Abstract
Several classes of antibiotics exert antimalarial activity. The mechanisms of action of antibiotics against malaria parasites have been unclear, and prior studies have led to conflicting results, in part because they studied antibiotics at suprapharmacological concentrations. We examined the antimalarial effects of azithromycin, ciprofloxacin, clindamycin, doxycycline, and rifampin against chloroquine-resistant (W2) and chloroquine-sensitive (3D7) Plasmodium falciparum strains. At clinically relevant concentrations, rifampin killed parasites quickly, preventing them from initiating cell division. In contrast, pharmacological concentrations of azithromycin, ciprofloxacin, clindamycin, and doxycycline were relatively inactive against parasites initially but exerted a delayed death effect, in which the progeny of treated parasites failed to complete erythrocytic development. The drugs that caused delayed death did not alter the distribution of apicoplasts into developing progeny. However, the apicoplasts inherited by the progeny of treated parasites were abnormal. The loss of apicoplast function became apparent as the progeny of antibiotic-treated parasites initiated cell division, with the failure of schizonts to fully mature or for erythrocyte rupture to take place. These findings explain the slow antimalarial action of multiple antibiotics.
Malaria, caused by infection with the protozoan parasite Plasmodium falciparum, causes half a billion illnesses and over a million deaths each year, mostly among children (6, 38). Several classes of antibiotics have potent antimalarial effects. Despite relatively slow antimalarial activity, some antibiotics, in particular doxycycline, are used for antimalarial prophylaxis (2) and in combination with more rapidly acting drugs to treat malaria (1). Multiple antibiotics with antimalarial activity at clinically achievable doses exert their antibacterial effects by interfering with targets specific to prokaryotes, including 70S ribosomes (tetracyclines, macrolides, and lincosamides) and prokaryotic RNA polymerases (rifampin) or DNA gyrases (fluoroquinolones) (10). Since plasmodia are eukaryotes, the specific antimalarial mechanisms of these antibiotics have been poorly defined. Previous reports have ascribed activities of antibiotics to action against the plasmodial mitochondrion or an unusual organelle called the apicoplast, which is similar to plant chloroplasts and unique to plasmodia and other apicomplexan parasites (42, 46). However, reconciling these reports has been difficult, due to differences in methodologies and large variations in the concentrations of antibiotics studied (8, 9, 12, 13, 19, 20, 22, 25, 26, 35-37, 44, 45). We recently demonstrated that at clinically relevant concentrations doxycycline specifically disrupted maintenance of the apicoplast during the asexual erythrocytic stages of the P. falciparum life cycle (12). Doxycycline did not block apicoplast segregation but caused nonfunctional apicoplasts to distribute into the progeny of treated parasites, which subsequently failed to complete cell division, explaining the slow action of tetracyclines.
The function of the apicoplast is poorly defined. Several hundred nuclear encoded proteins are predicted to localize to the apicoplast based on the presence of putative apicoplast targeting signals (17, 49). This has allowed for the construction of proposed apicoplast metabolic pathways, including those for type II fatty acid synthesis, non-mevalonate isoprenoid synthesis, and a portion of heme biosynthesis (33). The apicoplast also contains an independent genome, encoding prokaryote-like RNA polymerase subunits, 70S ribosomal subunits, tRNAs, and a small number of proteins (47). The presence of multiple putative antibiotic targets in the apicoplast suggests that antibiotics in addition to doxycycline may act against this organelle.
In this study, we sought to clarify the mechanisms by which clinically relevant doses of antibiotics that are commonly used to treat bacterial infections exert antimalarial effects. We therefore examined the efficacies of azithromycin, clindamycin, doxycycline, ciprofloxacin, and rifampin on cultured P. falciparum over two parasite life cycles. We found that antibiotics inhibiting either protein synthesis or DNA gyrase activity caused a delayed effect in P. falciparum, such that the progeny of antibiotic-treated parasites inherited nonfunctional apicoplasts, leading to a delayed but potent antimalarial effect.
MATERIALS AND METHODS
Malaria parasites and culture.
P. falciparum strains 3D7 and W2, both from the Malaria Research and Reference Reagent Resource Center, were cultured in human erythrocytes at 2% hematocrit in RPMI 1640 medium with 0.5% (wt/vol) AlbuMAX II (Invitrogen-Gibco) in 92% N2, 5% CO2, and 3% O2 (41). Synchrony was maintained by serial sorbitol treatments (23). Stably transfected 3D7 parasites expressing a green fluorescent protein fused to an acyl carrier protein apicoplast targeting signal (ACPl-GFP) were kindly provided by Geoff McFadden (University of Melbourne) (43), and were maintained in medium containing 100 nM pyrimethamine.
Sensitivity of parasites to antibiotics.
Stock solutions of azithromycin, clindamycin, doxycycline, and rifampin (all from Sigma) were dissolved in dimethyl sulfoxide. Ciprofloxacin (Fluka) and chloroquine (Sigma) were dissolved in water. For determining 50% inhibitory concentrations (IC50s), synchronized ring-stage parasites were cultured in 96-well plates at an initial parasitemia of 1% (for 48 h) or 0.2% (for 96 h). Serial dilutions of drugs (100 μM to 0.5 nM) were prepared in complete medium, and parasites were treated for 48 h. At the completion of the 48-h life cycle, new ring-stage parasites were either fixed immediately (to determine IC50s at 48 h) or were transferred to fresh medium and grown for an additional 48 h in the absence of drugs before fixing (to determine IC50s after 96 h). To determine IC50s, new ring-stage parasites produced after 48 h or 96 h were counted by flow cytometry. This method evaluates the ability of parasites to produce viable progeny, rather than measuring metabolic activities inferred by uptake of radiolabeled hypoxanthine or amino acids, resulting in slightly lower but comparable IC50s (12). Infected erythrocytes were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for at least 48 h, permeabilized with 0.1% Triton X-100, and stained with 1 nM YOYO-1 (Molecular Probes). Parasitemias were determined from dot plots (forward scatter versus fluorescence) acquired on a FACSort cytometer using CELLQUEST software (Becton Dickinson). Dose-response curves were generated by comparing parasite counts between treated and untreated cultures. IC50 values were calculated from variable-slope sigmoidal dose-response curves using GraphPad Prism version 3.00 for Windows (GraphPad Software).
For determining the timing of sensitivity to antibiotics, parasites grown in 200-μl cultures in 96-well plates were incubated with drugs for periods of 12, 24, 36, or 48 h covering different portions of the 48-h life cycle. At 48 h, rifampin-treated parasites were fixed in paraformaldehyde as described above. The remaining cultures were transferred to fresh, drug-free medium, grown for an additional 48 h, and then fixed in paraformaldehyde. Parasites were counted by flow cytometry as described above.
Microscopy.
Giemsa-stained thin smears were photographed using a SPOT Flex Color Mosaic digital camera (Diagnostic Instruments) on a Nikon Optiphot microscope. For fluorescence microscopy of live ACPl-GFP parasites, infected erythrocytes were rinsed in PBS, resuspended in PBS plus 5 μg/ml Hoechst nuclear stain (Invitrogen), and allowed to attach to a poly-l-lysine-coated microscope slide for 30 min. The slides were then rinsed in PBS, overlaid with a coverslip, and imaged immediately. Fluorescent images were captured digitally, and merged images were assembled and optimized (with background corrections and gamma adjustments) using SPOT software version 4.5 (Diagnostic Instruments). All final figures were prepared in Adobe Photoshop version 5.5.
Counting apicoplasts.
Late-ring-stage ACPl-GFP-labeled parasites were treated with azithromycin, clindamycin, ciprofloxacin, or doxycycline or not treated with an antibiotic until they reached the late schizont/early ring stage, then transferred to drug-free medium, and allowed to mature to trophozoites. Live parasites were resuspended in PBS for analysis by flow cytometry. For each culture, half the parasites were stained with 100 nM of the green fluorescent nuclear stain SYTO 16 (Molecular Probes), and parasites were counted by flow cytometry as described above. The remaining unstained infected erythrocytes containing GFP-labeled apicoplasts were evaluated by flow cytometry, and the percentage of parasites containing apicoplasts was determined by dividing the number of apicoplast-containing erythrocytes by the number of DNA-containing erythrocytes.
RESULTS
Antimalarial effects of different classes of antibiotics.
We cultured chloroquine-resistant strain W2 and chloroquine-sensitive strain 3D7 parasites with azithromycin, clindamycin, ciprofloxacin, doxycycline, rifampin, or chloroquine for 48 h, beginning at the early ring stage, and then determined the IC50s by comparing parasitemias with those of the control parasites after the 48-h period of treatment and after an additional 48 h of culture without drug (Table 1). Parasitemias were determined by counting new ring forms by fluorescence-activated cell sorting analysis after 48 h or 96 h, a technique that yields results comparable to those obtained based on comparisons of uptake of hypoxanthine (12). Clindamycin demonstrated no antimalarial activity at concentrations up to 100 μM when assessed after 48 h. Azithromycin, ciprofloxacin, doxycycline, and rifampin demonstrated effects after 48 h at relatively high concentrations. Azithromycin, clindamycin, and doxycycline were much more active when assessed after two parasite life cycles, a phenomenon referred to as “delayed death” (12, 15, 19, 20, 35). Ciprofloxacin was also more active when assessed at 96 h, but differences between 48 h and 96 h IC50s were less pronounced, particularly for strain 3D7. There was no difference in the IC50s at 48 and 96 h for chloroquine or rifampin. Activities of drugs against the two P. falciparum strains tested were similar except that chloroquine was more active against 3D7 and rifampin was much more active against W2. Considering the concentrations achieved by standard doses of the antibiotics studied, clinically meaningful antimalarial effects are probably achieved only after 96 h for all the antibiotics except rifampin (Table 2), consistent with demonstrated slow clinical antimalarial activity for doxycycline (39), clindamycin (24), and azithromycin (14).
TABLE 1.
Delayed effects of antibiotics on cultured P. falciparuma
| Treatment (first generation) | IC50 (μM) of the following strain at the indicated time:
|
|||
|---|---|---|---|---|
| 3D7
|
W2
|
|||
| 48 h (second-generation rings) | 96 h (third-generation rings) | 48 h (second-generation rings) | 96 h (third-generation rings) | |
| Azithromycin | 8.67 ± 0.02 | 0.0527 ± 0.0091 | 1.46 ± 0.21 | 0.0310 ± 0.0085 |
| Ciprofloxacin | 9.44 ± 0.06 | 3.04 ± 0.51 | 24.9 ± 8.6 | 1.75 ± 0.010 |
| Clindamycin | >100 | 0.00881 ± 0.0051 | >100 | 0.00312 ± 0.00011 |
| Doxycycline | 5.32 ± 0.53 | 0.453 ± 0.12 | 3.30 ± 0.95 | 0.211 ± 0.020 |
| Rifampin | 2.99 ± 1.1 | 2.83 ± 0.67 | 0.274 ± 0.066 | 0.528 ± 0.19 |
| Chloroquine | 0.00564 ± 0.0022 | 0.00849 ± 0.0030 | 0.0452 ± 0.0084 | 0.130 ± 0.036 |
Parasites were treated for 48 h, starting at the ring stage (first generation). Parasitemia of new rings (second generation) was determined by flow cytometry after 48 h or after an additional 48 h of growth in drug-free medium (third generation). Data are the averages ± standard deviations of two independent experiments, each performed in duplicate.
TABLE 2.
Pharmacokinetics of antibiotics and chloroquine after standard oral dosing
| Drug | Class | Antibacterial target | Dose (mg) (oral) | Avg peak plasma concn (μg/ml) | Avg peak plasma concn (μM) | Time to peak concn (h) | t1/2a | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Azithromycinb | Macrolide | 70S ribosome (50S subunit) | 1,000 | 0.8 | 1.1 | 2.4 | 74 h | 11 |
| Ciprofloxacinc | Fluoroquinolone | DNA gyrase | 500 | 2.5 | 7.6 | 0.6 | 3.3 h | 4, 40 |
| Clindamycind | Lincosamide | 70S ribosome (50S subunit) | 600 | 7.4 | 17 | 1 | 2.6 h | 28 |
| Doxycyclinee | Tetracycline | 70S ribosome (30S subunit) | 200 | 3.2 | 6.9 | 2 | 7-18 h | 29 |
| Rifampinf | Rifamycin | RNA polymerase | 600 | 6.5 | 8.0 | 1-3 | 3.5 h | 40 |
| Chloroquine | 8-Aminoquinoline | Not applicable | 300 | 0.076 | 0.24 | 6.6 | 10-24 days | 40 |
t1/2, half-life.
Given once daily for 3 days, alone or in combination with chloroquine for malaria.
Given twice daily for 3 days in patients with bronchitis.
Given twice daily for 7 days in combination with fosmidomycin for malaria.
Given to patients with severe malaria.
Given once daily for 15 to 18 days in patients with tuberculosis.
Stage specificity of antimalarial activity of antibiotics.
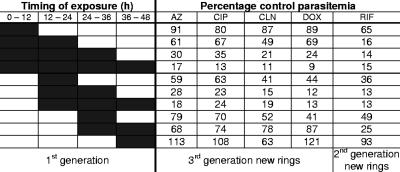
Intraerythrocytic P. falciparum parasites progress from relatively inert rings, to larger and more metabolically active trophozoites, to schizonts, which undergo nuclear and cellular division. At the end of the schizont stage, free merozoites rupture the host cell and invade new erythrocytes to begin a new cycle. To determine the stage specificity of antimalarial action, we cultured parasites for different portions of a 48-h life cycle with each antibiotic and then counted new rings after 96 h (48 h for rifampin) (Fig. 1). For these and subsequent experiments, antibiotics were studied at approximately twice the IC50 at 96 h (48 h for rifampin). We found that the antibiotics causing a delayed effect after 96 h were most effective against later trophozoites and early schizonts (24 to 36 h postinvasion), as we had observed with doxycycline (12), while rifampin was also active against earlier stages.
FIG. 1.
Trophozoites and early schizonts are most susceptible to the delayed action of antibiotics. P. falciparum 3D7 parasites were cultured in the presence of antibiotics at twice the IC50 at 96 h (100 nM azithromycin [AZ], 6 μM ciprofloxacin [CIP], 25 nM clindamycin [CLN], and 1 μM doxycycline [DOX]), except that rifampin-treated parasites were dosed at twice the IC50 at 48 h (6 μM rifampin [RIF]). The portions of the life cycle during which parasites were treated are indicated by dark shading. New rings were counted 96 h (AZ, CIP, CLN, and DOX) or 48 h (RIF) after the initiation of treatment. Percentages of control parasitemias are shown.
The progeny of antibiotic-treated parasites are unable to complete schizogeny.
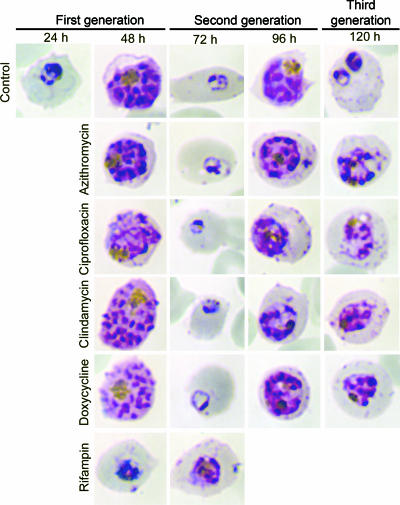
We treated parasites with antibiotics for one cycle and examined them every 24 h to determine when the effects of antibiotic treatment became apparent (Fig. 2). Parasites treated with rifampin arrested at the trophozoite stage and were unable to initiate schizogeny. Parasites treated with azithromycin, ciprofloxacin, clindamycin, and doxycycline formed healthy-appearing schizonts (48 h), ruptured host cells, and successfully invaded new erythrocytes. In drug-free medium, progeny progressed through a second life cycle, formed normal-appearing trophozoites (72 h), and progressed to form multinucleated schizonts (96 h). However, these parasites were unable to complete this cycle and remained arrested at the schizont stage for at least another 24 h (120 h), as untreated parasites completed schizogeny, invaded new erythrocytes, and progressed to early trophozoites.
FIG. 2.
Progeny of treated parasites produce schizonts that are unable to form functional merozoites. 3D7 strain parasites were treated with antibiotics at twice the IC50 at 96 h (100 nM azithromycin, 6 μM ciprofloxacin, 25 nM clindamycin, 1 μM doxycycline, and 6 μM rifampin), and Giemsa-stained thin smears were examined every 24 h for 120 h. The times shown above panels are the numbers of hours after invasion of the first generation of parasites. Rifampin-treated parasites arrested during the trophozoite stage of the first cycle, while parasites treated with doxycycline, clindamycin, azithromycin, and ciprofloxacin arrested during the schizont stage of the second cycle.
The progeny of antibiotic-treated parasites inherit morphologically abnormal apicoplasts.
To determine whether ciprofloxacin, clindamycin, doxycycline, and azithromycin disrupt apicoplast segregation, we treated 3D7 parasites that had been stably transfected with ACPl-GFP (43) at approximately twice the IC50 at 96 h and then examined apicoplast morphology in schizonts during treatment and in the progeny of treated parasites (Fig. 3). In parasites treated with azithromycin, clindamycin, doxycycline, and ciprofloxacin, apicoplasts branched and segregated normally into developing merozoites and were present in the progeny. We counted the number of progeny containing GFP-labeled apicoplasts by flow cytometry (Fig. 4) and found no significant difference in apicoplast number between the progeny of control and antibiotic-treated parasites (Table 3). However, at the schizont stage, when the progeny of untreated parasites contained multiple apicoplasts, the progeny of antibiotic-treated parasites contained only a single abnormal apicoplast (Fig. 3).
FIG. 3.
Apicoplasts distribute normally into the progeny of antibiotic-treated parasites but are abnormal in the following cycle. ACPl-GFP 3D7 strain transgenic parasites were treated with antibiotics at twice the IC50 at 96 h (100 nM azithromycin [AZ], 6 μM ciprofloxacin [CIP], 25 nM clindamycin [CLN], and 1 μM doxycycline [DOX]), and representative live parasites were imaged by fluorescence microscopy. GFP-labeled apicoplasts are green; Hoechst-stained nuclei are blue.
FIG. 4.
GFP-labeled apicoplasts are detectable in progeny by flow cytometry. ACPl-GFP 3D7 strain transgenic parasites were evaluated for the presence of a GFP-labeled apicoplast during the trophozoite stage by flow cytometry. For each sample, half of the live parasites were stained with SYTO 16 nuclear stain to evaluate parasitemia, and the other half were unstained to determine the percentage of cells containing a fluorescent apicoplast. Counts falling below the gray horizontal line reflect uninfected erythrocytes. Cells containing a GFP-labeled apicoplast appear above the gray line in the left panel. Cells containing a SYTO 16-stained nucleus appear above the gray line in the right panel. Forward scatter (FSC) is plotted against fluorescence (FL).
TABLE 3.
All surviving progeny of treated parasites inherit an apicoplasta
| Treatment (first generation) | % Control parasitemia (SD) in second-generation trophozoites | % of parasites containing apicoplasts (SD) in second-generation trophozoites |
|---|---|---|
| None (control) | 100 | 87.6 (11.4) |
| Azithromycin | 105 (12.3) | 83.4 (7.3) |
| Ciprofloxacin | 98.6 (8.0) | 81.4 (8.9) |
| Clindamycin | 99.3 (8.7) | 85.2 (7.9) |
| Doxycycline | 97.2 (5.6) | 83.6 (3.5) |
ACPl-GFP 3D7 parasites were treated at approximately twice the IC50 at 96 h from the late ring stage through the end of the life cycle (first generation). Progeny (second generation) were allowed to mature to the trophozoite stage in the absence of drug. Then live cells were analyzed to identify fluorescent apicoplasts, and SYTO 16-stained cells were counted to determine parasitemia, both using flow cytometry as described in the legend to Fig. 4. Data are means of at least three independent determinations.
DISCUSSION
Doxycycline, clindamycin, and azithromycin are effective antimalarials, though they are slow acting, and best used in combination with a more rapid acting drug (5, 14, 24, 27, 30, 31, 39). Consistent with their slow clinical action, all of these drugs are much more potent against cultured erythrocytic-stage parasites when assessed two asexual life cycles after the initiation of treatment (13, 19, 32, 48). This increased potency is seen even if the drugs are removed after completion of the first cycle (12, 20), similar to the “delayed-death” phenotype described in the related apicomplexan parasite Toxoplasma gondii (15). We demonstrate here that, at clinically relevant concentrations, clindamycin, ciprofloxacin, and azithromycin, but not rifampin, cause delayed death by acting against the apicoplast, as we have described previously for doxycycline (12). These drugs do not disrupt apicoplast segregation, as all the progeny of treated parasites contain an apicoplast. Rather, the drugs cause abnormal apicoplasts to distribute into developing merozoites.
A specific link between antibiotics and the apicoplast in T. gondii was first implied by the observation that the apicoplast genome begins to degrade following treatment with ciprofloxacin, clindamycin, or chloramphenicol (16). Also, in a transgenic T. gondii cell line carrying an apicoplast segregation defect (21), parasites missing apicoplasts died shortly after invading new cells. These results led to the assumption that multiple antibiotics exert antimalarial effects by blocking the segregation of apicoplasts into developing progeny. However, it is now clear that antibiotics causing a delayed-death phenotype do not block segregation of apicoplasts into new merozoites in P. falciparum. We showed previously that the progeny of doxycycline-treated parasites inherited apicoplasts, but these apicoplasts were nonfunctional, as indicated by their inability to properly import or process nuclear encoded proteins or to elongate or segregate during schizogeny (12). We now report that, at clinically achievable doses, clindamycin, azithromycin, and ciprofloxacin exerted effects similar to those of doxycycline when cultured with malaria parasites. Our results agree with a recent report that apicoplast segregation was unaffected by clindamycin or tetracycline (20) but disagree with another report (34), suggesting that clindamycin blocked apicoplast segregation. We did observe abnormal apicoplasts following treatment with high doses of antibiotics (twice the IC50 at 48 h), but these parasites did not produce progeny and were grossly abnormal, suggesting that at these doses antibiotics were interfering with multiple targets in addition to the apicoplast (data not shown).
Prior studies of the antimalarial mechanisms of antibiotics have been complicated by the use of suprapharmacological concentrations of these drugs. To help clarify the mechanisms by which antibiotics kill parasites when used clinically, we assessed their effects at concentrations below those achieved with routine dosing. Our results indicate that antibiotics inhibiting either translation (doxycycline, clindamycin, and azithromycin) or DNA gyrase activity (ciprofloxacin) cause delayed death through disrupting apicoplast function, though the precise mechanism remains uncertain. The apicoplast genome encodes 70S rRNA and proteins (18, 47), and selection for clindamycin-resistant T. gondii (7) and azithromycin-resistant P. falciparum (37) led to point mutations in apicoplast-encoded ribosomal components. Tetracyclines block the synthesis of proteins resistant to disruption by the eukaryotic ribosomal inhibitor cycloheximide in P. falciparum (22) and in T. gondii (3). It is likely that this pool of cycloheximide-resistant, tetracycline-inhibited proteins includes proteins translated by the prokaryote-like ribosomes of the apicoplast or mitochondrion, though they remain to be identified. Antibiotic effects on mitochondrial morphology and function are minimal until late in the second cycle of treatment and are likely secondary to the death of the parasites (12, 20, 22).
Rifampin has antimalarial activity and has been reported to show increased efficacy after prolonged treatment (13, 32). However, we saw no difference in the IC50 for rifampin when measured at 48 h or 96 h, agreeing with one study (20), but not with another (35). Parasites treated with rifampin arrested at the end of the trophozoite stage, suggesting that the primary mechanism of action of rifampin may involve targets independent of the apicoplast.
For ciprofloxacin, differences in antimalarial activity between 48-h and 96-h assessments were more modest than with other antibiotics, especially in 3D7 parasites, but nonetheless delayed death was demonstrated at clinically relevant concentrations of the drug. Of note, a recent report that demonstrated only rapid antimalarial activity, and not delayed death, with ciprofloxacin studied this antibiotic at concentrations far above those that are clinically meaningful (20). While we found only minor strain-specific differences in sensitivity at 96 h, we did note more striking strain-specific differences in the sensitivity to ciprofloxacin, azithromycin, and rifampin at 48 h. This agrees with other studies reporting strain-specific differences in sensitivity to azithromycin (37) and rifampin (32).
In summary, we have demonstrated that clinically achievable concentrations of antimalarial antibiotics that interfere with either prokaryotic translation or DNA gyrase activity exert a delayed antimalarial effect in which the progeny of treated parasites inherit abnormal apicoplasts and die before the completion of their cycle. Since antibiotics are well characterized and approved for human use, they can and have been incorporated into effective antimalarial treatment regimens. Understanding the mechanisms by which these drugs kill parasites will help guide their use in combination therapies to help bring this disease under control. In addition, characterization of the antimalarial mechanisms of these compounds may facilitate the identification of novel inhibitors of apicoplast function with potential roles in combination antimalarial regimens.
Acknowledgments
We thank Geoff McFadden from the University of Melbourne for the ACPl-GFP transgenic Plasmodium falciparum lines and Jiri Gut and Jenny Legac for excellent technical assistance.
This work was supported by grants from the National Institutes of Health (RO1 AI051800 and T32 A1060537). P.J.R. is a Doris Duke Charitable Foundation Distinguished Clinical Scientist.
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Baird, J. K. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565-1577. [DOI] [PubMed] [Google Scholar]
- 2.Baudon, D., G. Martet, B. Pascal, J. Bernard, A. Keundjian, and R. Laroche. 1999. Efficacy of daily antimalarial chemoprophylaxis in tropical Africa using either doxycycline or chloroquine-proguanil: a study conducted in 1996 in the French Army. Trans. R. Soc. Trop. Med. Hyg. 93:302-303. [DOI] [PubMed] [Google Scholar]
- 3.Beckers, C. J., D. S. Roos, R. G. Donald, B. J. Luft, J. C. Schwab, Y. Cao, and K. A. Joiner. 1995. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Investig. 95:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg, E. J., R. A. Robson, D. A. Saunders, G. G. Graham, R. C. Buttimore, A. M. Neill, and G. I. Town. 2000. The pharmacokinetics of oral fleroxacin and ciprofloxacin in plasma and sputum during acute and chronic dosing. Br. J. Clin. Pharmacol. 49:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrmann, S., S. Issifou, G. Esser, A. A. Adegnika, M. Ramharter, P. B. Matsiegui, S. Oyakhirome, D. P. Mawili-Mboumba, M. A. Missinou, J. F. Kun, H. Jomaa, and P. G. Kremsner. 2004. Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J. Infect. Dis. 190:1534-1540. [DOI] [PubMed] [Google Scholar]
- 6.Bremen, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):1-11. [DOI] [PubMed] [Google Scholar]
- 7.Camps, M., G. Arrizabalaga, and J. Boothroyd. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol. Microbiol. 43:1309-1318. [DOI] [PubMed] [Google Scholar]
- 8.Chaubey, S., A. Kumar, D. Singh, and S. Habib. 2005. The apicoplast of Plasmodium falciparum is translationally active. Mol. Microbiol. 56:81-89. [DOI] [PubMed] [Google Scholar]
- 9.Clough, B., M. Strath, P. Preiser, P. Denny, and I. R. Wilson. 1997. Thiostrepton binds to malarial plastid rRNA. FEBS Lett. 406:123-125. [DOI] [PubMed] [Google Scholar]
- 10.Clough, B., and I. R. Wilson. 2001. Antibiotics and the plasmodial plastid organelle, p. xi. In P. J. Rosenthal (ed.), Antimalarial chemotherapy: mechanisms of action, resistance, and new directions in drug discovery. Humana Press, Totowa, NY. [DOI] [PubMed]
- 11.Cook, J. A., E. J. Randinitis, C. R. Bramson, and D. L. Wesche. 2006. Lack of a pharmacokinetic interaction between azithromycin and chloroquine. Am. J. Trop. Med. Hyg. 74:407-412. [PubMed] [Google Scholar]
- 12.Dahl, E. L., J. L. Shock, B. R. Shenai, J. Gut, J. L. DeRisi, and P. J. Rosenthal. 2006. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob. Agents Chemother. 50:3124-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divo, A. A., T. G. Geary, and J. B. Jensen. 1985. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob. Agents Chemother. 27:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne, M. W., N. Singh, M. Shukla, N. Valecha, P. C. Bhattacharyya, V. Dev, K. Patel, M. K. Mohapatra, J. Lakhani, R. Benner, C. Lele, and K. Patki. 2005. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J. Infect. Dis. 191:1582-1588. [DOI] [PubMed] [Google Scholar]
- 15.Fichera, M. E., M. K. Bhopale, and D. S. Roos. 1995. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob. Agents Chemother. 39:1530-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 17.Foth, B. J., S. A. Ralph, C. J. Tonkin, N. S. Struck, M. Fraunholz, D. S. Roos, A. F. Cowman, and G. I. McFadden. 2003. Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science 299:705-708. [DOI] [PubMed] [Google Scholar]
- 18.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geary, T. G., and J. B. Jensen. 1983. Effects of antibiotics on Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 32:221-225. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, C. D., V. Su, and G. I. McFadden. 2007. The effects of antibacterials on the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 152:181-191. [DOI] [PubMed] [Google Scholar]
- 21.He, C. Y., M. K. Shaw, C. H. Pletcher, B. Striepen, L. G. Tilney, and D. S. Roos. 2001. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 20:330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiatfuengfoo, R., T. Suthiphongchai, P. Prapunwattana, and Y. Yuthavong. 1989. Mitochondria as the site of action of tetracycline on Plasmodium falciparum. Mol. Biochem. Parasitol. 34:109-115. [DOI] [PubMed] [Google Scholar]
- 23.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 24.Lell, B., and P. G. Kremsner. 2002. Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob. Agents Chemother. 46:2315-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, Q., K. Katakura, and M. Suzuki. 2002. Inhibition of mitochondrial and plastid activity of Plasmodium falciparum by minocycline. FEBS Lett. 515:71-74. [DOI] [PubMed] [Google Scholar]
- 26.McConkey, G. A., M. J. Rogers, and T. F. McCutchan. 1997. Inhibition of Plasmodium falciparum protein synthesis. Targeting the plastid-like organelle with thiostrepton. J. Biol. Chem. 272:2046-2049. [DOI] [PubMed] [Google Scholar]
- 27.Miller, R. S., C. Wongsrichanalai, N. Buathong, P. McDaniel, D. S. Walsh, C. Knirsch, and C. Ohrt. 2006. Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin-quinine combinations: a randomized, dose-ranging study. Am. J. Trop. Med. Hyg. 74:401-406. [PubMed] [Google Scholar]
- 28.Na-Bangchang, K., R. Ruengweerayut, J. Karbwang, A. Chauemung, and D. Hutchinson. 2007. Pharmacokinetics and pharmacodynamics of fosmidomycin monotherapy and combination therapy with clindamycin in the treatment of multidrug resistant falciparum malaria. Malaria J. 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton, P. N., J. F. Chaulet, A. Brockman, W. Chierakul, A. Dondorp, R. Ruangveerayuth, S. Looareesuwan, C. Mounier, and N. J. White. 2005. Pharmacokinetics of oral doxycycline during combination treatment of severe falciparum malaria. Antimicrob. Agents Chemother. 49:1622-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noedl, H., S. Krudsood, K. Chalermratana, U. Silachamroon, W. Leowattana, N. Tangpukdee, S. Looareesuwan, R. S. Miller, M. Fukuda, K. Jongsakul, S. Sriwichai, J. Rowan, H. Bhattacharyya, C. Ohrt, and C. Knirsch. 2006. Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: a randomized, phase 2 clinical trial in Thailand. Clin. Infect. Dis. 43:1264-1271. [DOI] [PubMed] [Google Scholar]
- 31.Noedl, H., S. Krudsood, W. Leowattana, N. Tangpukdee, W. Thanachartwet, S. Looareesuwan, R. S. Miller, M. Fukuda, K. Jongsakul, K. Yingyuen, S. Sriwichai, C. Ohrt, and C. Knirsch. 2007. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrob. Agents Chemother. 51:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pradines, B., C. Rogier, T. Fusai, J. Mosnier, W. Daries, E. Barret, and D. Parzy. 2001. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrob. Agents Chemother. 45:1746-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ralph, S. A., G. G. Van Dooren, R. F. Waller, M. J. Crawford, M. J. Fraunholz, B. J. Foth, C. J. Tonkin, D. S. Roos, and G. I. McFadden. 2004. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2:203-216. [DOI] [PubMed] [Google Scholar]
- 34.Ramya, T. N., K. Karmodiya, A. Surolia, and N. Surolia. 2007. 15-Deoxyspergualin primarily targets the trafficking of apicoplast proteins in Plasmodium falciparum. J. Biol. Chem. 282:6388-6397. [DOI] [PubMed] [Google Scholar]
- 35.Ramya, T. N., S. Mishra, K. Karmodiya, N. Surolia, and A. Surolia. 2007. Inhibitors of nonhousekeeping functions of the apicoplast defy delayed death in Plasmodium falciparum. Antimicrob. Agents Chemother. 51:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaberg, L. S., A. R. Parquette, I. Y. Gluzman, G. W. Phillips, Jr., T. F. Brodasky, and D. J. Krogstad. 1984. Clindamycin activity against chloroquine-resistant Plasmodium falciparum. J. Infect. Dis. 150:904-911. [DOI] [PubMed] [Google Scholar]
- 37.Sidhu, A. B., Q. Sun, L. J. Nkrumah, M. W. Dunne, J. C. Sacchettini, and D. A. Fidock. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 282:2494-2504. [DOI] [PubMed] [Google Scholar]
- 38.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor, W. R., H. Widjaja, T. L. Richie, H. Basri, C. Ohrt, Tjitra, E. Taufik, T. R. Jones, K. C. Kain, and S. L. Hoffman. 2001. Chloroquine/doxycycline combination versus chloroquine alone, and doxycycline alone for the treatment of Plasmodium falciparum and Plasmodium vivax malaria in northeastern Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 64:223-228. [DOI] [PubMed] [Google Scholar]
- 40.Thummel, K. E., and D. D. Shen. 2001. Design and optimization of dosage regimens: pharmacokinetic data, p. 1917-2023. In J. G. Hardman, L. E. Limbird, and A. G. Gilman (ed.), Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. McGraw-Hill, New York, NY.
- 41.Trager, W., and J. B. Jenson. 1978. Cultivation of malarial parasites. Nature 273:621-622. [DOI] [PubMed] [Google Scholar]
- 42.Waller, R. F., and G. I. McFadden. 2005. The apicoplast: a review of the derived plastid of apicomplexan parasites. Curr. Issues Mol. Biol. 7:57-79. [PubMed] [Google Scholar]
- 43.Waller, R. F., M. B. Reed, A. F. Cowman, and G. I. McFadden. 2000. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissig, V., T. S. Vetro-Widenhouse, and T. C. Rowe. 1997. Topoisomerase II inhibitors induce cleavage of nuclear and 35-kb plastid DNAs in the malarial parasite Plasmodium falciparum. DNA Cell Biol. 16:1483-1492. [DOI] [PubMed] [Google Scholar]
- 45.Williamson, D. H., P. R. Preiser, P. W. Moore, S. McCready, M. Strath, and R. J. Wilson. 2002. The plastid DNA of the malaria parasite Plasmodium falciparum is replicated by two mechanisms. Mol. Microbiol. 45:533-542. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, R. J. 2005. Parasite plastids: approaching the endgame. Biol. Rev. Camb. Philos. Soc. 80:129-153. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, R. J., P. W. Denny, P. R. Preiser, K. Rangachari, K. Roberts, A. Roy, A. Whyte, M. Strath, D. J. Moore, P. W. Moore, and D. H. Williamson. 1996. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261:155-172. [DOI] [PubMed] [Google Scholar]
- 48.Yeo, A. E., and K. H. Rieckmann. 1995. Increased antimalarial activity of azithromycin during prolonged exposure of Plasmodium falciparum in vitro. Int. J. Parasitol. 25:531-532. [DOI] [PubMed] [Google Scholar]
- 49.Zuegge, J., S. Ralph, M. Schmuker, G. I. McFadden, and G. Schneider. 2001. Deciphering apicoplast targeting signals-feature extraction from nuclear-encoded precursors of Plasmodium falciparum apicoplast proteins. Gene 280:19-26. [DOI] [PubMed] [Google Scholar]