Abstract
Necrotic enteritis (NE) in broiler chickens is caused by Clostridium perfringens. Currently, no vaccine against NE is available and immunity to NE is not well characterized. Our previous studies showed that immunity to NE followed oral infection by virulent rather than avirulent C. perfringens strains and identified immunogenic secreted proteins apparently uniquely produced by virulent C. perfringens isolates. These proteins were alpha-toxin, glyceraldehyde-3-phosphate dehydrogenase, pyruvate:ferredoxin oxidoreductase (PFOR), fructose 1,6-biphosphate aldolase, and a hypothetical protein (HP). The current study investigated the role of each of these proteins in conferring protection to broiler chickens against oral infection challenges of different severities with virulent C. perfringens. The genes encoding these proteins were cloned and purified as histidine-tagged recombinant proteins from Escherichia coli and were used to immunize broiler chickens intramuscularly. Serum and intestinal antibody responses were assessed by enzyme-linked immunosorbent assay. All proteins significantly protected broiler chickens against a relatively mild challenge. In addition, immunization with alpha-toxin, HP, and PFOR also offered significant protection against a more severe challenge. When the birds were primed with alpha-toxoid and boosted with active toxin, birds immunized with alpha-toxin were provided with the greatest protection against a severe challenge. The serum and intestinal washings from protected birds had high antigen-specific antibody titers. Thus, we conclude that there are certain secreted proteins, in addition to alpha-toxin, that are involved in immunity to NE in broiler chickens.
Necrotic enteritis (NE) is an economically important enteric disease of chickens caused by Clostridium perfringens. The disease is usually controlled by antimicrobial drugs administered at prophylactic doses either in water or in feed. However, there is concern about the routine prophylactic use of antimicrobial drugs in food animal production because of their contribution to antimicrobial resistance problems. If antimicrobial drugs were banned for such purposes in North America, there might be an increase in NE in broiler flocks, as has happened in Scandinavia (12).
Although vaccination offers an alternative approach to antimicrobial drugs for control of the disease, very little is known about immunity to NE. However, there has been considerable work on immunity to C. perfringens in other circumstances, since it is a cause of gas gangrene in people. That work has identified the alpha-toxin, a phospholipase C exoenzyme, both as a major virulence factor and as an important protective immunogen (5, 30, 34). In addition, based on naturally occurring antibodies or maternal vaccination, some studies suggest that antibodies to alpha-toxin are important in immunity to NE in chickens (10, 19). However, the role of alpha-toxin or any other protein in immunity to NE in chickens remains to be demonstrated, and one study has shown the immunizing effects of alpha-toxin-negative mutants (32). A recent study also demonstrated that an alpha-toxin-negative mutant produced NE experimentally in chickens, demonstrating that factors other than alpha-toxin are important in the pathogenesis of NE (14).
Recent studies from this laboratory showed that the immunizing ability for protection against NE was associated with infection with virulent rather than with avirulent C. perfringens strains (32). Several proteins apparently uniquely expressed by virulent, protective C. perfringens strains that reacted to serum and intestinal antibodies from infection-immunized birds were identified by mass spectrometry (15). These secreted proteins were alpha-toxin, glyceraldehyde-3-phosphate dehydrogenase (GPD), pyruvate: ferredoxin oxidoreductase (PFOR), fructose 1,6-biphosphate aldolase (FBA), and a hypothetical protein (HP). On the basis of these findings, the objective of the current study was to investigate the role of each of these proteins in immunizing broiler chickens against experimental challenges of various severities with virulent C. perfringens.
MATERIALS AND METHODS
Cloning, overexpression, and purification.
Escherichia coli strains were used to clone and express the genes of interest; E. coli DH5α (recA lacZΔM15) (Stratagene, La Jolla, CA) was used as the host for plasmid construction, and E. coli BL21-Star(DE3) (F− ompT hsdSB (rB− mB−) gal dcm rne-131) (Invitrogen, Carlsbad, CA) was used for the overexpression of histidine-tagged fusion proteins. These strains were grown in Luria-Bertani medium at 37°C, and when required, kanamycin was added to the medium at a concentration of 50 μg/ml. The chromosomal DNA of virulent, protective C. perfringens strain CP4 was used as the source of DNA for the expression of the secreted antigens. PCR amplifications were performed with a Platinum PCR SuperMix high-fidelity kit (Invitrogen, Burlington, ON, Canada) and the specific primers described in Table 1. After purification (PCR purification kit; QIAGEN, Mississauga, ON, Canada), the PCR products (alpha-toxin, HP, GPD, FBA, and truncated PFOR [tPFOR]) were cloned into vector pET28a (N′- and/or C′-terminal His tag vector, Kmr; Novagen Inc., Madison, WI) to generate proteins fused with histidine residues (six-His). The resulting plasmids were introduced into E. coli BL21-Star(DE3), following the manufacturer's instructions. The nucleotide sequences of the cloned PCR products were verified by sequencing both strands. Expression of recombinant proteins by E. coli was induced by isopropyl-β-d-thiogalactopyranoside at a final concentration of 1 mM. The histidine-tagged proteins were purified under native conditions by affinity chromatography on nickel-nitrilotriacetic acid (Ni-NTA) agarose, following the manufacturer's instructions (QIAGEN). Briefly, when the proteins were expressed as soluble proteins, the bacterial pellets were resuspended in a lysis/binding buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole) containing lyzozyme (1 mg/ml) and were incubated for 60 min on ice. The bacterial cells were lysed by using a French pressure cell (three to four cycles of 1,000 lb/in2). The supernatant was collected by centrifugation and was added to the Ni-NTA agarose. The washing and elution steps were performed with buffers containing increasing concentration of imidazole (20 mM to 250 mM). Finally, imidazole was removed from the eluted material by dialysis against phosphate-buffered saline (PBS; pH 7.2), the recombinant proteins were concentrated with an Amicon filter (pore size, 10 kDa; Millipore, Billerica, MA), and the protein concentration was determined by using a PlusOne 2-D Quant kit (Amersham Biosciences, San Francisco, CA).
TABLE 1.
Primers used to amplify genes encoding proteins used in immunization experiments
| Gene | Direction | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|---|
| Alpha-toxin | Forward | CCGCTCGAGTTGGGATGGAAAAATTGAT | 1,100 |
| Reverse | CCGGAATTCTTTATATTATAAGTTGAATTT | ||
| HP | Forward | CCGCTCGAGGAATAAGAGAAAAATAGCAG | 5,400 |
| Reverse | CCGGGTACCACGTTAAATAAATAGAACAT | ||
| GPD | Forward | CCGCTCGAGGGTAAAAGTAGCTATTAACGG | 1,000 |
| Reverse | CCGGGTACCTTAGAAACTAAGCATTTTAAA | ||
| FBA | Forward | CCGCGGATCCATGGCATTAGTTAACGCAAA | 900 |
| Reverse | CCGCCTCGAGAGCTCTGTTTACTGAACCGA | ||
| tPFOR | Forward | CCGCCTCGAGCACTTCATTAGAACCAGTTG | 1,600 |
| Reverse | CCGCGGATCCTAGCTAAGTAGTCTTGGTCTCCGCGGATCCTAGCTAAGTAGTCTTGGTCT |
SDS-PAGE and Western immunoblotting.
Purified recombinant proteins were separated by one-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) in a 12.5% acrylamide gel under denaturing conditions, as described by Laemmli (16). The proteins were transferred to a nitrocellulose membrane with a pore size of 0.45 μm by using a mini-gel transfer assembly (Bio-Rad Laboratories, Hercules, CA). After completion of the transfer, the nonspecific binding sites on the membranes were blocked for 1 h with blocking buffer containing 1% casein (Bio-Rad Laboratories) and the membranes were incubated with immune sera collected from infection-immunized birds in a previous study (32) for 1 h at a 1:1,000 dilution. Goat anti-chicken immunoglobulin Y (IgY; heavy and light chains; Cedarlane Laboratories, Hornby, ON, Canada) was used as the secondary antibody at a 1:2,000 dilution. The blots were developed, and specific immunoreactive protein bands were visualized by using an alkaline phosphatase-conjugated substrate kit (Bio-Rad Laboratories).
Immunization and challenge.
The experiments with chickens and the conditions for their use were approved by the University of Guelph Animal Care Committee, in accordance with the Canadian Council on Animal Care's guidelines. Commercial 1-day-old male White Plymouth Rock broiler chickens (Bonnie's Chick Hatchery, Elmira, ON, Canada) were fed an antibiotic-free chicken starter diet containing 20% protein for 13 days, followed by a formulated wheat-based grower feed containing 28% protein (Arkell Research Station, University of Guelph). The birds were immunized with purified recombinant proteins at different dose levels in the pectoral muscle in a volume of 0.2 ml per bird two to three times with an interval of 1 week and challenged a week after the last immunization, when the birds were 4 weeks old. For the experimental infection (challenge) of birds, virulent C. perfringens (CP4) was grown in cooked meat medium (Difco) for 24 h at 37°C. Fluid thioglycollate medium (Difco) was then inoculated with a 3% (vol/vol) inoculum from the C. perfringens-infected cooked meat medium and incubated at 37°C for 24 h. The growth at 24 h was 8.24 ± 0.09 C. perfringens log10 CFU/ml. The inoculated fluid thioglycollate medium was then mixed with feed at a ratio of 2:1 (vol/wt). The inoculated feed was freshly prepared twice per day and fed to chickens that were fasted for 20 h prior to challenge.
The general experimental design is summarized in Table 2. Quil-A (Superfos Biosector, Vedbaek, Denmark) was used as an adjuvant to immunize the chickens (50 μg/bird/injection), and the unimmunized controls in each experiment received only Quil-A, followed by a challenge similar to that used for the immunized groups. All the proteins were tested for their abilities to protect the birds against a gradient of severity of challenge (mild-moderate-severe). A “mild” challenge (experiment 1) was produced by a duration of challenge of 3 days; the mildness of the challenge was confirmed by the lesion scores for the nonimmunized birds. A “moderate” challenge (experiment 2) was produced by a duration of challenge of 5 days, in which the birds were fed a fixed amount of feed that sometimes ran out before the next feeding (feedings were given every 12 h); the moderateness of the challenge was confirmed by the lesion scores for the nonimmunized birds. A “severe” challenge (experiment 3) was produced by challenge for 5 days and by ensuring that the birds constantly had infected feed available; the severity of the challenge was confirmed by the lesion scores for the nonimmunized birds. In experiment 4A, the challenge was considered “mild-moderate,” since the birds in these groups that received virulent C. perfringens for 3 days but were necropsied on day 6 were found to have lesion scores higher than those of birds that were challenged for 3 days and necropsied on day 4. Alpha-toxin was used in both an active and a toxoid form to immunize the birds, and toxoid was prepared by a previously described protocol (11). Briefly, the purified toxin was incubated with 0.2% formalin and 0.05 M l-lysine at 30°C until its activity was completely lost, as confirmed by a 5% egg yolk agar plate assay. In all experiments, the number of birds in each group was between 10 and 20, and all birds were identified individually. Blood was collected from the wing vein from all the groups at three times: preimmunization (day 0), midexperiment (day 10), and prechallenge (day 20). Intestinal washings were collected at necropsy by using PBS.
TABLE 2.
Summary of experimental design
| Expt no. | Immunization groupa | Dosage (μg) of vaccine/bird | Frequency of administration | No. of days (severity) of oral challenge |
|---|---|---|---|---|
| 1 | VC, Sup, alpha-toxoid, GPD, HP, FBA, and tPFOR | 20 | Three times; days 7, 14, and 21 | 3 (mild) |
| 2 | VC, MC, GPD, HP, FBA, and tPFOR | 40 | Two times; days 7 and 14 | 5 (moderate) |
| 3 | VC, alpha-toxoid/alpha-toxin,b GPD, HP, tPFOR, and a combination of GPD and HP | 20 | Three times; days 7, 14, and 21 | 5 (severe) |
| 4A | VC and FBA | 20 | Three times; days 7, 14, and 21 | 3 (mild-moderate) |
| 4B | VC, alpha-toxin,c and FBA | 20 | Three times; days 7, 14, and 21 | 5 (severe) |
VC, vehicle-only controls; Sup, crude culture supernatant of virulent C. perfringens, in which the birds received 60 μg/injection of culture supernatant that was processed and concentrated following a protocol described earlier (15); MC, mock-immunized controls, in which the birds were mock immunized with an unrelated protein that was cloned, expressed, and purified from E. coli in the same manner as the C. perfringens-related proteins.
The birds received alpha-toxoid in the first two injections, followed by active alpha-toxin in the third.
The birds received three injections of alpha-toxin, in which the first and the third injections were with 20 μg and the second was reduced to 10 μg.
Necropsy.
The chickens were euthanized with carbon dioxide gas, and their small intestines (duodenum to ileum) were examined for grossly visible lesions. Any chickens that had reached a predetermined severity of clinical illness prior to necropsy were euthanized and later necropsied. Intestinal lesions in the small intestine (duodenum to ileum) were scored as follows: 0, no gross lesions; 1, thin or friable wall or very mild and superficial generalized inflammation; 2, focal necrosis or ulceration; 3, large patches of necrosis; 4, severe extensive necrosis; 5, death during the experiment with lesion scores of 4+ (25). Blind scoring was used to avoid scorer bias.
Measurement of antibody titers in chicken sera and intestinal washings.
The specific antibody titers were determined by the end-point dilution method by use of an enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Immulon-2; Dynatech Laboratories, Chantilly, VA) were coated with recombinant proteins (5 μg/ml in 0.1 M carbonate buffer, pH 9.6) for 60 min at 37°C, followed by an overnight incubation at 4°C. After the coated plates were blocked for 60 min at 37°C with PBS containing 3% bovine serum albumin (BSA; Sigma), sera from the immunized birds, along with sera from their concurrent controls, were serially diluted in PBS containing 1% BSA and incubated for 2 h with recombinant protein-coated plates at room temperature. After the plates were washed with PBS containing 0.1% Tween 20 (PBST), alkaline phosphatase-coupled goat anti-chicken IgY (heavy and light chains; diluted 1:5,000 in PBST-1% BSA) was added to the microplates and the mixture was incubated for 60 min at room temperature. After extensive washing of the plates with PBST, the color reaction was developed by using a alkaline phosphate substrate kit (Bio-Rad Laboratories), following the manufacturer's instructions. The reaction was stopped by adding 0.4 M NaOH. The absorbance at 405 nm was measured in an ELISA spectrophotometer. The specific antibody titer of the immune serum was expressed as the reciprocal of the serum dilution (log2 optical density) that gave an A405 value above the cutoff, which was defined as twice the absorbance value of the unimmunized and mock control wells run in duplicate.
The intestinal antibody response was also measured by ELISA by the procedure described above for serum. Intestinal washings from at least 10 chickens per group were pooled, and the total protein content was measured by using a PlusOne 2-D Quant kit (Amersham Biosciences). The total protein was used as the source of the primary antibody after the protein content of the initial dilution (1:10) was kept constant across all the groups. Alkaline phosphatase-conjugated goat anti-chicken IgY and IgA were used as the secondary antibodies and were used at dilutions of 1:4,000 and 1:2,000, respectively. The end-point titers were determined as described above for the serum ELISA.
Statistical analysis.
Statistical analysis was performed to determine whether there was a significant difference between the numbers of birds with lesions from the immunized groups and the numbers of birds with lesions from the unimmunized, vehicle-only controls. A two-tailed Fisher's exact test was used to determine whether the two groups differed in the proportions that fell into the two classifications of either lesions or no lesions, under the null hypothesis that the proportions were the same. The data were analyzed by use of a two-by-two contingency table, with the data for the unimmunized control group in one column and the data for the immunized groups in the other column. The lesion scores were ranked from 0 to 5+; however, a “protective” response was given to birds with lesions scores of ≤1+. The null hypothesis was rejected at α = 0.05. For the serum ELISA, a one-way analysis of variance was used to determine significant (P ≤ 0.01) differences in the antibody titers between preimmunized and immunized birds across all the groups. Statistical analysis could not be performed for the intestinal antibody response determined by ELISA, since the washings collected were pooled for each group.
RESULTS
Cloning, expression, and purification.
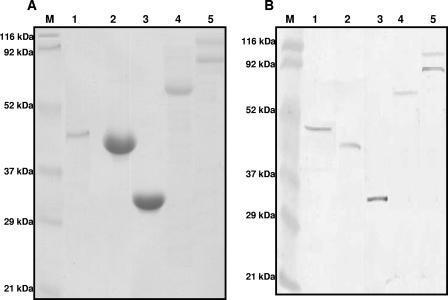
All five genes selected for the immunization study were successfully cloned, expressed, and purified to homogeneity. However, alpha-toxin appeared to be toxic to the host E. coli cells, such that it could not be obtained in a quantity sufficient for the immunization studies. Hence, commercially available purified alpha-toxin (Sigma Laboratories) (and alpha-toxoid) was used to the immunize the birds. HP (190 kDa) was found to be cleaved upon expression into two bands of 90 to 100 kDa. Attempts to express the entire protein by using different E. coli expression hosts were unsuccessful. Since both bands reacted strongly to antihistidine antibodies as well as to immune sera collected from infection-immunized birds (Fig. 1) from a previous study (32), both bands were further purified in large quantities and used for immunization.
FIG. 1.
Recombinant Clostridium perfringens histidine-tagged proteins purified from Escherichia coli cells. (A) Coomassie-stained purified proteins; (B) reactivities of purified proteins to immune serum from chickens immune to NE. Lanes 1, alpha-toxin (45 kDa); lanes 2, GPD (40 kDa); lanes 3, FBA (35 kDa); lanes 4, tPFOR (67 kDa); lanes 5, HP (90 to 100 kDa); lanes M, molecular mass standards.
Cloning of the pfor gene was not successful, despite several attempts. However, a portion of the gene that encoded a truncated protein (tPFOR) of 67 kDa that contained the iron-sulfur active sites of this enzyme was successfully cloned and purified in large quantities.
All the recombinant proteins purified from E. coli were visualized by Coomassie staining, and their reactivities to antihistidine antibodies as well as immune serum were confirmed by Western blotting in at least three separate experiments (Fig. 1).
Immunization experiments.
In experiment 1, HP, GPD, tPFOR, and FBA offered significant protection against a mild challenge (Table 3), with HP offering the greatest protection. Immunization with crude culture supernatant that contained all secreted proteins, including those that were purified, also offered significant protection. Alpha-toxoid did not protect the birds against challenge.
TABLE 3.
Intestinal lesion scores of birds immunized with three injections intramuscularly and then infected with a mild challenge with C. perfringens
| Protein | No. of chickens | No. of chickens with the following lesion scores:
|
Mean no. of chickens | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | 5+ | |||
| Vehicle-only controls | 10 | 1 | 3 | 4 | 1 | 1 | 0 | 1.55 |
| Culture supernatanta | 10 | 8 | 1 | 1 | 0 | 0 | 0 | 0.4 |
| Alpha-toxoid | 12 | 3 | 4 | 3 | 1 | 1 | 0 | 1.41 |
| HPa | 12 | 10 | 2 | 0 | 0 | 0 | 0 | 0.16 |
| GPDa | 10 | 7 | 2 | 1 | 0 | 0 | 0 | 0.4 |
| tPFORa | 10 | 4 | 5 | 1 | 0 | 0 | 0 | 0.7 |
| FBAa | 10 | 4 | 6 | 0 | 0 | 0 | 0 | 0.6 |
Immunized groups that had significantly fewer chickens with lesions than the unimmunized vehicle-only control group (Fisher's exact test, P ≤ 0.05).
In experiment 2, HP alone offered significant protection against a moderate challenge, whereas GPD, tPFOR, and FBA did not (Table 4). Mock immunization with an unrelated purified recombinant fusion protein resulted in a mean lesion score similar to that for the unimmunized controls. The increased mean lesion score for the controls compared to those for the birds in experiment 1 appeared to reflect the increased duration of challenge.
TABLE 4.
Intestinal lesion scores of birds immunized with two injections intramuscularly and then infected with a moderate challenge with C. perfringens
| Protein | No. of chickens | No. of chickens with the following lesion scores:
|
Mean no. of chickens | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | 5+ | |||
| Vehicle-only controls | 18 | 1 | 4 | 9 | 2 | 1 | 1 | 2.05 |
| Mock controls | 10 | 0 | 1 | 6 | 3 | 0 | 0 | 2.20 |
| HPa | 17 | 10 | 3 | 4 | 0 | 0 | 0 | 0.64 |
| GPD | 17 | 3 | 8 | 6 | 0 | 0 | 0 | 1.17 |
| tPFOR | 18 | 7 | 4 | 3 | 3 | 0 | 1 | 1.33 |
| FBA | 18 | 2 | 7 | 6 | 2 | 0 | 1 | 1.66 |
The immunized group had significantly fewer chickens with lesions then the vehicle-only control group (Fisher's exact test, P ≤ 0.05).
In experiment 3, birds that received two initial injections of alpha-toxoid but a third injection with active alpha-toxin had the greatest protection against a heavy challenge (Table 5). Birds immunized with either HP or tPFOR also had significant protection against severe challenge. Although birds immunized with GPD, FBA, and the combination of GPD and HP had mean lesion scores less than those for the nonimmunized controls, no statistically significant protection was observed.
TABLE 5.
Intestinal lesion scores of birds immunized with three injections intramuscularly and then infected with a severe challenge with C. perfringens
| Protein | No. of chickens | No. of chickens with the following lesion scores:
|
Mean no. of chickens | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | 5+ | |||
| Vehicle-only controls | 22 | 0 | 5 | 5 | 6 | 4 | 2 | 2.68 |
| Alpha toxoid/toxina,b | 19 | 10 | 8 | 1 | 0 | 0 | 0 | 0.53 |
| HPa | 20 | 8 | 6 | 4 | 2 | 0 | 0 | 1.0 |
| GPD | 18 | 4 | 4 | 6 | 1 | 1 | 1 | 1.64 |
| tPFORa | 19 | 9 | 2 | 6 | 2 | 0 | 0 | 1.05 |
| GPD + HP | 19 | 5 | 5 | 7 | 1 | 1 | 0 | 1.36 |
Immunized groups that have significantly fewer chickens with lesions than the unimmunized vehicle-only control group (Fisher's exact test, P ≤ 0.05).
The birds in this group received alpha-toxoid in the first two injections and alpha-toxin in the third.
In experiment 4A, birds immunized with FBA had significant protection against a mild-moderate challenge compared to the level of protection for the unimmunized controls (Table 6). The mean lesion scores for the unimmunized controls in experiment 4B were comparable to the scores for the unimmunized controls in experiment 3 that also received a severe challenge.
TABLE 6.
Intestinal lesion scores of birds immunized with three injections intramuscularly and then infected with a mild-moderate or a severe challenge with C. perfringens
| Group and protein | No. of chickens | No. of chickens with the following lesion scores:
|
Mean no. of chickens | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 4+ | 5+ | |||
| Chickens infected with mild-moderate challenge | ||||||||
| Vehicle-only controls | 10 | 0 | 5 | 2 | 0 | 1 | 2 | 2.3 |
| FBAa | 13 | 4 | 6 | 1 | 1 | 0 | 1 | 1.23 |
| Chickens infected with severe challenge | ||||||||
| Vehicle-only controls | 11 | 0 | 3 | 2 | 3 | 1 | 2 | 2.72 |
| Alpha-toxinb | 10 | 0 | 2 | 4 | 4 | 0 | 0 | 2.2 |
| FBA | 14 | 1 | 4 | 9 | 0 | 0 | 0 | 1.57 |
The immunized group had significantly fewer chickens with lesions than the unimmunized vehicle-only control group (Fisher's exact test, P ≤ 0.05).
The birds in this group received three injections of alpha-toxin, in which the first and the third injections were with 20 μg and the second was reduced to 10 μg.
A visual summary of the mean lesion scores for the birds from all immunized groups that received different doses of antigens and challenges across different experiments, together with those for the concurrent unimmunized controls, is shown in Fig. 2.
FIG. 2.
Summary of mean lesion scores for birds from all immunized groups across different experiments, together with those for the concurrent unimmunized controls. VC, vehicle-only controls, A-tox, alpha-toxin; Sup, culture supernatant of C. perfringens; G+H, combination of GPD and HP; Exp, experiment; +, the birds in this group were challenged for 3 days and necropsied on day 6; ++, the birds in this group were given a severe challenge, like the one used in experiment 3; *, the immunized group had significantly fewer chickens with lesions than the unimmunized vehicle-only control group (Fisher's exact test, P ≤ 0.05).
Antibody titers in chicken sera and intestinal washings.
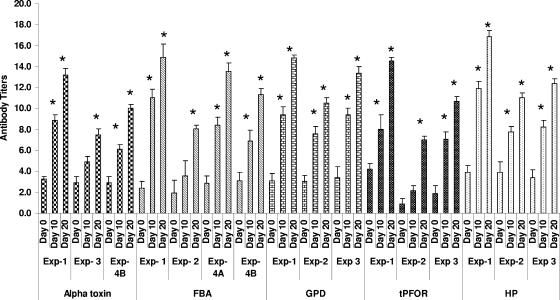
All the proteins used to immunize birds in the immunization experiments described produced significant antigen-specific serum antibody titers (Fig. 3) in comparison to the preimmunization titers. Birds immunized twice with a larger antigen quantity had lower titers than birds immunized three times with a smaller quantity. The protection provided in experiment 3 was not as marked as that provided in experiment 1, even though the antibody titers were generally similar. This difference was attributed to the difference in the severity of the challenge. There was a discrepancy between the titers of antibody to alpha-toxin and protection, since either alpha-toxoid- and alpha-toxin-immunized but nonprotected birds in experiments 1 and 4, respectively, had higher titers than the alpha toxoid- and alpha-toxin-immunized birds that were significantly protected in experiment 3.
FIG. 3.
Serum IgY ELISA titers of broiler chickens immunized intramuscularly with Clostridium perfringens purified proteins. Serum was collected at three time points: day 0, for determination of the preimmunization titer; day 10 (midexperiment); and day 20, for determination of the prechallenge titer. Exp, experiment. In experiment 4A, the birds were challenged for 3 days and necropsied on day 6. In experiment 4B, the birds were given a severe challenge, like the one used in experiment 3. *, significant titer values compared to the preimmunization titers (P ≤ 0.01).
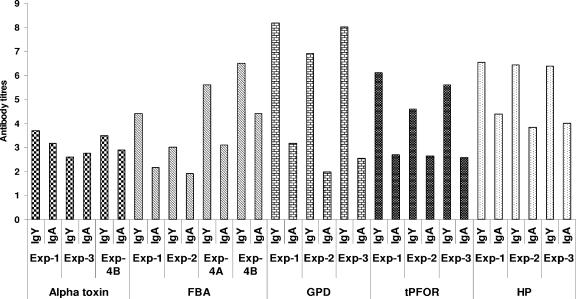
The intestinal antibody responses to all the proteins used for immunization showed higher IgY titers than IgA titers (Fig. 4), but the titers of both isotypes were markedly lower than those observed in the sera of the birds. However, the IgY and IgA titers were generally similar in birds immunized with alpha-toxoid and alpha-toxin (experiments 1, 3, and 4).
FIG. 4.
Intestinal IgY and IgA ELISA titers of broiler chickens immunized intramuscularly with Clostridium perfringens purified proteins. The samples analyzed were from pooled intestines collected from at least 10 chickens in each group. Exp, experiment. In experiment 4A, the birds were challenged for 3 days and necropsied on day 6. In experiment 4B, the birds were given a severe challenge, like the one used in experiment 3.
DISCUSSION
This study has shown, for the first time, that a degree of immunity to NE in broiler chickens can be produced by immunization with several different secreted C. perfringens proteins and that the degree of protection is a function of the severity of the challenge. All the proteins used in immunization, including alpha-toxin, offered significant protection, depending on the severity of the challenge. It seems that protection against NE lies in the secreted component of C. perfringens, since immunization with crude culture supernatant that included all proteins tested in the current study largely protected the birds against challenge. Of the five secreted proteins used for immunization, three proteins, namely, alpha-toxin, HP, and tPFOR, significantly protected the chickens against a heavy challenge, whereas the other two proteins, GPD and FBA, significantly protected the birds only against mild challenge. Nevertheless, a degree of protection against even severe challenge was apparent with the latter proteins.
The role of alpha-toxin in immunity to NE has been suspected but not previously clearly demonstrated. Priming with alpha-toxoid and boosting with active toxin offered the best protection, whereas immunization with three injections of either alpha-toxoid or of active toxin offered no protection (Tables 3 and 6). The failure of active toxin to protect birds against a heavy challenge may have resulted from the toxin's activity on immune system cells. The failure of alpha-toxoid to offer protection in experiment 1 may be the result of a degradation effect from creation of the toxoid on the protein that was observed on the SDS-polyacrylamide gel (data not shown). However, it is clear from Fig. 3 that use of the toxoid was adequate to induce antibodies sufficient for the birds to tolerate the active toxin given as a booster in experiment 3. The findings from the present study and an earlier study (15) suggest that antibodies to conformational (rather than linear) epitopes of alpha-toxin are critical for protection against NE. Achieving conformational but nontoxic epitopes in a vaccine may prove challenging (1, 7, 33). Although many studies have emphasized the importance of the nontoxic C-terminal domain in offering protection against experimental gas gangrene (5, 30, 34), some have shown that the neutralizing epitopes are on the N terminus (18). It seems likely that the positioning of the protective, neutralizing, conformational epitopes of alpha-toxin is subtle. For this reason, other immunogens such as those identified here may be more feasible candidates for use for immunization.
Perfringolysin O is a potent hemolytic cytolysin that mediates necrosis in the pathogenesis of clostridial gas gangrene (29) and is an important protective immunogen in mouse and guinea pig gas gangrene models (8). Our previous study suggested its possible role in NE immunity in broiler chickens (15). In the current study, the purified alpha-toxin (Sigma Laboratories) used to immunize birds had traces of perfringolysin O, which we identified using mass spectrometry (data not shown). However, the relative amounts in the otherwise apparently pure toxin preparation (as assessed by SDS-PAGE) were not quantified. It is possible that the protection observed in alpha-toxoid- and alpha-toxin-immunized birds (Table 5) can be partly attributed to perfringolysin O or even to traces of other but undetected immunogenic proteins and that a synergistic effect on the induction of neutralizing antibodies against both toxins may have contributed to better protection (2).
The observation that immunization with secreted proteins other than alpha-toxin provides to birds some immunity against NE highlights the likely involvement of several proteins in the pathogenesis of this infection. Both alpha-toxin and perfringolysin O are regulated in C. perfringens by the VirR-VirS two-component regulon (4, 26), a regulon that also controls the genes involved in energy metabolism, such as FBA, as well as others that may be indirectly involved in bacterial virulence (3, 13, 28). There is growing evidence that certain enzymes such as GPD and FBA, which are conventionally regarded as metabolic or “housekeeping” enzymes, may have a “dual role” in both the pathogenesis of and the immunity to other infections (6, 9, 17, 20, 22-24). Interestingly, a recent study showed that antibodies to FBA and GPD of Streptococcus pneumoniae showed age-dependent increased titers in the sera of children of different ages. Immunization of mice with recombinant GPD and FBA offered significant protection against respiratory challenge with virulent S. pneumoniae (17). A role for FBA in immunity to Onchocerca volvulus has also been suggested (21). Similarly, PFOR, an enzyme crucial for anaerobic energy metabolism, has been suggested to have a role in immunity to invasive amoebiasis (31). HP is a novel protein of C. perfringens of unknown function that has been identified in its genome (27) and that may have protease activity (zinc metallopeptidase), based on the analysis of its protein structure (15). It will be of interest to determine whether HP is a virulence determinant. It is apparent from the present immunization study that, besides alpha-toxin, other proteins (HP, GPD, tPFOR, and FBA) are important in some aspects of the host-pathogen interaction during the disease process. The recent demonstration that alpha-toxin is apparently not essential in the pathogenesis of NE (15) supports the suggestion that other proteins are involved.
Alpha-toxoid- and alpha-toxin-immunized, protected birds had lower antibody titers than toxoid-immunized birds that were not protected, suggesting the importance of conformational epitope-specific neutralizing antibodies in mounting a protective immune response. This implies the importance of the quality of the response in providing protection. The intestinal antibody response, as expected, was mainly dominated by IgY, since systemic immunization resulted in more antigen-specific IgY than antigen-specific IgA (Fig. 4) that reached the mucosal surfaces under inflammatory or necrotic conditions of the gut, allowing the seepage of serum IgY at the site of infection. It is also possible that a mucosal IgY response is more important in immunity to C. perfringens-induced NE, since a previous study showed that C. perfringens proteins to mucosal IgA in the intestinal washings collected from orally infection-immunized birds had weak reactivities (15). Immunization with HP, which significantly protected the birds against challenge doses of all severities, in all three experiments produced IgA titers higher than those in the other immunized groups. However, this association of IgA titers with protection was not evident in the groups immunized with either the alpha-toxoid and alpha-toxin or tPFOR, which also significantly protected the birds against a heavy challenge in experiment 3.
In conclusion, this is the first report that has demonstrated the immunizing ability of C. perfringens secreted proteins, including alpha-toxin, in protecting broiler chickens against NE. It seems likely that some of the secreted proteins that appear to be important in NE immunity also play a previously unsuspected role in the pathogenesis of the disease. This study also suggests that conformational epitopes of alpha-toxin are important in immunity and that antibody to alpha-toxin provides birds with better protection. Nevertheless, there are other proteins that might be suitable candidates for use in vaccines for the prevention of this important disease.
Acknowledgments
We thank the staff of the OMAFRA Isolation Facility, University of Guelph, for the housing and care of the broiler chickens.
This work was supported by the Ontario Ministry of Agriculture, Food, and Rural Affairs; by the Poultry Industry Council, Ontario, Canada; and by the Saskatchewan Chicken Industry Development Fund. We also thank the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada, and the Canadian Poultry Research Council of Canada for funding.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Alape-Giron, A., M. Flores-Diaz, I. Guillouard, C. E. Naylor, R. W. Titball, A. Rucavado, B. Lomonte, A. K. Basak, J. M. Gutierrez, S. T. Cole, and M. Thelestam. 2000. Identification of residues critical for toxicity in Clostridium perfringens phospholipase C, the key toxin in gas gangrene. Eur. J. Biochem. 2675191-5197. [DOI] [PubMed] [Google Scholar]
- 2.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 697904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banu, S., K. Ohtani, H. Yaguchi, T. Swe, S. T. Cole, H. Hayashi, and T. Shimizu. 2000. Identification of novel VirR/VirS-regulated genes in Clostridium perfringens. Mol. Microbiol. 35854-864. [DOI] [PubMed] [Google Scholar]
- 4.Ba-Thein, W., M. Lyristis, K. Ohtani, I. T. Nisbet, H. Hayashi, J. I. Rood, and T. Shimizu. 1996. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J. Bacteriol. 1782514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett, A. M., T. Lescott, R. J. Phillpotts, M. Mackett, and R. W. Titball. 1999. Recombinant vaccinia viruses protect against Clostridium perfringens alpha-toxin. Viral Immunol. 1297-105. [DOI] [PubMed] [Google Scholar]
- 6.Boel, G., H. Jin, and V. Pancholi. 2005. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect. Immun. 736237-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eaton, J. T., C. E. Naylor, A. M. Howells, D. S. Moss, R. W. Titball, and A. K. Basak. 2002. Crystal structure of the C. perfringens alpha-toxin with the active site closed by a flexible loop region. J. Mol. Biol. 319275-281. [DOI] [PubMed] [Google Scholar]
- 8.Efimova, M. G., V. A. Blagoveshchenskii, and B. V. Khatuntseva. 1982. Protective properties of theta-hemolysin obtained by affinity chromatography. Zh. Mikrobiol. Epidemiol. Immunobiol. 1287-92. [PubMed] [Google Scholar]
- 9.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, F. Randazzo, and G. Grandi. 2002. Gene expression profile in Neisseria meningitidis and Neisseria lactamica upon host-cell contact: from basic research to vaccine development. Ann. N. Y. Acad. Sci. 975202-216. [DOI] [PubMed] [Google Scholar]
- 10.Heier, B. T., A. Lovland, K. B. Soleim, M. Kaldhusdal, and J. Jarp. 2001. A field study of naturally occurring specific antibodies against Clostridium perfringens alpha-toxin in Norwegian broiler flocks. Avian Dis. 45724-732. [PubMed] [Google Scholar]
- 11.Ito, A. 1968. Alpha-toxoid of Clostridium perfringens. I. Purification and toxoiding of alpha-toxin of C. perfringens. Jpn. J. Med. Sci. Biol. 21379-391. [DOI] [PubMed] [Google Scholar]
- 12.Kaldhusdal, M., and A. Lovland. 2000. Necrotic enteritis: the economical impact of Clostridium perfringens is greater than anticipated. World Poultry 1650-51. [Google Scholar]
- 13.Kawsar, H. I., K. Ohtani, K. Okumura, H. Hayashi, and T. Shimizu. 2004. Organization and transcriptional regulation of myo-inositol operon in Clostridium perfringens. FEMS Microbiol. Lett. 235289-295. [DOI] [PubMed] [Google Scholar]
- 14.Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad, J. I. Rood, and R. J. Moore. 2006. The alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 746496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2006. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin. Vaccine Immunol. 131358-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 17.Ling, E., G. Feldman, M. Portnoi, R. Dagan, K. Overweg, F. Mulholland, V. Chalifa-Caspi, J. Wells, and Y. Mizrachi-Nebenzahl. 2004. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin. Exp. Immunol. 138290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan, A. J., E. D. Williamson, R. W. Titball, D. A. Percival, A. D. Shuttleworth, J. W. Conlan, and D. C. Kelly. 1991. Epitope mapping of the alpha-toxin of Clostridium perfringens. Infect. Immun. 594338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovland, A., M. Kaldhusdal, K. Redhead, E. Skjerve, and A. Lillehaug. 2004. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 3383-92. [DOI] [PubMed] [Google Scholar]
- 20.Madureira, P., M. Baptista, M. Vieira, V. Magalhaes, A. Camelo, L. Oliveira, A. Ribeiro, D. Tavares, P. Trieu-Cuot, M. Vilanova, and P. Ferreira. 2007. Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J. Immunol. 1781379-1387. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy, J. S., M. Wieseman, J. Tropea, D. Kaslow, D. Abraham, S. Lustigman, R. Tuan, R. H. Guderian, and T. B. Nutman. 2002. Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect. Immun. 70851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modun, B., and P. Williams. 1999. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect. Immun. 671086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pancholi, V., and G. S. Chhatwal. 2003. Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 293391-401. [DOI] [PubMed] [Google Scholar]
- 24.Pancholi, V., and V. A. Fischetti. 1997. Regulation of the phosphorylation of human pharyngeal cell proteins by group A streptococcal surface dehydrogenase: signal transduction between streptococci and pharyngeal cells. J. Exp. Med. 1861633-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott, J. F. 1979. The prevention of experimentally induced necrotic enteritis in chickens by avoparcin. Avian Dis. 231072-1074. [PubMed] [Google Scholar]
- 26.Shimizu, T., W. Ba-Thein, M. Tamaki, and H. Hayashi. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 1761616-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu, T., K. Shima, K. Yoshino, K. Yonezawa, T. Shimizu, and H. Hayashi. 2002. Proteome and transcriptome analysis of the virulence genes regulated by the VirR/VirS system in Clostridium perfringens. J. Bacteriol. 1842587-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens, D. L., and A. E. Bryant. 1993. Role of theta toxin, a sulfhydryl-activated cytolysin, in the pathogenesis of clostridial gas gangrene. Clin. Infect. Dis. 16(Suppl. 4)S195-S199. [DOI] [PubMed] [Google Scholar]
- 30.Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-Schroer, and A. E. Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 190767-773. [DOI] [PubMed] [Google Scholar]
- 31.Thammapalerd, N., D. Kotimanusvanij, M. Duchene, J. A. Upcroft, R. Mitchell, A. Healey, N. Samarawickrema, S. Tharavanij, G. Wiedermann, and P. Upcroft. 1996. Pyruvate: ferredoxin oxidoreductase from Entamoeba histolytica recognized by a monoclonal antibody. Southeast Asian J. Trop. Med. Public Health 2763-70. [PubMed] [Google Scholar]
- 32.Thompson, D. R., V. R. Parreira, R. R. Kulkarni, and J. F. Prescott. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 11325-34. [DOI] [PubMed] [Google Scholar]
- 33.Titball, R. W., A. M. Fearn, and E. D. Williamson. 1993. Biochemical and immunological properties of the C-terminal domain of the alpha-toxin of Clostridium perfringens. FEMS Microbiol. Lett. 11045-50. [DOI] [PubMed] [Google Scholar]
- 34.Williamson, E. D., and R. W. Titball. 1993. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene. Vaccine 111253-1258. [DOI] [PubMed] [Google Scholar]