Abstract
Using our high-throughput hepatitis C replicon assay to screen a library of over 8,000 novel diversity-oriented synthesis (DOS) compounds, we identified several novel compounds that regulate hepatitis C virus (HCV) replication, including two libraries of epoxides that inhibit HCV replication (best 50% effective concentration, < 0.5 μM). We then synthesized an analog of these compounds with optimized activity.
Hepatitis C virus (HCV) infects over 170 million people worldwide and frequently leads to cirrhosis, liver failure, and hepatocellular carcinoma (1). Currently, the best therapy for the treatment of chronic hepatitis C is a combination of pegylated interferon and ribavirin, which has suboptimal efficacy and has an unfavorable side effect profile (14). The identification of more-effective and better-tolerated agents is therefore a high priority.
We have recently reported the successful adaptation of the Huh7/Rep-Feo replicon cell line (18) to a high-throughput screening assay system (8). Using this system, we previously screened a library of 2,568 well-known compounds whose biological activity is fully characterized (8). In order to discover novel regulators of HCV replication, we then screened a library of 8,064 diversity-oriented synthesis (DOS) compounds (15, 16). This library, known as the DOS set, is ameta-library comprised of DOS libraries from chemists throughout the United States and Canada. Information about the DOS set is available at http://www.broad.harvard.edu/chembio/platform/screening/compound_libraries/index.htm.
The high-throughput primary screen and the secondary validation assays were performed as described in our previous publication (8).
Computational data analysis of the primary screening results was performed as previously described (8) except for the hit criteria. As the characteristics of this data set are different from those generated by our previous screen (8), different threshold values were chosen to assure optimal hit selection. Compounds were considered hits for inhibiting replication if they had a composite Z score of <−2.57 in the reporter gene screen, a reproducibility of >0.9 or <−0.9 in that screen, and a composite Z score of >−2.00 in the cell viability screen. Compounds were considered hits for stimulating luciferase production if they had a composite Z score of >2.50 in the reporter gene screen, a reproducibility of >0.9 or <−0.9 in that screen, and a composite Z score of <1.00 in the cell viability screen.
Full synthetic experimental procedures and spectroscopic data for the SM library compounds discussed in this publication are provided in the supplemental material. The synthesis of the full SM library, including compounds not discussed here, will be the subject of an upcoming report.
The synthesis of the BUCMLD epoxyquinol library has been previously described (10, 17).
Full experimental details regarding the JFH1 HCVcc system (11) are provided in the supplemental material. We identified 41 antiviral compounds that inhibited HCV replication and 20 proviral compounds that increased luciferase production (Table 1). In our analysis of the antiviral hit compounds from the DOS set, a striking finding was that 21 of the 41 compounds contained an epoxide moiety. Moreover, the most potent of these compounds were epoxides. Further analysis revealed that these epoxides came from only two DOS libraries, SM and BUCMLD epoxyquinol (10, 17), with very high sublibrary hit rates of 35% and 33%, respectively (Table 1). Of note, the non-hit members of these two libraries did exhibit antiviral activity but failed to meet the formal hit criteria.
TABLE 1.
Hits by library from the primary high-throughput screening with the DOS seta
| Library | Increased luciferase signal hit libraries
|
Antiviral hit libraries
|
||||
|---|---|---|---|---|---|---|
| Hits | Members | Reference(s) | Hits | Members | Reference(s) or sources | |
| FPA | 11 | 319 | 5 | |||
| BUCMLD | 4 | 880 | 10, 17 | 4 | 880 | 10, 17, Fig. 1, Table 2 |
| JMM | 4 | 544 | 13 | |||
| UGISS | 1 | 319 | 2 | |||
| BUCMLD epoxyquinol | 12 | 34 | 10, 17, Fig. 1 and 2, Table 2 | |||
| SM | 9 | 27 | Fig. 1 and 2, Table 2 | |||
| SpOx | 6 | 612 | 6, 12 | |||
| BEA | 3 | 238 | 3 | |||
| ICCB6 | 3 | 352 | 4 | |||
| YKK | 2 | 281 | 9 | |||
| RTE | 2 | 159 | 19 | |||
The total number of compounds which comprise each library is listed in the “Members” columns.
As we were especially intrigued by these epoxide-bearing compounds, we restricted our hit validation to these compounds (Table 2 and Fig. 1). SM_A6B5_2P100 was the most active member of the SM library, while BUCMLD-B10A11 was the most potent member of the BUCMLD epoxyquinol library (Table 2 and Fig. 2).
TABLE 2.
Results of secondary screening with antiviral hit compounds from the SM and BUCMLD librariesa
| Compound name | EC50 | CC50 |
|---|---|---|
| BUCMLD-B10A11 | <0.5 (<0.5-0.5) | 19.5 (19.4-22.4) |
| BUCMLD-B10A3 | 0.7 (<0.5-5.2) | 9.0 (7.1-10.0) |
| BUCMLD-XL-184 | 1.4 (0.8-3.9) | >50 |
| BUCMLD-B10A5 | 1.5 (<0.5-5.4) | 39.3 (28.6->50) |
| BUCMLD-XL-190 | 2.5 (<0.5-10) | >50 |
| BUCMLD-B10A1 | 2.6 (1.0-5.0) | 18.0 (15.9-19.5) |
| SM_A14B5 | 3.5 (2.7-4.4) | 27.1 (18.0-44.6) |
| BUCMLD-XL-189 | 3.8 (2.2-7.0) | >50 |
| SM_A6B5_2P100 | 6.6 (4.0-15.3) | >50 |
| BUCMLD-B10A8 | 7.0 (0.9-30.0) | >50 |
| BUCMLD-B10A10 | 7.0 (5.4-30.0) | >50 |
| BUCMLD-B10A14 | 7.6 (1.0-23.0) | >50 |
| BUCMLD-B10A7 | 7.75 (1.0-30.0) | 35.3 (33.6-36.7) |
| SM_A4B6_2P123 | 8.0 (6.3-10.0) | >50 |
| SM_A5B5_2P118 | 9.1 (2.5-16.7) | >50 |
| SM_A7C2_2P155 | 12.7 (7.0-24.0) | >50 |
| BUCMLD-B13A2 | 14.2 (6.4-45.0) | >50 |
| SM_A1B2_1P32 | 19.6 (11.7-28.2) | >50 |
| SM_A1B5_2P24 | 19.7 (6.25-50.0) | >50 |
| BUCMLD-B13A1 | 21.1 (7.5-36.7) | >50 |
| SM_A5B3_2P141 | 25.7 (18.3-39.8) | >50 |
| SM_A5B2_2P142 | 26.7 (9.1-50.0) | >50 |
| BUCMLD-NTM-EN2-67A | 30.0 (0.7-46.1) | >50 |
| SM_A12B3 | >30 | >50 |
| BUCMLD-B10A13 | 42.9 (25.2-59.4) | >50 |
| BUCMLD-XL-130 | >100 | >50 |
Note that structure-activity relationship SM library compounds are also included. The EC50 and 50% cytotoxic concentration (CC50) are reported in μM with 95% confidence intervals in parentheses. A value of <0.5 indicates a concentration of less than 0.5 μM; >30 indicates a concentration of greater than 30 μM; >50 indicates a concentration of greater than 50 μM; and >100 indicates a concentration of greater than 100 μM.
FIG. 1.
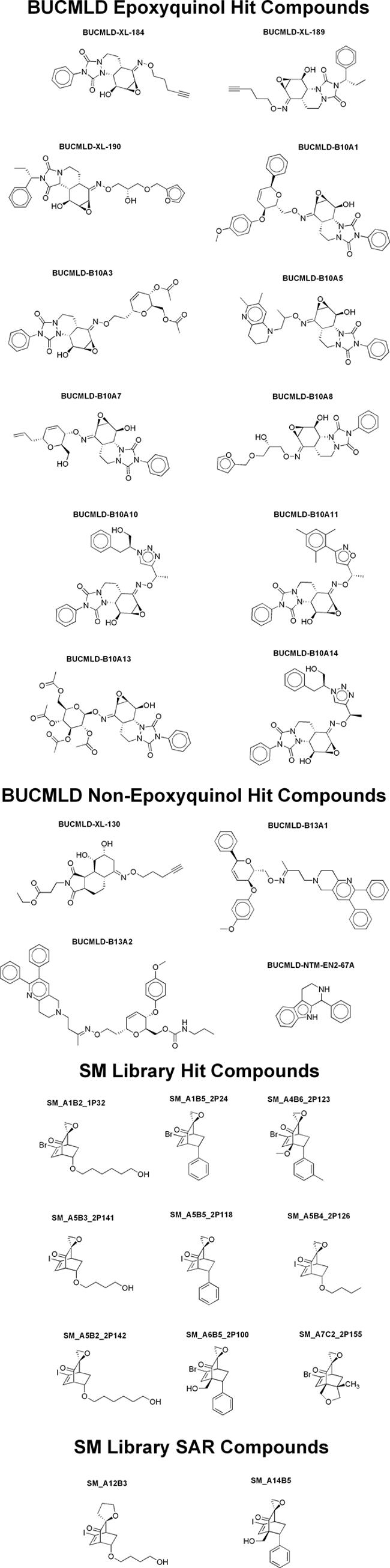
Structures of antiviral hit compounds from the BUCMLD and SM libraries. SAR, structure-activity relationship.
FIG. 2.
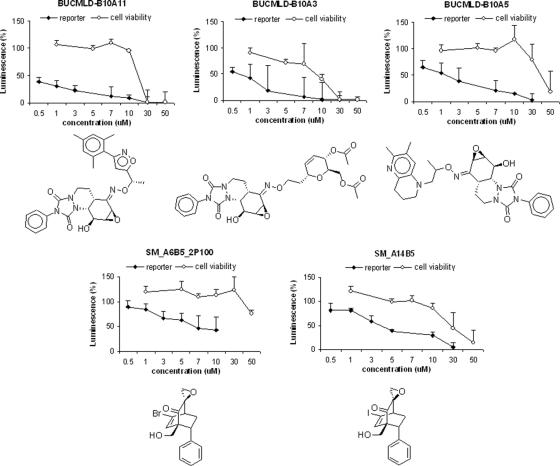
Selected graphical results of secondary screening with antiviral hit compounds from the SM and BUCMLD epoxyquinol libraries. Luciferase activity for HCV RNA replication levels is shown as a percentage of control. Cell viability is also shown as a percentage of control. Each point represents the average of triplicate data points with standard deviation represented as the error bar.
Structure-activity relationship analysis of the SM library reveals the structural elements most important for antiviral activity (Table 2 and Fig. 1). Comparing SM_A5B5_2P118 to SM_A1B5_2P24, iodinated compounds are more active than brominated ones. Comparing SM_A5B5_2P118 to SM_A5B3_2P141 and SM_A5B2_2P142, compounds with a phenyl substituent are more active than those with aliphatic chains. Finally, the most active compounds, SM_A4B6_2P123 and SM_A6B5_2P100, have a bridgehead substituent. Thus, we hypothesized that the most active compound should bear an iodine, a phenyl substituent, and a bridgehead substituent.
SM_A14B5, which incorporates all of these elements, was therefore synthesized, as it was reasoned to be the most active SM library compound. Indeed, SM_A14B5 had a 50% effective concentration (EC50) of approximately 3.5 μM, which is about half that of SM_A6B5_2P100 (Table 2 and Fig. 2).
The most potent compounds from each library, SM_A14B5 and BUCMLD-B10A11, underwent further validation in the infectious JFH1 HCVcc system (11). They were tested at concentrations of 5 μM and 1 μM, respectively, and inhibited HCV replication 48.4% ± 5.9% and 45.1% ± 5.2%, respectively, relative to the level of inhibition achieved by interferon at a concentration of 1 ng/ml. These data roughly approximate the EC50 validation data derived from the OR6 system (7) in which inhibition was also measured relative to that of interferon at a concentration of 1 ng/ml.
Our observations suggest that the epoxide moiety is essential for potent antiviral activity. Analyzing the BUCMLD compounds, those compounds that bear an epoxide moiety are, in general, more-potent antivirals than those that do not (Table 2 and Fig. 1). Furthermore, all of the compounds from the SM library bear epoxides. SM_A12B3, an analog of SM_A5B3_2P141, which bears a tetrahydrofuran moiety in place of an epoxide, was therefore synthesized to further test this hypothesis. SM_A12B3 had negligible antiviral activity (Table 2), while SM_A5B3_2P141 displayed modest antiviral activity. Other analogs of SM compounds bearing tetrahydrofuran rings in place of epoxides showed similar attenuation of antiviral activity relative to their parent compounds. Unfortunately, attempts to synthesize the tetrahydrofuran analog of the most potent SM compound, SM_A14B5, have so far been unsuccessful.
It is interesting to note that it is the urazole-containing epoxyquinol constituents of the BUCMLD epoxyquinol library, rather than the maleimide-derived ones, that demonstrated anti-HCV activity in the primary screen. It is therefore likely that the combination of a urazole with the epoxide is necessary for the activity of the BUCMLD epoxyquinol compounds.
Although none of our most potent antiviral DOS compounds showed significant cytotoxicity at their EC50s, all of them ultimately proved to be cytotoxic at higher concentrations (Table 2 and Fig. 2). Therefore, future modifications should not only aim to improve anti-HCV activity but should also attempt to decrease cytotoxicity, in order to widen the therapeutic window.
It is tempting to hypothesize that these epoxides exert their antiviral effects through a common pathway. Presumably, they act as electrophiles, with the nucleophilic target making a covalent bond by attacking and opening the epoxides. Studies to elucidate their mechanism of action are under way.
Supplementary Material
Acknowledgments
We thank the National Cancer Institute and the Initiative for Chemical Genetics, who provided support for this publication, and the Chemical Biology Platform of the Broad Institute of Harvard and MIT for their assistance in this work.
The project has been funded in whole or in part with federal funds from the National Cancer Institute's Initiative for Chemical Genetics, National Institutes of Health, under contract no. N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Financial support was provided by the following: The American Gastroenterological Association FDHN/TAP Pharmaceuticals FFT Award (L.F.P.), The GlaxoSmithKline Research Fund of the Korean Association for The Study of The Liver (S.S.K.), NIH 5T32DK07191-31 (L.F.P.), NIH NS050854-01 (R.T.C.), and the NIGMS CMLD Initiative P50 GM067041 (J.A.P.).
Footnotes
Published ahead of print on 6 August 2007.
This publication is dedicated to Yoshito Kishi on the occasion of his 70th birthday.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alter, M. J. 2006. Epidemiology of hepatitis C. Hepatology 43:S207-S220.16447262 [Google Scholar]
- 2.Andreana, P. R., C. C. Liu, and S. L. Schreiber. 2004. Stereochemical control of the Passerini reaction. Org. Lett. 6:4231-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittain, D. E. A., B. L. Gray, and S. L. Schreiber. 2005. From solution-phase to solid-phase enyne metathesis: crossover in the relative performance of two commonly used ruthenium pre-catalysts. Chem. Eur. J. 11:5086-5093. [DOI] [PubMed] [Google Scholar]
- 4.Burke, M. D., E. M. Berger, and S. L. Schreiber. 2004. A synthesis strategy yielding skeletally diverse small molecules combinatorially. J. Am. Chem. Soc. 126:14095-14104. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C., X. Li, C. Neumann, M. M.-C. Lo, and S. L. Schreiber. 2005. Convergent diversity-oriented synthesis of small-molecule hybrids. Angew. Chem. 117:2-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C., X. Li, and S. L. Schreiber. 2003. Catalytic asymmetric [3+2] cycloaddition of azomethine ylides. Development of a versatile stepwise, three-component reaction for diversity-oriented synthesis. J. Am. Chem. Soc. 125:10174-10175. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda, M., K. Abe, H. Dansako, T. Nakamura, K. Naka, and N. Kato. 2005. Efficient replication of a full-length hepatitis C virus genome, strain O, in cell culture, and development of a luciferase reporter system. Biochem. Biophys. Res. Commun. 329:1350-1359. [DOI] [PubMed] [Google Scholar]
- 8.Kim, S. S., L. F. Peng, W. Lin, W.-H. Choe, N. Sakamoto, S. L. Schreiber, and R. T. Chung. 2007. A cell-based, high-throughput screen for small molecule regulators of HCV replication. Gastroenterology 132:311-320. [DOI] [PubMed] [Google Scholar]
- 9.Kim, Y.-K., M. A. Arai, T. Arai, J. O. Lamenzo, E. F. Dean, N. Patterson, P. A. Clemons, and S. L. Schreiber. 2004. Relationship of stereochemical and skeletal diversity of small molecules to cellular measurement space. J. Am. Chem. Soc. 126:14740-14745. [DOI] [PubMed] [Google Scholar]
- 10.Lei, X., N. Zaarur, M. Y. Sherman, and J. A. Porco. 2005. Stereocontrolled synthesis of a complex library via elaboration of angular epoxyquinol scaffolds. J. Org. Chem. 70:6474-6483. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 12.Lo, M. M.-C., C. S. Neumann, S. Nagayama, E. O. Perlstein, and S. L. Schreiber. 2004. A library of spirooxindoles based on a stereoselective three-component coupling reaction. J. Am. Chem. Soc. 126:16077-16086. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell, J. M., and J. T. Shaw. 2006. A structurally diverse library of polycyclic lactams resulting from systematic placement of proximal functional groups. Angew. Chem. 45:1722-1726. [DOI] [PubMed] [Google Scholar]
- 14.Pawlotsky, J. M. 1997. Therapy of hepatitis C: from empiricism to eradication. Hepatology 26:S62-S65. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber, S. L. 2000. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287:1964-1969. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber, S. L. 2003. Chemical genetics. Chem. Eng. News 81:51-61. [Google Scholar]
- 17.Su, S., D. E. Acquilano, J. Arumugasamy, A. B. Beeler, E. L. Eastwood, J. R. Giguere, P. Lan, X. Lei, G. K. Min, A. R. Yeager, Y. Zhou, J. S. Panek, J. K. Snyder, S. E. Schaus, and J. A. Porco. 2005. Convergent synthesis of a complex oxime library using chemical domain shuffling. Org. Lett. 7:2751-2754. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe, Y., N. Sakamoto, N. Enomoto, M. Kurosaki, E. Ueda, S. Maekawa, T. Yamashiro, M. Nakagawa, C. H. Chen, N. Kanazawa, S. Kakinuma, and M. Watanabe. 2004. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon. J. Infect. Dis. 189:1129-1139. [DOI] [PubMed] [Google Scholar]
- 19.Taylor, A. M., and S. L. Schreiber. 2006. Enantioselective addition of terminal alkynes to isolated isoquinoline iminiums. Org. Lett. 8:143-146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.