Abstract
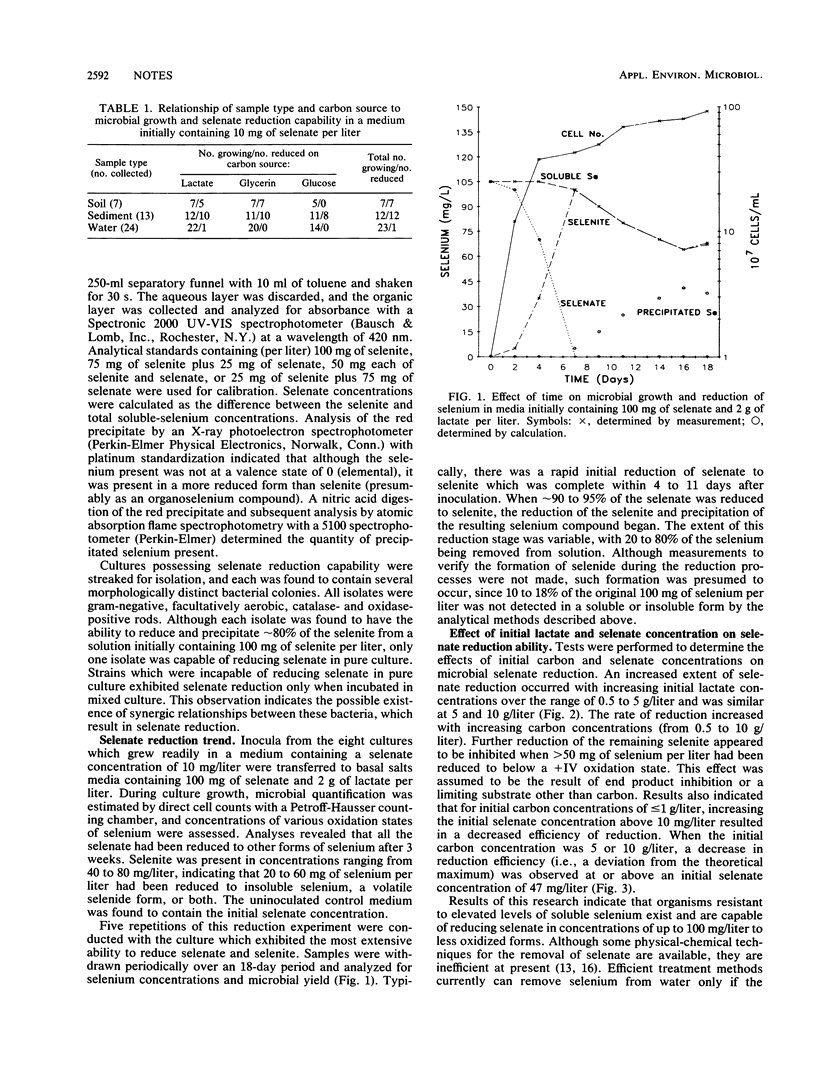
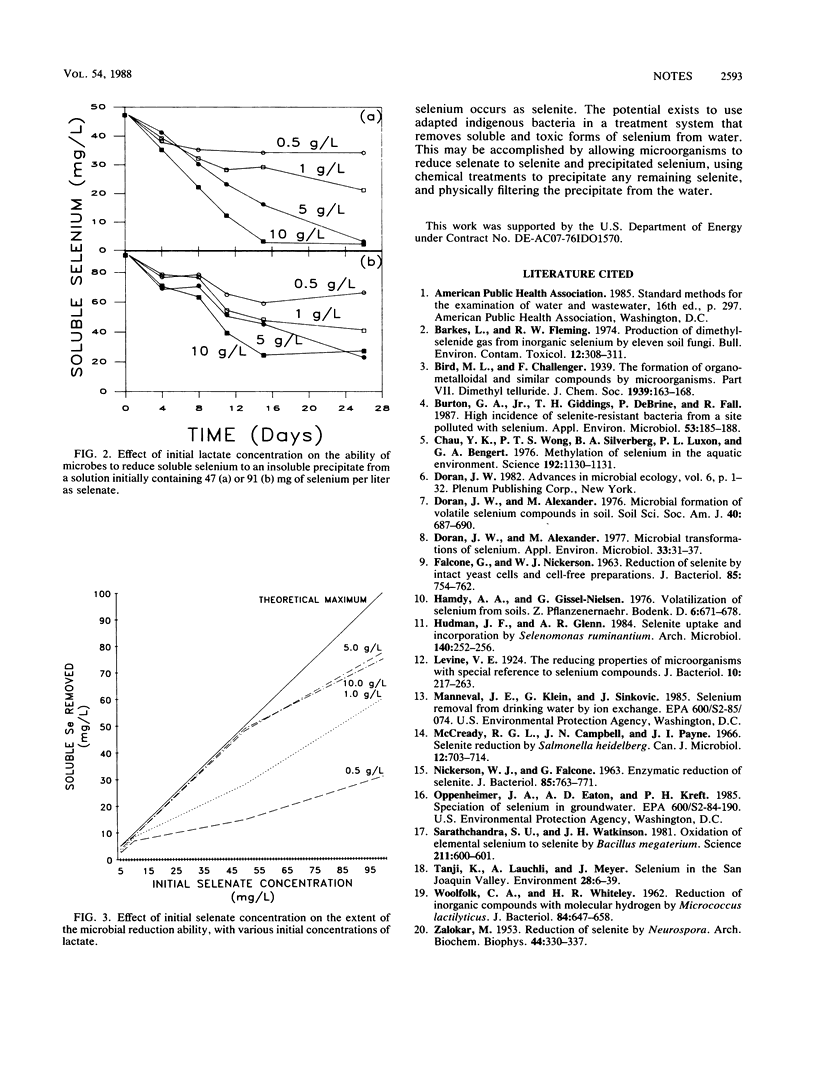
Samples collected from Kesterson Reservoir were screened for bacterial presence and selenate reduction capability. Selenate concentrations of 100 mg/liter were not toxic to indigenous bacteria. Of the 44 samples collected, 20 possessed microbial populations capable of reducing selenate. Reduction was observed in 4% of the water samples, 92% of the sediment samples, and 100% of the soil samples. Microbial reduction of 100 mg of selenate per liter was complete within 1 week of incubation. Up to 75 mg of selenate per liter was reduced beyond selenite to an insoluble red precipitate. Data collected indicate that indigenous bacteria have a significant role in the biogeochemical cycling of selenium.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkes L., Fleming R. W. Production of dimethylselenide gas from inorganic selenium by eleven soil fungi. Bull Environ Contam Toxicol. 1974 Sep;12(3):308–311. doi: 10.1007/BF01709124. [DOI] [PubMed] [Google Scholar]
- Burton G. A., Jr, Giddings T. H., DeBrine P., Fall R. High incidence of selenite-resistant bacteria from a site polluted with selenium. Appl Environ Microbiol. 1987 Jan;53(1):185–188. doi: 10.1128/aem.53.1.185-188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau Y. K., Wong P. T., Silverberg B. A., Luxon P. L., Bengert G. A. Methylation of selenium in the aquatic environment. Science. 1976 Jun 11;192(4244):1130–1131. doi: 10.1126/science.192.4244.1130. [DOI] [PubMed] [Google Scholar]
- Doran J. W., Alexander M. Microbial transformations of selenium. Appl Environ Microbiol. 1977 Jan;33(1):31–37. doi: 10.1128/aem.33.1.31-37.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. REDUCTION OF SELENITE BY INTACT YEAST CELLS AND CELL-FREE PREPARATIONS. J Bacteriol. 1963 Apr;85:754–762. doi: 10.1128/jb.85.4.754-762.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Keller C., Morawa R., Schmidt N., Siemens H. J., Laufs R. Plasmids of human strains of Yersinia enterocolitica: molecular relatedness and possible importance for pathogenesis. J Infect Dis. 1983 Jan;147(1):107–115. doi: 10.1093/infdis/147.1.107. [DOI] [PubMed] [Google Scholar]
- Hudman J. F., Glenn A. R. Selenite uptake and incorporation by Selenomonas ruminantium. Arch Microbiol. 1984 Dec;140(2-3):252–256. doi: 10.1007/BF00454937. [DOI] [PubMed] [Google Scholar]
- Levine V. E. THE REDUCING PROPERTIES OF MICROORGANISMS WITH SPECIAL REFERENCE TO SELENIUM COMPOUNDS. J Bacteriol. 1925 May;10(3):217–263. doi: 10.1128/jb.10.3.217-263.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCready R. G., Campbell J. N., Payne J. I. Selenite reduction by Salmonella heidelberg. Can J Microbiol. 1966 Aug;12(4):703–714. doi: 10.1139/m66-097. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J., FALCONE G. ENZYMATIC REDUCTION OF SELENITE. J Bacteriol. 1963 Apr;85:763–771. doi: 10.1128/jb.85.4.763-771.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilici M. L., Guilvout I., Mazigh D., Mollaret H. H. Repeated sequences of 3-kb DNA fragment of a plasmid from Yersinia enterocolitica 09 in plasmids from Y. pestis and Y. pseudotuberculosis. Contrib Microbiol Immunol. 1987;9:324–331. [PubMed] [Google Scholar]
- Ross A. J., Rucker R. R., Ewing W. H. Description of a bacterium associated with redmouth disease of rainbow trout (Salmo gairdneri). Can J Microbiol. 1966 Aug;12(4):763–770. doi: 10.1139/m66-103. [DOI] [PubMed] [Google Scholar]
- Simonet M., Mazigh D., Berche P. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt 47 X 10(6). J Med Microbiol. 1984 Dec;18(3):371–375. doi: 10.1099/00222615-18-3-371. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Colwell R. R., Hetrick F. M. Characterization of plasmids in bacterial fish pathogen. Infect Immun. 1983 Jan;39(1):184–192. doi: 10.1128/iai.39.1.184-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLFOLK C. A., WHITELEY H. R. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. I. Stoichiometry with compounds of arsenic, selenium, tellurium, transition and other elements. J Bacteriol. 1962 Oct;84:647–658. doi: 10.1128/jb.84.4.647-658.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willshaw G. A., Smith H. R., Anderson E. S. Application of agarose gel electrophoresis to the characterization of plasmid DNA in drug-resistant enterobacteria. J Gen Microbiol. 1979 Sep;114(1):15–25. doi: 10.1099/00221287-114-1-15. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Reduction of selenite by Neurospora. Arch Biochem Biophys. 1953 Jun;44(2):330–337. doi: 10.1016/0003-9861(53)90051-4. [DOI] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]


