Abstract
Eight weeks of treatment with rifampin-streptomycin sterilizes Mycobacterium ulcerans infection in mice. Because the bactericidal activity against M. ulcerans of the combination rifampin-moxifloxacin, rifampin-clarithromycin, or moxifloxacin-clarithromycin was similar to that of rifampin-streptomycin, these combinations might be considered as alternative, orally administered combined regimens for treatment of Buruli ulcer in humans.
The combination of rifampin (RIF) and streptomycin (STR) is highly effective for treatment of Buruli ulcer (Mycobacterium ulcerans disease) (1, 2, 6; A. Chauty, presented at the 8th Annual World Health Organization Advisory Group Meeting on Buruli Ulcer), but ambulatory treatment requiring daily intramuscular injection of STR for 8 weeks is operationally demanding in most countries where the disease is endemic. To simplify the treatment under field conditions, there is an urgent need to develop effective combined regimens that may be administered orally. We have attempted to identify such regimens in mouse experiments.
The left hind footpad of each of the 310 female BALB/c mice was inoculated subcutaneously with 0.03 ml of a bacterial suspension containing 1.2 × 104 CFU of M. ulcerans isolate CU001. The MICs of RIF, STR, and moxifloxacin (MXF) against this isolate were, respectively, 2, 0.5, and 0.25 μg/ml on 7H11 agar medium, and that of clarithromycin (CLR) was 0.5 μg/ml on Mueller-Hinton agar medium. Seven weeks later, when all mice developed a “lesion index” (4) of 2 (definite inflammatory swelling limited to footpad) or 3 (inflammatory swelling involving the entire inoculated foot), 10 mice were sacrificed for enumeration of CFU in the inoculated footpads to establish the pretreatment (day 0) value. The remaining 300 mice were randomly allocated among 10 groups, including 1 untreated control group and 9 treated groups (Table 1), and treatment was begun immediately. All antimicrobial agents were given five times weekly by gavage, except STR was injected subcutaneously. Mice were sacrificed at regular intervals. After 8 weeks of treatment, 20 mice each from groups 5 to 8 were held without treatment for an additional 28 weeks to detect relapse of M. ulcerans infection (4). Severity of infection and effectiveness of treatment were assessed by the mean number (log10) of CFU per inoculated footpad (3). To enumerate CFU, tissues of the footpad were removed aseptically at sacrifice and homogenized in Hanks' solution in a final volume of 2 ml. To monitoring relapse during 28 weeks of posttreatment follow-up, footpads were examined weekly to observe the evolution of the lesion index, which was scored from 0 to 5 (4). If there was a rebound of the lesion index to ≥3 after completion of 8 weeks of treatment (4), the affected mouse was immediately sacrificed and the entire volume of undiluted tissue suspension from the footpad was plated on 10 tubes of Löwenstein-Jensen medium for cultivation of M. ulcerans. At the end of follow-up, all surviving mice were also sacrificed for cultivation by the same technique. Cultures were examined after 90 days of incubation at 30°C. A positive culture indicated relapse of the infection.
TABLE 1.
Mean numbers of CFU per footpad in various groups of mice
| Group | Regimena | Result at:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0b (log10 CFU/footpad for group)c | 2 wk
|
4 wk
|
8 wk
|
||||||||
| No. of mice culture positive/total | Log10 CFU/culture-positive footpadc | Log10 CFU/footpad for groupc | No. of mice culture positive/total | Log10 CFU/culture-positive footpadc | Log10 CFU/footpad for groupc | No. of mice culture positive/total | Log10 CFU/culture-positive footpadc | Log10 CFU/footpad for groupc | |||
| 1 | Untreated control | 6.24 ± 0.45 | 10/10 | 5.94 ± 0.51 | 5.94 ± 0.51 | 7/7d | 6.03 ± 0.76 | 6.03 ± 0.76 | |||
| 2 | RIF alone | 3/10f | 1.52 ± 1.01 | 0.46 ± 0.87 | 0/10f | All 10 pads negative | |||||
| 3 | MXF alone | 9/10 | 3.23 ± 0.87 | 2.99 ± 1.12 | 7/10 | 1.72 ± 0.95 | 1.25 ± 1.08 | ||||
| 4 | CLR alone | 5/10 | 3.43 ± 1.03 | 2.53 ± 1.21 | 0/10e | <1.82 | |||||
| 5 | RIF-STR | 7/20f | 0.88 ± 0.73 | 0.31 ± 0.59 | 0/10f | All 10 pads negative | |||||
| 6 | RIF-STR for 4 wk followed by RIF-MXF for 4 wk | 0/20f | All 20 pads negative | ||||||||
| 7 | RIF-STR for 2 wk followed by RIF-MXF for 6 wk | 7/10 | 2.50 ± 1.23 | 1.88 ± 1.43 | 0/20f | All 20 pads negative | |||||
| 8 | RIF-MXF | 4/10f | 1.07 ± 0.65 | 0.43 ± 0.67 | 0/10f | All 10 pads negative | |||||
| 9 | RIF-CLR | 0/10f | All 10 pads negative | 0/10f | All 10 pads negative | ||||||
| 10 | MXF-CLR | 8/10f | 1.07 ± 0.83 | 0.86 ± 0.86 | 1/10f | 0 | −1 | ||||
All drugs were administered by gavage, except STR was injected subcutaneously, 5 days per week. The dosages for each treatment were 10 mg/kg RIF, 150 mg/kg STR, 100 mg/kg MXF, and 100 mg/kg CLR.
Treatments were begun 7 weeks after inoculation, when all mice developed a lesion index of 2 or 3 (4).
Values are means ± standard deviations, unless otherwise noted.
One mouse had already succumbed to M. ulcerans infection, and two of the remaining nine footpads were contaminated during enumeration of CFU.
Although all 10 pads were culture negative, only 0.1 ml of a 1:10-, 1:100-, or 1:1,000-diluted suspension of the inoculated footpad had been plated in triplicate on Löwenstein-Jensen medium.
The entire volume (2 ml) of the undiluted tissue suspension from each footpad had been plated onto 10 tubes of Löwenstein-Jensen medium.
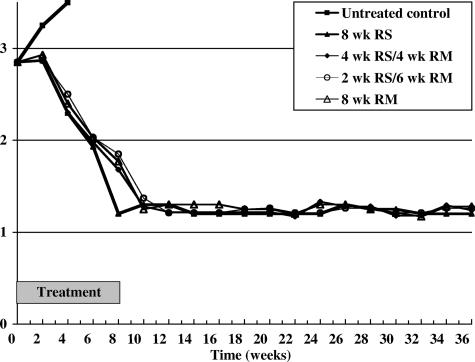
After starting treatment, among untreated controls, the inoculated footpads were invariably culture positive, the mean numbers of CFU per footpad were similar to the pretreatment value, and the average lesion index increased progressively, as shown in Table 1 and Fig. 1. In contrast, all of these parameters declined progressively in all treated groups. After 4 weeks of treatment, the mean numbers of CFU per footpad of all treated groups were significantly smaller than the pretreatment value (P < 0.01); the mean CFU of mice treated with MXF was similar to that treated with CLR, and the reduction of CFU in mice treated with MXF or CLR was significantly less than that in mice treated with RIF (P < 0.01). After 8 weeks of treatment, all footpads of mice treated with RIF monotherapy or any of the tested combinations were culture negative, except a single colony was detected in one of 10 footpads from mice treated with MXF-CLR. Although all footpads of mice treated with CLR monotherapy were also culture negative, the dilution of tissue suspension employed to enumerate CFU was different from those in mice treated with RIF monotherapy or any of the tested combinations. In the former group, only 0.1 ml of 1:10-, 1:100-, and 1:1,000-diluted tissue suspensions had been plated, respectively, in triplicate on Löwenstein-Jensen medium, whereas in the latter groups, the entire volume (2 ml) of undiluted tissue suspension was plated on 10 tubes of Löwenstein-Jensen medium. Therefore, a culture-negative result in the CLR monotherapy group did reveal significant bactericidal activity, but it is unclear whether the treatment had killed all viable organisms. During 28 weeks of posttreatment follow-up, no rebound of lesion index was observed in any mouse that had been treated with 8 weeks of RIF-STR, 4 or 2 weeks of RIF-STR followed by 4 or 6 weeks of RIF-MXF, or 8 weeks of RIF-MXF (groups 5 to 8). At the end of follow-up, all footpads of 20, 17, 19, and 18 surviving mice from these four groups were culture negative.
FIG. 1.
Evolution of the average lesion indexes in various groups of mice after starting treatment. RS and RM refer, respectively, to the combination RIF-STR and RIF-MXF.
That 7 of 20 mice remained culture positive after 4 weeks of treatment with RIF-STR, whereas all were culture negative after 8 weeks of treatment, with no relapse thereafter, confirmed our earlier findings that 4 weeks of RIF-STR was unable to kill all viable M. ulcerans cells, whereas 8 weeks of treatment sterilized the infection in mice (4).
Although MXF was significantly less bactericidal than STR against M. ulcerans when both drugs were administered as monotherapy (3), in terms of CFU and relapse rate, the bactericidal activity of 8 weeks of treatment with any of the three regimens in which MXF had been partially (groups 6 and 7) or completely substituted (group 8) for the STR component of the combination RIF-STR was identical to that of RIF-STR, indicating that the bactericidal activity of RIF-STR was not compromised by substitution of MXF for STR and confirming that the combination RIF-MXF was as effective as RIF-STR against M. ulcerans in mice (3). That all footpads were culture negative after 8 weeks of treatment with RIF-CLR suggests that the bactericidal activity of RIF-CLR is indistinguishable from that of RIF-STR or RIF-MXF.
Because the reductions in both the proportion of culture-positive mice and the mean number of CFU per footpad during treatment with RIF-containing combined regimens (groups 5 to 9) were virtually identical with those of RIF monotherapy group (group 2), one may attribute the promising bactericidal activities of the RIF-containing combinations to their RIF component. However, this finding by no means indicates that M. ulcerans infection could be treated with RIF monotherapy, because RIF-resistant mutants of M. ulcerans multiply selectively over the course of RIF monotherapy (5). The moderate bactericidal activity of MXF or CLR against M. ulcerans may play an important role in preventing the selection of RIF-resistant mutants, thus justifying their combination with RIF. We therefore conclude that the combinations RIF-MXF and RIF-CLR may be considered as orally administered alternatives to the combination RIF-STR for treatment of Buruli ulcer in humans.
The results also revealed that the bactericidal activity of the combination MXF-CLR is similar to that of RIF-STR, suggesting that MXF-CLR may be employed as an orally administered combined regimen for treatment of Buruli ulcer among patients who cannot be treated with RIF due to either RIF resistance, a severe hypersensitivity reaction, or a serious adverse event.
Acknowledgments
This investigation was funded by the Foundation Raoul Follereau, Paris, France, and by Université Paris 6 (EA 1541).
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Chauty, A., M.-F. Ardant, A. Adeye, H. Euverte, A. Guedenon, C. Johnson, J. Aubry, E. Nuermberger, and J. Grosset. 25 May 2007. Promising clinical efficacy of the combination streptomycin-rifampin for the treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob. Agents Chemother. doi: 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed]
- 2.Etuaful, S., B. Carbonnelle, J. Grosset, S. Lucas, C. Horsfield, R. Phillips, M. Evans, D. Ofori-Adjei, E. Klustse, J. Owusu-Boateng, G. K. Amedofu, P. Awuah, E. Ampadu, G. Amofah, K. Asiedu, and M. Wansbrough-Jones. 2005. Efficacy of the combination of rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 49:3182-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji, B., S. Lefrançois, J. Robert, A. Chauffour, C. Truffot, and V. Jarlier. 2006. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob. Agents Chemother. 50:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefrançois, S., J. Robert, A. Chauffour, B. Ji, and V. Jarlier. 2007. Curing Mycobacterium ulcerans infection in mice with combination of rifampin-streptomycin or rifampin-amikacin. Antimicrob. Agents Chemother. 51:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsollier, L., N. Honoré, P. Legras, A. L. Manceau, H. Kouakou, B. Carbonnelle, and S. T. Cole. 2003. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob. Agents Chemother. 47:1228-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2004. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). World Health Organization, Geneva, Switzerland.