Abstract
Zidovudine (ZDV) and lamivudine (3TC) metabolism to triphosphates (TP) is necessary for antiviral activity. The aims of this study were to compare ZDV-TP and 3TC-TP concentrations in adolescents receiving twice daily (BID) and once daily (QD) regimens and to determine the metabolite concentrations of ZDV and 3TC during chronic therapy on a QD regimen. Human immunodeficiency virus-infected patients (12 to 24 years) taking ZDV (600 mg/day) and 3TC (300 mg/day) as part of a highly active antiretroviral therapy regimen received QD and BID regimens of ZDV and 3TC for 7 to 14 days in a crossover design. Serial blood samples were obtained over 24 h on the QD regimen. Intracellular mono-, di-, and triphosphates for ZDV and 3TC were measured. The median ratio of BID/QD for ZDV-TP predose concentrations was 1.28 (95% confidence interval [CI] = 1.00 to 2.45) and for 3TC-TP was 1.12 (95% CI = 0.81 to 1.96). The typical population estimated half-lives (± the standard error of the mean) were 9.1 ± 0.859 h for ZDV-TP and 17.7 ± 2.8 h for 3TC-TP. Most patients had detectable levels of the TP of ZDV (24 of 27) and 3TC (24 of 25) 24 h after dosing, and half-lives on a QD regimen were similar to previously reported values when the drugs were given BID. Lower, but not significantly different, concentrations of ZDV-TP were demonstrated in the QD regimen compared to the BID regimen (P = 0.056). Although findings were similar between the BID and QD groups, the lower concentrations of ZDV and the number of patients below the level of detection after 24 h suggests that ZDV should continue to be administered BID.
Since its introduction, zidovudine (ZDV) treatment has undergone many changes in dosage and frequency of administration. Initially, the adult recommended dosage was 250 mg every 4 h for a total daily dose of 1,500 mg (16, 24). Both the total daily dose and the frequency have decreased, and the current recommended adult dosage is 600 mg daily, administered as 300 mg twice daily (BID) (5, 23). Initial dosing regimens were based on the short half-life, approximately 1 h, of ZDV in plasma (4, 12, 20). Subsequent clinical trials have demonstrated that lower total daily doses of ZDV are less toxic than higher doses with no apparent decrease in efficacy (25-27). The reason for this is that ZDV, like all of the nucleoside reverse transcriptase inhibitors (NRTIs), undergoes a series of three sequential phosphorylation reactions producing monophosphates (MP), diphosphates (DP), and triphosphates (TP) within the cell. The TP is the active metabolite of both ZDV and lamivudine (3TC), another NRTI.
Recent studies of the intracellular metabolism of ZDV in subjects have demonstrated that ZDV-TP is present within the cell for an extended period of time, which suggests that antiviral activity will be present with less frequent dosing (1, 2, 8, 15, 19, 20, 21). Likewise, 3TC is given on a twice-daily schedule based in part on a systemic half-life of 5 to 7 h in adults and children and a longer half-life for 3TC-TP (11, 14, 21). The adult and pediatric dosage schedules are supported by clinical studies demonstrating efficacy and safety (7, 13). Similar to changes in the ZDV dosing schedule, 3TC has been approved for once daily (QD) administration. This change was based on studies demonstrating that 300 mg once daily produced a similar systemic area under the concentration-time curve (AUC) to 150 mg twice daily (28).
An adolescent living with human immunodeficiency virus (HIV) infection is a challenge to manage and responses to highly active antiretroviral therapy (HAART) are not as successful as in adult trials. In a trial of initial HAART in adolescents, only 59% had achieved and maintained a viral load <400 copies/ml after 24 weeks of therapy (9). Adherence was the only factor that correlated with virologic outcome. There are significant problems with adherence to complex regimens in this population. Frequent daily dosing and the numbers of medications are listed as reasons for nonadherence among adolescents enrolled in the REACH (for reaching for excellence in adolescent care and health) project (A. Rogers, unpublished data). Reducing the dose frequency while preserving viral suppression, is a major goal of adolescent caretakers. Combination medications, including Combivir (GlaxoSmithKline) that contains ZDV and 3TC, are popular agents among adolescent caretakers. Less frequent drug administration could potentially demonstrate improvements in adherence among adolescents and thus improve the outcome of HAART therapy.
Thus, we undertook the present study to compare the steady-state concentrations of ZDV-TP and 3TC-TP in peripheral blood mononuclear cells when the same total daily dose of Combivir is administered QD versus BID. We also sought to describe the kinetics of phosphorylation of ZDV and 3TC in peripheral blood mononuclear cells over 24 h at steady state when Combivir is administered as a single daily dose and to describe any differences in adherence that could be demonstrated between a QD and a BID regimen. Our working hypothesis was that a <2-fold fluctuation in ZDV-TP and 3TC-TP between QD and BID administration would support further investigation to determine whether this simpler dosing regimen is appropriate for general clinical use.
MATERIALS AND METHODS
Study design.
PACTG P1012 was a phase I, randomized, crossover study of two schedules of combination NRTI therapy with ZDV and 3TC, administered as the commercially available combination product Combivir. HIV-infected adolescents, ages 12 to 24 years, who had received at least 4 weeks of ZDV and 3TC given individually or as Combivir immediately prior to study entry were eligible for enrollment. Subjects also had to weigh more than 40 kg, have a hemoglobin level of ≥9 g/dl, and have CD4+ cell counts greater than 250 cells/mm3. Women of childbearing age were required to be on adequate birth control. Subjects were excluded from participation if they had underlying toxicities greater than or equal to grade 3 according to the Division of AIDS Pediatric Toxicity Table (http://rcc-tech-res.com/DAIDS%20RCC%20Forms/ToxicityTables_Pediatric_Over3MonthsAge_v03.pdf) or had an active malignancy or opportunistic infection. Subjects received a HAART regimen that contained a minimum of three drugs that included ZDV and 3TC as two of the agents and either a protease inhibitor or a non-NRTI as additional agents. NRTIs other than ZDV and 3TC were not allowed. The Institutional Review Board at each participating center approved the study.
Study procedure.
After enrollment, subjects completed a 7-day adherence assessment. If adherence to Combivir was <70% (defined as taking fewer than 10 of the prescribed 14 Combivir tablets during the 7 days) or if all scheduled doses in the 24 h prior to the assessment were not taken, the subject was removed from the study. Subjects that were able to achieve at least 70% adherence were randomized to one of two groups: (i) initial therapy with zidovudine (600 mg) plus lamivudine (300 mg) administered QD for the initial phase followed by zidovudine (300 mg) and lamivudine (150 mg) administered BID (group A) or (ii) BID dosing of these agents followed by QD dosing (group B). Subjects were maintained on their QD regimen for 7 days and on the BID regimen for 7 to 14 days. After completion of the designated pharmacokinetic studies on the first randomized regimen, the subjects were switched to the other regimen, and a second pharmacokinetic assessment performed. The goal was to accrue 10 adherent subjects in each group with evaluable pharmacokinetic data (20 subjects overall).
Pharmacokinetic sampling for all subjects included a baseline trough sample following the 7 days of BID Combivir used to assess adherence. After this sample, the subjects began their first randomized regimen. For subjects receiving the BID Combivir regimen, a predose sample was obtained after 7 to 14 days of BID therapy prior to the morning dose. For subjects receiving the QD Combivir regimen, a predose sample was taken in the morning at the time of the regularly scheduled QD dosing, a single dose of two Combivir tablets (600 mg of zidovudine and 300 mg of lamivudine) was administered, and samples for intracellular pharmacokinetic analysis were obtained 2, 4, 6, 12, and 24 h afterward.
Adherence assessment.
In addition to the initial 7-day adherence assessment after enrollment, adherence to the study medication regimen was determined by the self-reported number of missed doses in the 3 days prior to each study visit using the standard PACTG modules (9).
Analytical methods: laboratory studies.
Blood for intracellular pharmacokinetic studies was collected in cell preparation tubes (Becton Dickinson, Franklin Lakes, NJ). A 48-ml portion of blood was obtained for predose pharmacokinetic assessments when the subject was on a BID regimen. A 24-ml portion of blood was obtained at each assessment for samples obtained when the subject was taking the QD regimen. The cell preparation tubes were processed according to the manufacturer's instructions to recover mononuclear cells that were then pelleted by centrifugation at 4°C. The cells were suspended in 1 ml of the supernatant and recentrifuged. The supernatant was removed. Then the cells were lysed by suspension in 70% methanol-30% MilliQ water (final concentration of 15 mM Tris at pH 7.0) with 200 μl of methanol for every 10 million cells for at least 15 min on ice. Cell counts were verified by using a hemacytometer. The samples were centrifuged, and the supernatant was placed in a cryo-storage vial and stored at −70°C. The samples were transferred to the PACTG Core Pharmacology laboratory at St. Jude Children's Research Hospital for analysis.
The extract was split with 80 μl (4 million cells) used for 3TC analysis and the remainder used for ZDV metabolite analysis. Both samples were analyzed by cartridge separation of metabolites, followed by dephosphorylation and by radioimmunoassay (RIA) analysis according to procedures similar to those previously described (17-19), but with the following modifications. Samples used in the analysis of 3TC were eluted from QMA cartridges (Waters Accell Plus QMA; Millipore, Milford, MA) at the following volumes and concentrations of KCl. The uncharged/parent compound was eluted with 3.0 ml of 5 mM KCl, 3TC ethanolamine-choline was eluted with 6.0 ml of 25 mM KCl, 3TC-MP was eluted with 7.0 ml of 50 mM KCl, 3TC-DP was eluted with 15 ml of 75 mM KCl, and 3TC-TP was eluted with 5.0 ml of 700 mM KCl. The fractions were dephosphorylated with 1 U of acid phosphatase (sweet potato type XA, P1435; Sigma, St. Louis)/ml, dried overnight with a SpeedVac, and reconstituted with water. The samples were cleaned, and the salt was removed with SepPak C-18 cartridges (Waters Division, Millipore Corp.). The ZDV samples were separated by using a QMA cartridge with the following elution procedure. ZDV/uncharged were eluted with 3 ml of 5 mM KCl, ZDV-MP was eluted with 12.0 ml of 50 mM KCl, ZDV-DP was eluted with 15 ml of 75 mM KCl, and the ZDV-TP was eluted with 4.0 ml of 700 mM KCl. These samples were then dephosphorylated with acid phosphatase, dried, and reconstituted with 3.0 ml of water and cleaned with SepPak C-18 cartridges. The resultant ZDV and 3TC were measured with their respective RIA.
The limit of quantitation for both the ZDV and 3TC RIA assays was 0.15 ng/ml. Because of the expected concentration differential between intracellular metabolites of ZDV, the final fractions containing the ZDV and ZDV-MP were diluted more than ZDV-DP and ZDV-TP so that 0.15-ng/ml equaled concentrations of 28 fmol of ZDV-MP, 7.5 fmol of ZDV-DP, and 7.5 fmol of cells ZDV-TP/106 cells (17, 18). The 0.15 ng of LOQ/ml for the 3TC RIA equaled 0.16 pmol/106 cells for all 3TC metabolites because they were all diluted to the same volume. Triplicate control concentrations of 0.25, 2.5, and 15 ng/ml were run for the ZDV assay and of 0.15, 0.5, 1, and 10 ng/ml were run for the 3TC assay.
The AUC of the intracellular metabolite concentrations were calculated by using the trapezoidal method, and the half-life determined by a mixed-effects modeling of the terminal portion of the log concentration profile for ZDV-TP and 3TC-TP (4-6 through 24-h samples) using the program NONMEM (FOCE subroutine with interaction). Several subjects had ZDV-TP concentrations approaching or falling below the limit of quantitation near the end of the dose interval. To prevent potential bias through exclusion of these patients with rapid elimination of the ZDV-TP, ZDV-TP samples with concentrations below the limit of quantitation were included with reduced weight compared to other samples. The ZDV-TP concentrations that were below the limit of quantitation but above the limit of detection were set at their estimated concentration, while those below the limit of detection were set at half of the limit of detection. An additional additive random error, fixed to half of the limit of quantitation, was applied to these samples. Individual subject parameter estimates were generated by empirical Bayesian post-hoc subroutine.
Analytical methods: statistical.
We calculated noncompartmental pharmacokinetic parameters for all metabolites on the QD regimen, substituting half the lower limit of quantification for any samples where the metabolite concentrations were undetectable. Since there are no established therapeutic ranges for intracellular amounts of either ZDV-TP or 3TC-TP, we were unable to directly compare measured values to established ranges. We compared the within-subject predose and 24-h measurements on the QD regimen by using a one-sample signed-rank test and compared the predose values for all metabolites on the QD and BID regimens using nonparametric Wilcoxon rank-sum tests to test for treatment (dosing frequency) by order interactions, order effects, and treatment effects.
We also calculated the ratios of predose levels of all metabolites to assess our hypothesis that <2-fold differences in the ratios of predose ZDV-TP and 3TC-TP between BID and QD schedules would support additional clinical investigation. Ratios are sensitive to extreme values, so medians and 95% confidence intervals (CIs) for the median were used as summary statistics. CIs were calculated by using the nonparametric CIPCTLDF option in SAS, version 9 (10). A signed rank test was used to test whether the ratio of metabolite levels were significantly different from 1 and from 2 - the ‘acceptable’ difference. Correlations based on ranks rather than actual values were used as they are less sensitive to extreme values.
RESULTS
Subject characteristics.
Thirty-six subjects were accrued to the present study between June 2001 and July 2002. One subject did not achieve the targeted 70% adherence during the first week and was removed from the study. Of the 35 remaining subjects, 21 were randomized to group A (QD followed by BID) and 14 to group B (BID followed by QD). Because of an imbalance in the number of evaluable subjects by group, the randomization for order was shut down in June 2002 after the enrollment of 26 subjects. At that time, there were 4 of 11 evaluable in group A and 11 of 15 evaluable in group B. At the conclusion of the study, evaluable pharmacokinetic data on adherent subjects was available for analysis on 14 of the 21 subjects in group A and in 13 of the 14 subjects in group B. Subject characteristics are shown in Table 1. There were no statistically significant differences between the evaluable and nonevaluable patients nor those randomized or assigned to group A or group B. Reasons for nonevaluability of the pharmacokinetic data included four subjects with samples insufficient to assay and four who did not come for the visit or who had been given the incorrect dose or regimen of the study drugs.
TABLE 1.
Subject characteristics for the 36 patients enrolled and the 27 patients with evaluable pharmacokinetic sampling
| Characteristica | All accrued subjects (n = 36)
|
Subjects with adequate pharmacokinetic assessments (n = 27)
|
||
|---|---|---|---|---|
| Group A (n = 21) | Group B (n = 15) | Group A (n = 14) | Group B (n = 13) | |
| Gender (no. of subjects) | ||||
| Male | 12 | 9 | 9 | 8 |
| Female | 9 | 6 | 5 | 5 |
| Race or ethnicity (no. of subjects) | ||||
| White, non-Hispanic | 1 | 2 | 1 | 1 |
| Black, non-Hispanic | 16 | 11 | 12 | 10 |
| Hispanic | 4 | 2 | 1 | 2 |
| No. of subjects at age (yr): | ||||
| <15 | 2 | 3 | 1 | 2 |
| 15 to <18 | 5 | 2 | 3 | 2 |
| ≥18 | 14 | 10 | 10 | 9 |
| Median* | 19.7 (17.0, 21.4) | 20.0 (17.2, 22.7) | 19.8 (17.8, 21.4) | 20.0 (18.0, 22.3) |
| Baseline CD4+ count (cells/mm3) | ||||
| 250-500 | 9 | 6 | 6 | 4 |
| >500-1,000 | 11 | 5 | 8 | 5 |
| >1,000 | 0 | 2 | 0 | 2 |
| Missing | 1 | 0 | 0 | 2 |
| Median* | 550 (425, 640) | 531 (421, 830) | 550 (378, 620) | 670 (421, 935) |
| Treatment regimen | ||||
| PI | 9 | 2 | 5 | 2 |
| Non-NRTI | 11 | 12 | 8 | 10 |
| Both PI and non-NRTI | 1 | 1 | 1 | 1 |
*, The 25th and 75th percentiles are given in parentheses. PI, protease inhibitor.
Safety.
Both study regimens were well tolerated. During the initial week of observation for adherence, two subjects reported adverse effects. One subject reported a grade 2 diarrhea that resolved prior to the beginning the first randomized regimen. Another reported back pain that persisted for the remainder of the study. While receiving the QD regimen, three subjects developed a new adverse event greater than or equal to grade 2. These included low back pain (grade 2), increased bilirubin (grade 2), and decreased serum glucose (grade 2). On the BID regimen, two subjects had new adverse events of increased bilirubin: one grade 2 and one grade 3.
Pharmacokinetic data.
Because patients receiving the QD dosing regimens had predose and 24-h measurements that should be similar for all metabolites tested, we compared the two values using a one-sample signed-rank test of the within-patient differences. There were no statistically significant differences between the two measurements (P ≥ 0.300 for all), so we used the predose values for all subsequent analyses.
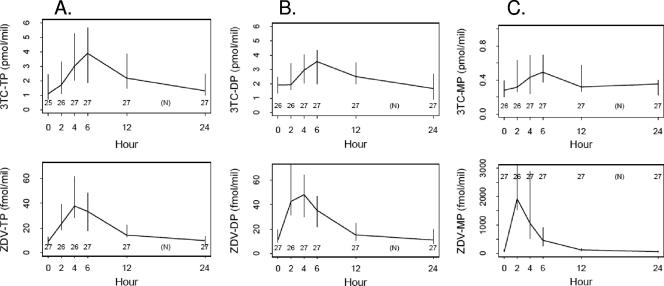
Using values from the frequent sampling of patients receiving the QD regimen, noncompartmental pharmacokinetic parameters were calculated and are shown in Table 2. The population estimate for half-life of ZDV-TP was 9.1 ± 0.9 h (standard error of the mean) and 17.7 ± 2.8 h for 3TC-TP. Post-hoc estimates of individual subjects ZDV-TP and 3TC-TP half-lives were similar to the population estimate model (medians of 8.4 and 18.2 h for ZDV-TP and 3TC-TP, respectively). There was no statistically significant correlation between ZDV-TP AUC and 3TC-TP AUC (Pearson correlation coefficient ρ = 0.12, P = 0.558). Figure 1 shows the concentration curves for intracellular ZDV and 3TC MP, DP, and TP.
TABLE 2.
Noncompartmental pharmacokinetic parameters for ZDV and 3TC metabolites in subjects receiving 600 mg of ZDV and 300 mg of 3TC QD (n = 27)
| Parameter | ZDV-MP | ZDV-DP | ZDV-TP | 3TC-MP | 3TC-DP | 3TC-TP |
|---|---|---|---|---|---|---|
| Median Cmax (fmol/106 cells) | 2,997.65 | 62.47 | 43.90 | 760 | 4,424 | 4,840 |
| Median Cmin (fmol/106 cells) | 38.82 | 7.95 | 6.26 | 249 | 1,055 | 1,002 |
| Median Tmax (h) | 2 | 4 | 4 | 6 | 6 | 4 |
| Mean Tmax (h) | 2.44 | 3.48 | 4.74 | 6.30 | 6.37 | 6.67 |
| Median AUC tra | 11,883 | 627 | 502 | 10.42 | 70.01 | 65.19 |
| Mean half-life (h) ± SEM | 9.1 ± 0.85 | 17.7 ± 2.8 |
ZDV metabolite AUCs were calculated in femtomoles, and 3TC metabolite AUCs were calculated in picomoles.
FIG. 1.
Time-concentration curves for ZDV and 3TC TP (A), DP (B), and MP (C) after a single dose of 600 mg of ZDV and 300 mg of 3TC showing median and 95% CI values for each metabolite.
We assessed (i) treatment by order, (ii) order, and (iii) treatment effects in all subjects with complete data on both the QD and BID regimen (n = 26 for all outcomes except 3TC-TP with n = 25) using Wilcoxon rank-sum tests. A statistically significant interaction (P = 0.031) for 3TC-DP was found, but there were no other significant interactions or order effects. Since median 3TC-DP levels were lower on the QD regimen in both order groups and since the one interaction was only marginally significant, we show summaries for all metabolites collapsed over order and include all subjects with available data on either the QD or BID regimens in Table 3. There was considerable intersubject variability in the concentrations of metabolites. On the QD regimen, 24 of 27 (89%) of the subjects had predose measurable concentrations of ZDV-TP, and 24 of 25 (96%) had measurable concentrations of 3TC-TP. On the BID regimen, 23 of 26 (88%) of the subjects had predose measurable concentrations of ZDV-TP, and 26 of 27 (96%) had a measurable concentration of 3TC-TP. A marginally significant difference in ZDV-TP between the QD and the BID regimens (P = 0.056) was demonstrated with higher ZDV-TP values on the BID regimen. The median predose ZDV-TP concentration on the BID regimen was 15.4 fmol/106 cells compared to 9.3 fmol/106 cells on the QD regimen.
TABLE 3.
Comparison of predose (and 24 h for QD) values for ZDV and 3TC metabolites on QD and BID regimens collapsed over order of administration
| Variable | Dosing regimen | n | <LLOQ (n)a | Medianb (95% CI) | CVc (%) | P (dose effect)d |
|---|---|---|---|---|---|---|
| ZDV | QD | 27 | 5 | 23.5 (12.1-40.2) | 191.1 | 0.560 |
| BID | 26 | 3 | 23.5 (8.7-40.8) | 251.8 | ||
| ZDV-MP | QD | 27 | 2 | 76.2 (57.7-131.5) | 183.0 | 0.666 |
| BID | 26 | 2 | 75.3 (32.4-172.6) | 153.8 | ||
| ZDV-DP | QD | 27 | 4 | 11.5 (9.2-19.1) | 108.7 | 0.594 |
| BID | 26 | 1 | 12.2 (7.7-22.9) | 90.2 | ||
| ZDV-TP | QD | 27 | 3 | 9.3 (6.4-12.9) | 74.2 | 0.056 |
| BID | 26 | 3 | 15.4 (7.7-23.6) | 96.5 | ||
| 3TC | QD | 26 | 2 | 302 (204-441) | 195.8 | 0.142 |
| BID | 27 | 1 | 437 (284-632) | 113.6 | ||
| 3TP-MP | QD | 26 | 2 | 282 (205-390) | 92.8 | 0.107 |
| BID | 27 | 2 | 396 (238-529) | 129.8 | ||
| 3TC-DP | QD | 26 | 1 | 1,934 (1,359-2,476) | 87.5 | 0.170 |
| BID | 27 | 1 | 2,393 (1,194-2,931) | 92.5 | ||
| 3TC-TP | QD | 25 | 1 | 1,115 (724-2,445) | 90.1 | 0.090 |
| BID | 27 | 1 | 2,163 (671-3,790) | 97.3 | ||
| 24 h | QD | |||||
| ZDV | 27 | 2 | 23.6 (11.4-38.4) | 134.3 | ||
| ZDV-MP | 27 | 0 | 60.5 (38.8-84.4) | 107.3 | ||
| ZDV-DP | 27 | 2 | 11.1 (7.9-19.9) | 79.5 | ||
| ZDV-TP | 27 | 4 | 9.8 (4.9-13.2) | 84.2 | ||
| 3TC | 27 | 3 | 262 (208-452) | 80.5 | ||
| 3TC-MP | 27 | 1 | 353 (225-401) | 62.8 | ||
| 3TC-DP | 27 | 0 | 1,684 (932-2,683) | 63.2 | ||
| 3TC-TP | 27 | 1 | 1,315 (988-2,463) | 88.0 |
That is, the number of measurements where one-half the lower limit of quantification (LLOQ) was imputed.
Median and 95% CI reported in fmol/106 cells for ZDV and in pmol/106 cells for 3TC.
CV, coefficient of variation.
Significance value as determined by the Wilcoxon rank-sum test for differences in median values on the QD versus the BID regimen.
The ratio of BID concentrations relative to the QD concentrations was calculated for 26 of the 27 evaluable subjects for all metabolites except 3TC-TP (n = 25). All 95% CI values for the median ratios of the metabolites covered the value of 1. The ZDV-TP ratio (median BID/QD ratio = 1.28 [95% CI = 1.00 to 2.45; interquartile range = 0.86 to 2.80]) was statistically significantly different from the value of 1 (signed-rank test P = 0.005) but not significantly different from a value of 2 (P = 0.405). Therefore, predose ZDV-TP values were not outside our targeted acceptable difference of twofold for the two regimens. The median ratio for the BID/QD 3TC-TP was 1.12 (95% CI = 0.81 to 1.96; interquartile range = 0.65 to 2.04). We saw no evidence that background antiviral medications (protease inhibitors or non-NRTIs) influenced TP values (data not shown).
Finally, we demonstrated that ZDV-TP measurements on the standard BID regimen were correlated with values on the QD regimen (Spearman correlation coefficient ρ = 0.60, P = 0.001).
Adherence.
Adherence at all study visits was excellent with no more than one patient reporting a missed Combivir dose over the 3 days prior to the study visit at which their pharmacokinetic data were collected for both the QD and the BID regimens. Because subjects could not proceed through the steps of the study without very good adherence, the study population was probably not representative of the general adolescent population, so these results need to be interpreted with care.
DISCUSSION
This study demonstrates that the intracellular active metabolites, ZDV-TP and 3TC-TP, persist within the cell for long periods of time at detectable levels that are likely to inhibit viral replication. Although the ZDV-MP concentration fluctuated greatly over the 24-h dose interval, the other metabolites, including ZDV-TP and 3TC-TP, only showed modest fluctuation over this interval. Pharmacokinetic modeling yielded a half-life for ZDV-TP of 9.1 h, while that for 3TC-TP was 17.7 h. These half-lives are similar to previously reported values from adult populations: 7 to 11 h for ZDV-TP (1, 21) and 16 to 32 h for 3TC-TP (1, 14, 21, 28).
We witnessed considerable intersubject variability in the rates of phosphorylation, with the ZDV-TP and 3TC-TP predose concentrations spanning an ∼40-fold range (coefficients of variation of 74% for ZDV-TP QD, 97% for ZDV-TP BID, 90% for 3TC-TP QD, and 97% for 3TC-TP BID). This broad variability is a concern when trying to extend the dose interval since a substantial number of subjects would have very low concentrations with the QD dosing.
A priori, we had chosen a ratio of BID/QD predose concentrations of ZDV-TP and 3TC-TP of <2 to support additional studies of QD ZDV and 3TC. When the same total daily doses were administered either QD or BID, the ratios of predose levels between all of the 3TC metabolites all differed by less than a factor of 2. The same was true for ZDV, ZDV-MP, and ZDV-DP. The ZDV-TP ratio was significantly different from 1 but not different from 2. Despite the ratios of predose concentrations falling below our threshold value of 2 for all of the metabolites, the lower levels of the active metabolite ZDV-TP, the substantial intersubject variability, the relatively short ZDV-TP half-life, and findings from preclinical and relatively recent adult clinical studies require reassessment of these criteria. Preclinical studies using ZDV as a continuous infusion or as a QD dose in a murine model of AIDS encephalopathy demonstrated improved antiviral effect of continuous infusion ZDV compared to QD dosing (3). Similarly, Drusano et al. reported findings that did not support a QD dosing of ZDV in an in vitro hollow-fiber model system that has been predictive for other nucleosides (6).
In addition, clinical studies of pharmacokinetics and pharmacodynamics comparing ZDV at 300 mg BID versus 600 mg QD have been performed in adults. The COD10001 study demonstrated that mean half-lives were similar in the two dosing regimens (6.3 h for the QD regimen versus 5.48 h for the BID regimen), and both groups had similar declines in viral loads over 14 days of monotherapy (22). In COD20002, 32 antiretroviral naive subjects were randomized, in a 1:1 ratio, to receive either ZDV 600 mg QD or 300 mg BID as monotherapy. Viral loads over the first 14 days demonstrated a trend suggesting that the subjects on the BID dosing regimen achieved lower viral loads (P = 0.056) and more rapid declines in viral load (P = 0.065) compared to the QD group (22). These studies differ from the present study in several significant ways. In the present study, the QD and BID regimen were given in addition to either a protease inhibitor or a non-NRTI or both as part of a HAART regimen. In addition, we opted to study subjects who had been receiving their current HAART regimen for at least 4 weeks and were at steady state. Although our ZDV-TP half-life was somewhat longer at 9.1 h in adolescents, it was still short enough to result in lower predose ZDV-TP concentrations with QD dosing. In addition, we did not investigate viral dynamics since many of our subjects had undetectable viral loads at the start of the study. Thus, despite a potential gain in adherence, the lower levels of ZDV-TP taken in consideration with findings from other studies have dissuaded us from pursuing further study of a QD ZDV regimen.
In our study, we were able to demonstrate that ZDV-TP measurements on the standard BID dosing regimen were correlated with ZDV-TP concentrations on a QD dose. This finding may be important if future technologies allow easy measurement of ZDV-TP. If monitoring of ZDV-TP becomes practical, one may be able to individualize patient dosing regimens in patients with high ZDV-TP levels on a BID regimen and to assess a QD regimen. In adolescent patients, removing barriers to adherence by simplifying dosing regimens is critically important and requires additional research efforts. However, because the measurement of ZDV-TP is a complex research laboratory procedure, extending the ZDV dose interval in patients with high predose ZDV-TP is not practical or readily available.
Another possible strategy to make daily ZDV more effective would be to use increased doses of ZDV to achieve higher ZDV-TP levels. Although we did not study doses other than 600 mg QD and 300 mg BID in our study, Fletcher and coworkers demonstrated systemic exposure to ZDV-TP increased using a 700-mg daily dose compared to a 500-mg daily dose (1, 8). In addition, Anderson et al. noted a 125% increase in ZDV-TP concentrations when the ZDV dose was increased by 130% (1). These data indicate that, despite substantial subject variability, measurements of ZDV-TP do change as a function of dose. However, higher doses may be associated with greater toxicity and cost and would need to be studied.
This is the first evaluation of intracellular NRTI metabolites in adolescent HIV-infected subjects. Our findings are in accord with previous studies in older populations. The estimates of the ZDV and 3TC metabolites were in the range of those previously reported, and peak ZDV-TP concentrations were in the range previously reported (1, 19, 28; Rogers, unpublished). Likewise, 3TC-TP concentrations were similar to those reported (1, 14, 28; Rogers, unpublished). The proportion of 3TC metabolites as the 3TC-TP moiety was higher than seen by Moore et al. (14). In that study 3TC accounted for only 15 to 20% of the intracellular phosphates compared to the ca. 45% observed in the present study. Unlike Anderson et al. (1), we were unable to find a correlation between concentrations of ZDV-TP and 3TC-TP. Given variability in measurements and different methodologies among studies, these findings suggest similar metabolic handling of ZDV and 3TC in this young population.
Our study had several limitations. Because of imbalance in the evaluability of pharmacokinetic data during the early stages of accrual, randomization to the two groups was stopped, compromising the crossover design and limiting the interpretability of the tests for interaction and order effects. Given the high variability in metabolite concentrations, our study was too small to assess for potential age or gender effects as reported by Anderson et al., and no analyses by gender were performed (1). In addition, the BID predose sample was obtained prior to the scheduled morning dose and did not follow an observed dose.
In conclusion, we have demonstrated that most patients have detectable levels of the TP of ZDV and 3TC 24 h after dosing and that half-lives on a QD regimen were similar to previously reported values when the drugs were given BID. There was considerable variability, and some patients had nonmeasurable concentrations of ZDV-TP after 24 h. The predose intracellular MP and DP metabolite concentrations of ZDV and 3TC on QD were all within 70% of those seen with BID therapy. However, ZDV-TP and 3TC-TP concentrations with QD therapy were lower as a percentage of the BID values, with the ZDV-TP changes reaching marginal significance due to its shorter half-life. Despite these similarities between the QD and BID ZDV dosing regimens, the lower concentrations of ZDV-TP and the lack of a targeted nadir linked with virologic efficacy, previously published literature, and the increasing number of QD antiviral agents do not support further study of a QD dose of 600 mg of ZDV.
Acknowledgments
Thus study was supported in part by National Institutes of Health grants P41-EB001978 and UO1 AI41089, by the Pediatric AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases, and by the American Lebanese-Syrian Associated Charities.
Staff at participating PACTG 1012 sites included J. Martinez and K. Bojan, The CORE Center for the Prevention, Care, and Research of Infectious Disease, Chicago, IL; S. Nesheim and R. Dennis, Emory University Hospital, Atlanta, GA; A. Kovacs and J. Homans, Los Angeles County Medical Center (USC), Los Angeles, CA; K. Knapp and S. DiScenza, St. Jude Children's Research Hospital, Memphis, TN; R. B. Van Dyke and M. Sillio, Tulane University Charity Hospital, New Orleans, LA; P. Palumbo, University of Medicine and Dentistry of New Jersey, Newark, NJ; and J. F. Rodriguez, University of Puerto Rico, San Juan, Puerto Rico.
Footnotes
Published ahead of print on 30 July 2007.
This study is presented in honor of John Rodman, who passed away in April 2006.
REFERENCES
- 1.Anderson, P. L., T. N. Kakuda, S. Kawle, and C. V. Fletcher. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159-2168. [DOI] [PubMed] [Google Scholar]
- 2.Barry, M. G., S. H. Khoo, G. J. Veal, P. G. Hoggard, S. E. Gibbons, E. G. Wilkins, O. Williams, A. M. Breckenridge, and D. J. Back. 1996. The effect of zidovudine dose on the formation of intracellular phosphorylated metabolites. AIDS 10:1361-1367. [DOI] [PubMed] [Google Scholar]
- 3.Bilello, J. A., J. L. Eiseman, H. C. Standiford, and G. L. Drusano. 1994. Impact of dosing schedule upon suppression of a retrovirus in a murine model of AIDS encephalopathy. Antimicrob. Agents Chemother. 38:628-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum, M. R., S. H. Liao, S. S. Good, and P. de Miranda. 1988. Pharmacokinetics and bioavailability of zidovudine in humans. Am. J. Med. 85:89-94. [PubMed] [Google Scholar]
- 5.Collier, A. C., S. Bozzette, R. W. Coombs, D. M. Causey, D. A. Schoenfeld, S. A. Spector, C. B. Pettinelli, G. Davies, D. D. Richman, and J. M. Leedom. 1990. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N. Engl. J. Med. 323:1015-1021. [DOI] [PubMed] [Google Scholar]
- 6.Drusano, G. L., P. A. Bilello, W. T. Symonds, D. S. Stein, J. McDoweell, A. Bye, and J. A. Bilello. 2002. Pharmacodynamics of abacavir in an in vitro hollow-fiber model system. Antimicrob. Agents Chemother. 46:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, and M. Rubin. 1995. Treatment with lamivudine, zidovudine or both in HIV-positive subjects. N. Engl. J. Med. 333:1662-1669. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, C. V., E. P. Acosta, K. Henry, L. M. Page, C. R. Gross, S. P. Kawle, R. P. Remmel, A. Erice, and H. H. Balfour, Jr. 1998. Concentration-controlled zidovudine therapy. Clin. Pharm. Ther. 64:331-338. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, P. M., B. J. Rudy, S. D. Douglas, J. Lathey, S. A. Spector, J. Martinez, M. Silio, M. Belzer, L. Friedman, L. D'Angelo, J. McNamara, J. Hodge, M. D. Hughes, J. C. Lindsey, et al. 2004. Virologic and immunologic outcomes after 24 weeks in HIV-1-infected adolescents on highly active antiretroviral therapy. J. Infect. Dis. 190:271-279. [DOI] [PubMed] [Google Scholar]
- 10.Hahn, G. J., and W. Q. Meeker. 1991. Statistical intervals: a guide for practitioners. John Wiley & Sons, Inc., New York, NY.
- 11.Johnson, M. A., K. H. P. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 12.Laskin, O. L., P. de Miranda, and M. R. Blum. 1989. Azidothymidine steady-state pharmacokinetics in subjects with AIDS and AIDS-related complex. J. Infect. Dis. 159:745-747. [DOI] [PubMed] [Google Scholar]
- 13.McKinney, R. E., Jr., G. M. Johnson, K. Stanley, F. H. Yong, A. Keller, K. J. O'Donnell, P. Brouwers, W. G. Mitchell, R. Yogev, D. W. Wara, A. Wiznia, L. Mofenson, J. McNamara, and S. A. Spector. 1998. A randomized study of combined zidovudine-lamivudine versus didanosine monotherapy in children. J. Pediatr. 133:500-506. [DOI] [PubMed] [Google Scholar]
- 14.Moore, K. H., J. E. Barrett, S. Shaw, G. E. Pakes, R. Churchus, A. Kapoor, J. Lloyd, M. G. Barry, and D. Back. 1999. The pharmacokinetics of lamivudine triphosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS 13:2239-2250. [DOI] [PubMed] [Google Scholar]
- 15.Peter, K., and J. G. Gambertoglio. 1998. Intracellular phosphorylation of zidovudine and other nucleoside reverse transcriptase inhibitors for HIV infection. Pharm. Res. 15:819-825. [DOI] [PubMed] [Google Scholar]
- 16.Richman, D. 1991. Antiviral therapy of HIV infection. Annu. Rev. Med. 42:69-90. [DOI] [PubMed] [Google Scholar]
- 17.Robbins, B. L., T. T. Tran, F. H. Pinkerton, Jr., F. Akeb, R. Guedj, J. Grassi, D. Lancaster, and A. Fridland. 1998. Development of a new cartridge radioimmunoassay for determination of intracellular levels of lamivudine triphosphate in the peripheral blood mononuclear cells of human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 42:2656-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins, B. L., B. H. Waibel, and A. Fridland. 1996. Quantitation of intracellular zidovudine phosphates by use of combined cartridge-radioimmunoassay methodology. Antimicrob. Agents Chemother. 40:2651-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodman, J. H., P. M. Flynn, B. Robbins, E. Jimenez, A. D. Bardeguez, J. F. Rodriguez, S. Blanchard, and A. Fridland. 1999. Systemic pharmacokinetics and cellular pharmacology of in human immunodeficiency virus type-1-infected women and newborn infants. J. Infect. Dis. 180:1844-1850. [DOI] [PubMed] [Google Scholar]
- 20.Rodman, J. H., B. Robbins, P. M. Flynn, and A. Fridland. 1996. A systemic and cellular model for zidovudine plasma concentrations and intracellular phosphorylation in subjects. J. Infect. Dis. 74:490-499. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez, J. F., J. L. Rodriguez, J. Santana, H. Garcia, and O. Rosario. 2000. Simultaneous quantitations of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob. Agent Chemother. 44:3097-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruane, P. J., G. J. Richmond, E. DeJesus, C. E. Hill-Zabala, S. C. Danehower, Q. Liao, J. Johnson, M. S. Shaefer, et al. 2004. Pharmacodynamic effects of zidovudine 600 mg once/day versus 300 mg twice/day in therapy-naive patients infected with human immunodeficiency virus. Pharmacotherapy 24:307-312. [DOI] [PubMed] [Google Scholar]
- 23.Shepp, D. H., C. Ramirez-Ronda, L. Dall, R. B. Pollard, R. L. Murphy, H. Kessler, R. Sherer, G. Mertz, G. Perez, D. J. Gocke, S. B. Greenberg, E. Petersen, I. Frank, M. D. Moore, R. McKinnis, and J. F. Rooney. 1997. A comparative trial of zidovudine administered every four versus every twelve hours for the treatment of advanced HIV disease. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:283-288. [DOI] [PubMed] [Google Scholar]
- 24.Smiley, L. 1989. Zidovudine treatment of subjects with acquired immune deficiency syndrome and acquired immune deficiency syndrome-related complex: long-term experience. J. Infect. 18(Suppl. 1):77. [DOI] [PubMed] [Google Scholar]
- 25.Volberding, P. A., S. W. Lagakos, J. M. Grimes, D. S. Stein, H. H. Balfour, Jr., R. C. Reichman, J. A. Bartlett, M. S. Hirsch, J. P. Phair, R. T. Mitsuyasu, et al. 1994. The duration of zidovudine benefit in persons with asymptomatic HIV infection. JAMA 272:437-442. [PubMed] [Google Scholar]
- 26.Volberding, P. A., S. W. Lagakos, J. M. Grimes, D. S. Stein, J. Rooney, T. C. Meng, M. A. Fischl, A. C. Collier, J. P. Phair, M. S. Hirsch, et al. 1995. A comparison of immediate with deferred zidovudine therapy for asymptomatic HIV-infected adults with CD4 cell counts of 500 or more per cubic millimeter. N. Engl. J. Med. 333:401-407. [DOI] [PubMed] [Google Scholar]
- 27.Volberding, P. A., S. W. Lagakos, M. A. Koch, C. Pettinelli, M. W. Myers, D. K. Booth, H. H. Balfour, Jr., R. C. Reichman, J. A. Bartlett, M. S. Hirsch, et al. 1990. Zidovudine in asymptomatic human immunodeficiency virus infection: a controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. N. Engl. J. Med. 322:941-949. [DOI] [PubMed] [Google Scholar]
- 28.Yuen, G. J., Y. Lou, N. F. Bumgarner, J. P. Bishop, G. A. Smith, V. R. Otto, and D. D. Hoelscher. 2004. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob. Agents Chemother. 48:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]