The CC50s for the test materials were derived by measuring the reduction of the tetrazolium dye XTT in replicate microtiter plates containing cell and compound without virus according to the manufacturer's instructions (Roche Molecular Biochemicals, Mannheim, Germany). Microsoft Excel 2000 was used to analyze and graph data; the EC50, CC50, and selectivity index (CC50/EC50) were calculated from the data plots. The EC50 andCC50 were expressed as the means ± standard deviations from triplicate determinations.
(iii) HBV assays in 2.2.15 cells.
Confluent cultures of 2.2.15 cells were maintained on 96-well flat-bottomed tissue culture plates in RPMI1640 medium with 2% fetal bovine serum as previously described (18, 19). Cultures were treated with nine consecutive daily doses of the test compound (six for each concentration on two replicate plates). The culture medium was changed every day with medium containing the indicated concentration of CMX157. HBV DNA levels were assessed by quantitative blot hybridization 24 h after the last treatment. Cytotoxicity was assessed in confluent 2.2.15 cells by uptake of neutral red dye and semiquantitative analysis of the absorbance of internalized dye at 510 nM at 24 h following the last treatment (three cultures per test concentration) (18, 19).
(iv) HBV assays in HepAD38 cells.
Anti-HBV activity was determined in the tetracycline-responsive cell line HepAD38, a hepatoma cell line that has been stably transfected with a cDNA copy of the pregenomic RNA of wild-type virus (20). Withdrawal of tetracycline from the culture medium results in the initiation of viral replication. Cells were cultured at 37°C in a humidified 5% CO2-air atmosphere in seeding medium (Dulbecco modified Eagle medium-Ham's F-12 [50:50] supplemented with 10% [vol/vol] heat-inactivated fetal bovine serum, 100 IU/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml kanamycin, 400 μg/ml G418, and 0.3 μg/ml tetracycline). Cells were seeded in 48-well plates at a density of 5 × 105/well, and after 2 to 3 days the cultures were induced for viral production by washing with prewarmed phosphate-buffered saline and were fed with 200 μl assay medium (seeding medium without tetracycline and G418) with or without the antiviral compounds. The medium was changed after 3 days. The antiviral effect was quantified by measuring levels of intracellular viral DNA at day 6 postinduction by a real-time quantitative PCR method. Total cellular DNA was extracted from the cells by use of a commercial kit. The quantitative PCR is performed in a reaction volume of 25 μl using the TaqMan Universal PCR Master Mix (Applied Biosystems, Branchburg, NJ) with forward primer 5′-CCG TCT GTG CCT TCT CAT CTG-3′ (final concentration, 600 nM), reverse primer 5′-AGT CCA AGA GTY CTC TTA TRY AAG ACC TT-3′ (final concentration, 600 nM), and TaqMan probe (6-FAM-CCG TGT GCA CTT CGC TTC ACC TCT GC-TAMRA; final concentration, 150 nM). The reaction was analyzed using an SDS 7000 instrument (Applied Biosystems, Foster City, CA). A plasmid containing the full-length insert of the HBV genome was used to prepare the standard curve. The amount of viral DNA produced in treated cultures is expressed as a percentage of that in the mock treated samples. The cytostatic effects of the various compounds were assessed by employing the parent hepatoma cell line HepG2. The effect of the compounds on exponentially growing HepG2 cells was evaluated by means of the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] method according to the manufacturer's instructions (Promega Benelux, BV, Leiden, The Netherlands).
Toxicology and pharmacokinetic methods.
Sprague-Dawley rats (3/sex/group) approximately 8 to 12 weeks of age were administered vehicle or 10, 30, or 100 mg/kg/day CMX157 by the oral route once daily for 7 days. Mortality, morbidity, clinical observations, body weight, and food consumption were recorded daily. Ophthalmology examinations were performed prior to treatment initiation and prior to termination. Blood for evaluation of hematology, coagulation parameters, and clinical chemistry and urine for urinalysis were collected on day 8. All animals were sacrificed on day 8, and representative sections were obtained from 46 tissues. At necropsy, control and high-dose-receiving rats were examined grossly, weighed, and processed for microscopic examination. Histopathology examination of all tissues from all rats in groups 1 and 4 was conducted by Charles River Laboratories Pathology Associates.
Blood for pharmacokinetic analysis was obtained at 0.5, 1, 2, 4, 12, and 24 h (1 rat/sex/time point) after dosing on days 1 and 7. The rats were not fasted prior to dosing or during sample collection. CMX157 and tenofovir in deproteinized plasma samples were separated by high-pressure liquid chromatography on a mixed-mode column (Primesep B4) using a buffered aqueous-organic mobile phase; concentrations were determined by MS/MS (El, negative ion). The assay had a limit of quantitation of 1 ng/ml.
All plasma samples were used in the pharmacokinetic analysis. Values measuring below the lower limit of quantitation (<1 ng/ml) were treated as zero. Noncompartmental analysis of plasma concentration data was accomplished using PK Solutions 2.0 (Summit Research Services, Montrose CO). For the oral route, the maximum concentration of drug in plasma (Cmax) and the time to Cmax (Tmax) were determined by direct observation of the plotted data, and the area under the concentration-time curve (AUC) was calculated using the linear-log trapezoidal rule.
RESULTS
Antiviral activity.
CMX157 was highly active against the LAI and clade B strains of HIV-1 in vitro (Table 1), with EC50s of <10 picomolar against HIV-1LAI and 12 nanomolar against HIV-1clade B. The antiviral activity was 267-fold greater than that determined for tenofovir itself against HIV-1clade B in PMBCs and >65,000-fold greater than that of tenofovir in MT-2 cells infected with HIV-1LAI. Although CMX157 was more cytotoxic than tenofovir, its selectivity index was substantially greater than that of tenofovir in both MT-2 cells and PBMCs (Table 1).
TABLE 1.
Effects of PMPA and CMX157 on HIV-1 replication in MT-2 cells and human PBMCs in vitroa
| Compound | Mean ± SD (no. of replicates)
|
Selectivity index (EC50/CC50)
|
||||
|---|---|---|---|---|---|---|
| EC50 (μM)
|
CC50 (μM)
|
|||||
| MT-2 cells | PBMCs | MT-2 cells | PBMCs | MT-2 cells | PBMCs | |
| PMPA | 0.65 ± 0 (2) | 3.20 ± 0.89 (3) | >100 (4) | 1,270 ± 660 (3) | >154 | 396 |
| CMX157 | <1 × 10−5 (3) | 0.012 ± 0.004 (3) | 54 ± 29 (3) | 144 ± 40 (3) | >5.4 × 106 | 12,000 |
HIV-1 infection in MT-2 cells was with HIV-1LAI, and infection in PBMCs was with HIV-1clade B, as described in Materials and Methods. PBMC data were generated by ImQuest Biosciences, Frederick, MD.
The activities of tenofovir and CMX157 against replicating HBV were evaluated in two different assay systems employing either 2.2.15 or HepAD38 cells (Table 2). The EC50 of tenofovir was 7.2 μM in 2.2.15 cells and 2.25 μM in HepAD38 cells. CMX157 was 4.5- to 4.6-fold more active, with EC50s of 1.6 and 0.49 μM, respectively. In confluent 2.2.15 cells, the CC50s of tenofovir and CMX157 were both >300 μM, but in rapidly dividing HepG2 cells, tenofovir had a CC50 of >100, compared with 18.8 μM for CMX157. Selectivity indexes for tenofovir ranged from >41.7 to >133, versus 38.4 to >133 for CMX157 (Table 2).
TABLE 2.
Effect of PMPA and CMX157 on HBV replication in 2.2.15 cells and HepAD38 cells in vitro
| Compound | Mean ± SD (no. of replicates)
|
Selectivity index (CC50/EC50)
|
||||
|---|---|---|---|---|---|---|
| EC50 (μM)
|
CC50 (μM)a
|
|||||
| 2.2.15 cells | HepAD38 cells | 2.2.15 cells | HepG2 cells | 2.2.15 cells | HepG2 cells | |
| PMPA | 7.2 ± 0.8 (3) | 2.25 ± 1.24 (3) | >300 (3) | >100 (3) | >41.7 | >133 |
| CMX157 | 1.6 ± 0.2 (3) | 0.49 ± 0.24 (4) | >300 (3) | 18.8 ± 4.7 (5) | >187.5 | 38.4 |
The CC50s were determined in confluent 2.2.15 cells using neutral red and in rapidly dividing HepG2 cells using MTS, as described in Materials and Methods.
Toxicology and toxicokinetics.
Oral administration of CMX157 to Sprague-Dawley rats at doses of 10, 30, or 100 mg/kg/day for 7 days resulted in no mortality, clinical signs of toxicity, changes in body weights, body weight gain, or food consumption. There were no CMX157-related ophthalmological findings noted during the study. There were no CMX157-related changes in hematology or coagulation parameters or erythrocyte morphology and no changes in clinical chemistry parameters. There were no CMX157-related changes in qualitative or quantitative urinalysis parameters. No gross necropsy findings related to treatment were observed. Finally, there were no CMX157-related changes in organ weights or microscopic lesions. Based on these findings, the no-observed-adverse-effect level in rats given CMX157 for 7 days exceeds 100 mg/kg/day.
Approximately dose-proportional increases in the CMX157 Cmax were observed after single or multiple doses of 10, 30, or 100 mg/kg/day CMX157 (Table 3). On day 1 the Cmax of CMX157 was achieved in 0.5 to 2 h, with the Tmax occurring later at the 30- and 100-mg/kg doses than at the 10-mg/kg/day dose. On day 7 the Cmax was achieved in 0.5 to 4.0 h, again later at the 30- and 100-mg/kg doses than at the 10-mg/kg dose. After peaking, plasma levels of CMX157 declined quickly, with an estimated half-life of elimination ranging from 1.6 to 2.5 h. Plasma concentrations of CMX157 at 24 h approached the lower limit of quantitation on both days 1 and 7. Systemic exposure to CMX157 increased greater than in proportion to the dose and was similar on days 1 and 7.
TABLE 3.
Toxicokinetic parameters on days 1 and 7 after oral administration of CMX157 to ratsa
| Day | Dose (mg/kg) | CMX157 (prodrug)
|
Tenofovir (metabolite)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | Tmax (h) | AUC0-24 (ng · h/ml) | AUC0-∞ (ng · h/ml) | t1/2elim (h) | Cmax (ng/ml) | Tmax (h) | AUC0-24 (ng · h/ml) | AUC0-∞ (ng · h/ml) | t1/2elim (h) | ||
| 1 | 10 | 84.85 | 0.5 | 441.7 | ND | ND | 55.35 | 2.0 | 510.8 | 775.2 | 10.9 |
| 7 | 10 | 110.90 | 0.5 | 435.2 | ND | ND | 49.55 | 2.4 | 964.7 | 1,226.9 | 9.2 |
| 1 | 30 | 379.00 | 2.0 | 1,832.8 | ND | ND | 109.10 | 4.0 | 1,456.1 | 1,808.1 | 10.1 |
| 7 | 30 | 202.50 | 1.0 | 1,543.9 | ND | ND | 149.25 | 4.0 | 2,363.4 | 2,790.5 | 7.6 |
| 1 | 100 | 905.00 | 2.0 | 5,408 | 5,429.8 | 2.5 | 181.40 | 12.0 | 3,039.3 | 3,639.5 | 7.2 |
| 7 | 100 | 685.50 | 2.0 | 8,046.3 | 8,050.6 | 1.6 | 322.00 | 12.0 | 5,715.4 | 7,373.4 | 9.0 |
Plasma was obtained at 0.5, 1, 2, 4, 12, and 24 h after dosing and assayed for the prodrug and metabolite as described in Materials and Methods. Pharmacokinetic parameters were estimated using mean data from one male and one female in each group at each time point. AUC0-24, AUC from 0 to 24 h; t1/2elim, elimination half-life; ND, not determined.
Less-than-proportional increases in the metabolite (tenofovir) Cmax were observed after single or multiple doses of 10, 30, or 100 mg/kg/day CMX157 (Table 3). On days 1 and 7, the Cmax of tenofovir was achieved in 2, 4, and 12 h at the 10-, 30-, and 100-mg/kg/day doses, respectively. After peaking, plasma levels of tenofovir declined steadily, with an estimated half-life of elimination ranging from 7.2 to 10.9 h. Plasma concentrations of tenofovir at 24 h remained quantifiable on both days 1 and 7. Systemic exposure to tenofovir increased less than in proportion to dose and was greater on day 7 than on day 1.
DISCUSSION
Conversion of tenofovir to its HDP ester, HDP-tenofovir (CMX157), increased antiviral activity in HIV-1 infected MT-2 cells and in PBMCs by at least 260-fold in vitro (Table 1). This is likely due to increased cellular uptake of CMX157 and conversion to its diphosphate, as we reported previously for HDP-CDV (CMX001) (1). In two different HBV assays in 2.2.15 cells and in HepAD38 cells, the antiviral activity of CMX157 was 4.5- to 4.6-fold greater than that of tenofovir (Table 2). Although cytotoxicity also increased, the selectivity index of CMX157 in MT-2 cells and PBMCs was dramatically increased because of the marked decrease in the EC50. The HDP esters of CDV and HPMPA have been shown to exhibit increased cytotoxicity (2, 3) due to their greatly increased cellular uptake and conversion to their diphosphates (1; K. Y. Hostetler and K. A. Aldern, unpublished observations).
These results are in agreement with our recent reports on the ability of alkoxyalkyl esterification to increase oral availability and in vitro activity of other acyclic nucleoside phosphonate antivirals. For example, (S)-HPMPA was previously reported by several groups to be inactive against HIV-1. However, the HDP-(S)-HPMPA prodrug had an EC50 of 7 nanomolar in HIV-1-infected MT-2 cells (15). The HDP esters of phosphonomethoxyethyl-diaminopurine and phosphonomethoxyethyl-N6-cyclopropyl-diaminopurine were over 3 logs more active than the corresponding unmodified acyclic nucleoside phosphonates against HIV-1-infected MT-2 cells, with EC50 values in the range of 20 picomolar (23). The 5-phosphono-pent-2-en-yl nucleosides had no significant antiviral activity in vitro against herpesviruses and HBV unless they were converted to their HDP esters, which showed low-micromolar activity against herpesviruses and HBV (7). Thus, esterification of antiviral acyclic nucleoside phosphonates to their alkoxyalkyl esters appears to be a broadly applicable strategy to increase antiviral activity and selectivity.
Our previous studies with HDP-CDV indicated that it is orally available (8) and orally active against lethal poxvirus infections (6, 22), lethal murine cytomegalovirus infection (16), and human cytomegalovirus in SCID-hu mice with infected thy/liv implants (4). In the present study, HDP-PMPA (CMX157) was orally available in rats (Table 3). After a single dose of 10 mg/kg/day CMX157, systemic exposure to CMX157 was well in excess of that required to treat HIV-1 infection based on the 12 nanomolar EC50. By contrast, doses of up to 100 mg/kg/day for 7 days resulted in no toxicity, suggesting the potential for a favorable therapeutic index in humans. Exposure to tenofovir, an active metabolite of CMX157, was also good, with a Tmax several hours longer than that of CMX157 and a much longer terminal half-life, resulting in less potential for nephrotoxicity. Indeed, a toxicology study in rats given 10, 30, or 100 mg/kg/day CMX157 by the oral route for 7 days did not reveal any evidence of toxicity and, notably, no effects on the kidney, the typical target of CDV and tenofovir toxicity. Body weight, food consumption, serum biochemistry, hematology and urinalysis parameters, and gross and microscopic anatomic pathology examinations were normal at 7 days in all rats in all dose groups compared with vehicle-inoculated controls. Thus, the no-observed-adverse-effect level in this study exceeded 100 mg/kg/day, the highest dose tested. Additional toxicology studies are planned to assess the safety of longer periods of administration.
In conclusion, the antiviral activity of tenofovir can be increased at least 267-fold against HIV-1 and 4.5-fold against HBV by conversion to its CMX157 prodrug. CMX157 is orally bioavailable and has no toxicity in rats treated for 7 days at doses ranging from 10 to 100 mg/kg/day. These characteristics warrant the additional development of CMX157 for the treatment of HIV infections and potentially HBV infections.
Acknowledgments
These studies were supported in part by NIH grants AI-066499 and AI-071803 (to K.Y.H.), by the VIRGIL European Network of Excellence on Antiviral Drug Resistance supported by a grant (LSHM-CT-2004-503359, 6th Framework Programme of the EU) from the Priority 1 “Life Sciences, Genomics and Biotechnology for Health” and FWO grant no. G.0267.04 (to J.N. and E.D.C.), and by NIAID contract NO1-AI-45179 (to B.A.K.). K.Y.H. is a consultant and equity holder in Chimerix Inc. The terms of this relationship have been reviewed and approved by the University of California, San Diego, in accordance with their conflict-of-interest policies.
Footnotes
Published ahead of print on 23 July 2007.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. The increased antiviral activity of 1-O-hexadecyloxypropyl-cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Beadle, J. R., N. Rodriquez, K. A. Aldern, C. Hartline, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beadle, J. R., W. B. Wan, S. L. Ciesla, K. A. Keith, C. Hartline, E. R. Kern, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine (HPMPA) against cytomegalovirus and orthopoxviruses. J. Med. Chem. 49:2010-2015. [DOI] [PubMed] [Google Scholar]
- 4.Bidanset, D. J., J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2004. Oral activity of ether lipid prodrugs of cidofovir against experimental human cytomegalovirus infections. J. Virol. 190:499-503. [DOI] [PubMed] [Google Scholar]
- 5.Buckheit, R. W., V. Fliakas-Boltz, W. D. Decker, J. L. Roberson, T. L. Stup, C. A. Pyle, E. L. White, J. B. McMahon, M. J. Currens, M. R. Boyd, and J. P. Bader. 1995. Comparative anti-HIV evaluation of diverse HIV-1-specific reverse transcriptase inhibitor-resistant virus isolates demonstrates the existence of distinct phenotypic subgroups. Antiviral Res. 26:117-132. [DOI] [PubMed] [Google Scholar]
- 6.Buller, R. M., G. Owens, J. Schriewer, L. Melman, J. R. Beadle, and K. Y. Hostetler. 2004. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology 318:474-481. [DOI] [PubMed] [Google Scholar]
- 7.Choo, H., J. R. Beadle, E. R. Kern, M. N. Prichard, K. A. Keith, C. B. Hartline, J. Trahan, K. A. Aldern, B. E. Korba, and K. Y. Hostetler. 2007. Antiviral activities of novel 5-phosphono-pent-2-en-1-yl nucleosides and their alkoxyalkyl phosphonoesters. Antimicrob. Agents Chemother. 51:611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciesla, S. L., J. Trahan, K. L. Winegarden, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 9.Connelly, M. C., B. L. Robbins, and A. Fridland. 1993. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine (HPMPC) in Vero cells. Biochem. Pharmacol. 46:1053-1057. [DOI] [PubMed] [Google Scholar]
- 10.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogs cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq, E., and A. Holý. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928-940. [DOI] [PubMed] [Google Scholar]
- 12.Hammond, J. L., D. Koontz, H. Z. Bazmi, J. R. Beadle, S. E. Hostetler, G. D. Kini, K. A. Aldern, D. D. Richman, K. Y. Hostetler, and J. W. Mellors. 2001. Alkylglycerol prodrugs of phosphonoformate are potent in vitro inhibitors of nucleoside resistant human immunodeficiency virus type 1 and select for resistance mutations that reverse zidovudine resistance. Antimicrob. Agents Chemother. 45:1621-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, H. T., K. L. Woods, J. J. Bronson, H. De Boeck, J. C. Martin, and M. J. M. Hitchcock. 1992. Intracellular metabolism of the antiherpesvirus agent (S)-1-[3-hydroxy-2-(phosphonyl-methoxyl)propyl]cytosine. Mol. Pharmacol. 41:197-202. [PubMed] [Google Scholar]
- 14.Holý, A. 2003. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharm. Des. 9:2567-2592. [DOI] [PubMed] [Google Scholar]
- 15.Hostetler, K. Y., K. A. Aldern, W. B. Wan, S. L. Ciesla, and J. R. Beadle. 2006. Alkoxyalkyl esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl] adenine are potent inhibitors of the replication of wild-type and drug-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 50:2857-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern, E. R., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and D. C. Quenelle. 2004. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korba, B. E., and J. L. Gerin. 1992. Use of a standardized cell culture assay to determine activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 19:55-70. [DOI] [PubMed] [Google Scholar]
- 19.Korba, B. E., and G. Milman. 1991. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antiviral Res. 15:217-228. [DOI] [PubMed] [Google Scholar]
- 20.Ladner, S. K., M. J. Otto, C. S. Barker, K. Zaifert, G. H. Wang, J. T. Guo, C. Seeger, and R. W. King. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 41:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Painter, G. R., and K. Y. Hostetler. 2004. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 22:423-427. [DOI] [PubMed] [Google Scholar]
- 22.Quenelle, D. C., D. J. Collins, K. Y. Hostetler, J. R. Beadle, W. B. Wan, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valiaeva, N., J. R. Beadle, K. A. Aldern, J. Trahan, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl esters of acyclic purine and pyrimidine nucleoside phosphonates against HIV-1 in vitro. Antiviral Res. 72:10-19. [DOI] [PubMed] [Google Scholar]
- 24.Wan, W. B., J. R. Beadle, C. B. Hartline, E. R. Kern, S. L. Ciesla, N. Valiaeva, and K. Y. Hostetler. 2005. Comparison of the antiviral activity of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 49:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]