Abstract
We evaluated whether four recombinant antigens previously used for vaccination against experimental infection with Leishmania (Leishmania) major could also induce protective immunity against a challenge with Leishmania (Viannia) braziliensis, the species responsible for 90% of the 28,712 annual cases of cutaneous and mucocutaneous leishmaniasis recorded in Brazil during the year of 2004. Initially, we isolated the homolog genes encoding four L. (V.) braziliensis antigens: (i) homologue of receptor for activated C kinase, (ii) thiol-specific antioxidant, (iii) Leishmania elongation and initiation factor, and (iv) L. (L.) major stress-inducible protein 1. At the deduced amino acid level, all four open reading frames had a high degree of identity with the previously described genes of L. (L.) major being expressed on promastigotes and amastigotes of L. (V.) braziliensis. These genes were inserted into the vector pcDNA3 or expressed as bacterial recombinant proteins. After immunization with recombinant plasmids or proteins, BALB/c mice generated specific antibody or cell-mediated immune responses (gamma interferon production). After an intradermal challenge with L. (V.) braziliensis infective promastigotes, no significant reduction on the lesions was detected. We conclude that the protective immunity afforded by these four vaccine candidates against experimental cutaneous leishmaniasis caused by L. (L.) major could not be reproduced against a challenge with L. (V.) braziliensis. Although negative, we consider our results important since they suggest that studies aimed at the development of an effective vaccine against L. (V.) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World, should be redirected toward distinct antigens or different vaccination strategies.
The protozoan parasite Leishmania (Viannia) braziliensis commonly causes localized cutaneous leishmaniasis (CL); however, a chronic mucosal leishmaniasis (ML) may develop in some infected individuals, with severe and progressive manifestations (reviewed in reference 11). At present, this species accounts for more than 90% of the 28,712 annual cases of CL and ML recorded in Brazil in 2004 (7). Although chemotherapies for CL and ML do exist, there are several limitations: (i) drug treatment is rarely affordable by those who need them, (ii) drug treatment requires daily injections of the drug for weeks, (iii) drug treatment can be associated with side effects, and (iv) drug resistance is becoming an increasing problem (13, 24; reviewed in references 3 and 32). To make matters worse, control of CL and ML is problematic due to the sylvatic nature of both vectors and reservoirs, making insecticide spraying and the elimination of reservoirs particularly difficult (26). Due to these difficulties, in the long run, the development of an effective vaccine may help both the prevention and the treatment of CL and ML caused by L. (V.) braziliensis.
Most studies conducted thus far in vaccination against CL used genes and/or antigens isolated and characterized from Leishmania (Leishmania) major or L. (L.) amazonensis (8, 31; reviewed in references 9 and 21). Among the leading candidates to the development of a vaccine against CL, there are homologues of the receptor for activated C kinase protein (LACK or p36), Leishmania elongation and initiation factor (LeIF), L. (L.) major stress inducible protein 1 (LmSTI1), and thiol-specific antioxidant (TSA) from L. (L.) major (5, 6, 10, 14, 15, 28, and 30).
Based on these promising prospects, the present study was designed to test whether four recombinant antigens previously used for vaccination against experimental infection with L. (L.) major (LACK, LmSTI1, LeIF, and TSA) could also generate protective immunity against an intradermal (i.d.) challenge with L. (V.) braziliensis. We considered this question very important because, as mentioned above, this species is responsible for most cases of CL and ML in Brazil and in the New World.
MATERIALS AND METHODS
Parasites and mice.
L. (V.) braziliensis (MHOM/BR/1975/M2903 and MHOM/BR/01/BA788) and L. (L.) major (Friedlin strain) promastigotes were grown at 26°C in 199 medium (Life Technologies) supplemented with 40 mM HEPES, 0.1 mM adenine, 2 mM l-glutamine, 5 mg of hemin/ml (in 50% triethanolamine), 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 10% heat-inactivated fetal bovine serum (all from Life Technologies). To L. (V.) braziliensis promastigote culture was added 2% of sterile human male urine. The parasites were isolated from stationary phase in culture (5 to 6 days old). L. (V.) braziliensis amastigotes were obtained from infected mouse bone marrow-derived macrophage culture (1). Amastigote suspensions of L. (V.) braziliensis were prepared by disruption of infected macrophages scraped from the flask 48 h after infection with promastigotes. These cells were ruptured through a 22-gauge needle and were centrifuged at 250 × g for 10 min; the resulting supernatant was centrifuged at 1,400 × g for 10 min, and the pellet was resuspended in RPMI. The suspension was kept under agitation for 4 h at room temperature and centrifuged at 250 × g for 10 min. The final pellet contained purified amastigotes that were essentially free of contamination by other cells (2).
BALB/c mice were obtained from the Universidade de São Paulo, São Paulo, Brazil. The mice were maintained under pathogen-free conditions and used at 6 to 8 weeks of age. The protocols for the experiments with mice were approved by the Committee of Ethics of the Federal University of São Paulo-Escola Paulista de Medicina.
Genomic DNA isolation, PCR amplification, DNA cloning, and sequencing.
Genomic DNA from promastigote forms of L. (V.) braziliensis was extracted essentially as described previously (4). Amplification of the open reading frames (ORFs) of the antigens from L. (V.) braziliensis was carried out with specific primers designed according to the sequences available at GenBank. Briefly, for PCR we used 500 ng of L. (V.) braziliensis genomic DNA, 200 pmol of each primer, 10 mM deoxynucleoside triphosphate mix, 3 mM MgCl2, and Platinum Taq DNA polymerase (Gibco-BRL) in a final volume of 50 μl. The reaction was performed for a first step of 5 min at 94°C and 35 cycles, with the following thermal profile: 30 s at 94°C, 30 s at 50°C, and 1 to 2 min at 68°C, with a final step of 7 min at 4°C. PCR products were placed on to a 1% agarose gel and excised from it, and the DNA was purified with GeneClean II kit (Bio 101). The products were cloned into the pMOSBlue vector (Amersham-Pharmacia). The recombinant clones selected were sequenced.
Automatic sequencing of double-stranded DNA was performed by using the BigDye terminator cycle sequencing kit (Perkin-Elmer) on an ABI Prism 377 sequencer (Perkin-Elmer). DNA and predicted amino acid sequences were analyzed by using the DNASTAR package version 5.00 (DNASTAR, Inc.). Alignments were produced by using the CLUSTAL W program.
For a bacterial expression vector, we used the pHIS, plasmid kindly provided by Peter Sheffield (University of Virginia) (27). The ORFs of the genes containing the described restriction site sequences, for directional cloning, were removed from pMOSBlue vector by treatment with the specific restriction enzymes and linked into the pHIS vector treated with the same enzymes. For eukaryotic expression the commercially available plasmid pcDNA3 (Invitrogen, San Diego, CA) was used as the mammalian expression vector. The recombinant plasmids were obtained as described above using the ORFs of the genes containing the antigens. Immunization plasmids were obtained by inserting the nucleotide sequence encoding the mouse immunoglobulin κ chain signal peptide (IgSP) in frame with the 5′ nucleotide sequence of the genes. The nucleotide sequence encoding IgSP was generated as described previously (4). The recombinant plasmids were produced in Escherichia coli DH5α and purified on cesium chloride density gradients according to standard protocols (25). The DNA concentration was estimated to be 260 nM. Each plasmid DNA was diluted in sterile phosphate-buffered saline (PBS) to a concentration of 1 mg/ml.
Northern blot analysis.
Total RNA was isolated from 108 promastigotes and amastigotes of L. (V.) braziliensis and promastigotes of L. (L.) major using TRIzol reagent (Gibco-BRL) according to the manufacturer's instructions. For Northern blot analyses, total RNA was resuspended in RNase-free water; equilibrated in a buffer containing formamide, formaldehyde, and morpholinepropanesulfonic acid; heated for 30 min at 70°C; and fractioned in 1% agarose formaldehyde gels (26). An RNA ladder ranging from 0.24 to 9.5 kb was used as size marker (Life Technologies). Gels were photographed and transferred to membranes using 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and the RNA was fixed and cross-linked to Hybond-N membranes by UV irradiation. Filters were prehybridized for 2 h at 42°C in a hybridization solution containing 50% (vol/vol) formamide (Sigma), 5× SSC, 5× Denhardt solution, 10 μg of tRNA (Sigma) per ml, and 100 μg of salmon testes DNA (Sigma) per ml. Filters were hybridized overnight at the same temperature with the indicated probes labeled with [α-32P]dCTP (Amersham) by using an oligolabeling kit (Pharmacia Biotech) according to the manufacturer's instructions. The filters were washed in SSC solutions (2× to 0.1×) containing 0.1% (wt/vol) sodium dodecyl sulfate (SDS) from 42 to 50°C and exposed to Hyperfilm (Amersham-Pharmacia) or a Typhoon system (Amersham-Pharmacia).
Expression and purification of the recombinant proteins.
The recombinant plasmids obtained were transformed into E. coli BL21(DE3) host cells (Novagen, Madison, WI). Protein expressions were performed as described previously (30). Cells were harvested from 500-ml batch cultures by centrifugation and resuspended in 20 ml of binding buffer (0.1 M sodium phosphate [pH 8.0], 10 mM Tris-HCl [pH 8.0]) containing 2 mM phenylmethylsulfonyl fluoride. E. coli was lysed by adding 15 mg of lysozyme and rocking for 30 min at 4°C, following sonication (four times for 30 s each time), and then spun at 12,000 rpm for 30 min to pellet the cells. Only the TSA protein was obtained as a soluble protein. The protocol was continued to obtain the insoluble proteins LACK, LeIF, and LbSTI1. Briefly, the inclusion bodies were washed three times in 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} in 10 mM Tris-HCl (pH 8.0). The samples were spun at 12,000 rpm for 30 min to pellet the inclusion bodies. These were finally solubilized in 20 ml of binding protein containing 8 M urea.
All of the recombinant antigens with His tag residues were batch bound to Ni-nitrilotriacetic acid agarose resin (5 ml per 500-ml induction) by rocking at room temperature for 1 h, and the complexes were passed over a column. The flowthrough was passed twice over the same column, and it was washed three times with 30 ml each of wash buffer (0.1 M sodium phosphate and 10 mM Tris-HCl [pH 6.3]) also containing 8 M urea. Bound protein was eluted with 30 ml of 100 mM imidazole in wash buffer, and 3-ml fractions were collected. At this final step the samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).
Immunizations.
To perform the indirect immunofluorescence assay (IIA), BALB/c mice were injected in the hind footpad (subcutaneously) with 10 μg of each purified recombinant proteins (His6-LACK, His6-TSA, His6-LbSTI, and His6-LeIF) in Freund complete adjuvant (CFA). After 2 weeks the mice were injected at a location 1 cm distal to the rump with 10 μg of each recombinant protein with incomplete Freund adjuvant (IFA). Mice were bled with 7 days after the first dose and 10 days after the last dose. The plasma fraction was obtained and stored at −20°C.
DNA immunization was performed as described earlier (4), using the recombinant DNA plasmids either alone or together. Empty pcDNA3 plasmid was used as a control. Both tibialis anterioris muscles were injected with 3.5 μg of cardiotoxin (Sigma). Five days later, 50 μg of plasmid DNA were injected intramuscularly at the same sites as for the cardiotoxin injection (a total of 100 μg of plasmid DNA per mouse). The subsequent doses consisted of the same amount of plasmid DNA injected 2 and 4 weeks after the first immunizing dose.
The protocol for immunization with the recombinant proteins was performed by the intraperitoneal administration of 25 μg of each purified protein in the presence of CpG ODN 1826 (10 μg/dose/mouse; Coley Pharmaceuticals, Wellesley, MA) and 2% aluminum hydroxide (alum; Superfos Biosector, Denmark), i.e., 2.5 μl/μg of recombinant protein. Some animals received all four proteins together depending on the immunization strategy. The control group received only CpG ODN 1826 and alum in PBS. Immunizations were performed by the administration of three doses given 2 weeks apart.
The immunization protocol before the i.d. challenge consisted of two doses of the recombinant DNA plasmids followed by one or two doses of the purified recombinant proteins. At 2 weeks after the last dose, mice were challenged with promastigotes of L. (V.) braziliensis. BALB/c mice were challenged i.d. as described previously (20). Briefly, cultured promastigote forms of L. (V.) braziliensis (MHOM/BR/01/BA788) were adjusted to a concentration of 107 parasites/ml in PBS, and 10 μl was injected into the ear dermis of each mouse. The infection was monitored for 9 to 10 weeks, and lesion formation was measured once a week with a direct caliper.
IIA.
Macrophages (105 cells/coverslip) were infected with stationary promastigotes of L. (V.) braziliensis (at a 10:1 parasite/cell ratio); 48 h later the coverslips were used in IIA. Promastigotes of L. (V.) braziliensis or infected macrophages were fixed for 40 min in 3.5% paraformaldehyde-PBS and washed three times with PBS, and the solution was adjusted to a concentration of 107 parasites per ml. Portions (10 μl) of the parasite suspension were layered onto round, coated coverslips and allowed to stand overnight at room temperature. Coverslips were stored at 20°C until use. Slides containing promastigotes or amastigote-infected macrophages were blocked with PBS containing 2% gelatin and 0.1% NaN3 and permeabilized with 0.1% saponin (BDH; Amersham, United Kingdom) in 0.2 gelatin and 0.1% NaN3 (PGN) and then incubated with different sera from mice immunized with recombinant proteins (LACK, TSA, LbSTI, or LeIF) diluted to 1:100 in PGN for 1 h at room temperature, washed three times with PBS, and developed with rabbit immunoglobulin G (IgG) Cy3-conjugated anti-mouse IgG (Sigma Chemical Co., St. Louis, MO) diluted 1:100 in PGN with 10 μM DAPI (4′,6′-diamidino-2-phenylindole) for an 1 h at room temperature.
Infected macrophages were incubated with a pool of sera from patients with mucocutaneous leishmaniasis diluted 1:100 for 1 h at room temperature, washed three times with PBS, and then incubated with goat IgG fluorescein isothiocyanate (FITC)-conjugated anti-human IgG (Sigma) diluted 1:50 for 1 h in the presence of 10 μM DAPI. This DNA dye preferentially labels condensed DNA and clearly demonstrates host cell nucleoli and parasite kinetoplasts. Control uninfected macrophages cells were subjected to the same procedure. After three washes with PBS, the coverslip and promastigotes onto coverslips were mounted in glycerol buffered with 0.1 M Tris (pH 8.6) and 0.1% paraphenylenediamine to reduce bleaching and observed by using an Optishot-2 fluorescence microscope (Nikon). Images were acquired on a Bio-Rad 1024-UV confocal system attached to a Zeiss Axiovert microscope using a 63× N.A. 1.4 Phan-Apochromatic (differential interference contrast) oil immersion objective.
Immunoblot.
Extracts from L. (V.) braziliensis promastigotes (30 μg of protein/lane) were subjected to SDS-12% PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% powdered skim milk in PBS and incubated for 1 h with sera from BALB/c mice immunized with the recombinant proteins diluted 1:50. After a washing step with 0.05% Tween 20 in PBS, the nitrocellulose strips were incubated with peroxidase-conjugated anti-mouse and then developed with diaminobenzidine and H2O2.
Immunological assays.
Enzyme-linked immunosorbent assay (ELISA) was performed essentially as described earlier (4).
Spleen cells collected from immunized BALB/c mice 2 weeks after the third immunizing dose were cultured in RPMI medium supplemented with 10 mM HEPES, 2 mM l-glutamine, 5 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, 1% (vol/vol) nonessential amino acid solution, and 1% (vol/vol) minimal essential medium vitamin solution (all purchased from Life Technologies); 100 U of penicillin and streptomycin (Sigma) per ml; and 10% (vol/vol) of fetal bovine serum (pH 7.4; HyClone, Logan, UT). The cultures were maintained at 37°C in an atmosphere containing 5% CO2. The concentration of cells was 5 × 106 viable cells per ml. The assay was performed in 96-well flat-bottom plates (Costar); 0.2 ml of cell suspension was pipetted into each well, and the respective antigens (recombinant proteins) were added at a concentration of 10 μg/ml. We collected supernatants for cytokine determination after 3 days in culture. Each determination was done in triplicate; the results are reported as means ± the standard deviations (SD). The concentration of gamma interferon (IFN-γ) was estimated by a capture ELISA using commercially available antibody and recombinant cytokine (Pharmingen, San Diego, CA) as described previously (23). The concentration of cytokine in each sample was determined from standard curves executed in parallel with known concentrations of IFN-γ. The detection limit of the assay was 0.2 ng/ml.
GenBank accession numbers.
The GenBank accession numbers for the lack gene are as follows: AF363978.1, L. (V.) braziliensis; AF034804, L. (L.) major; AF363974.1, L. (L.) donovani; L. (L.) amazonensis, EU016104; and L. (L.) chagasi, EU016105. The GenBank accession numbers for the tsa gene are as follows: AF069386, L. (L.) major; AF312397, L. (L.) chagasi; and AAL25847.1, L. (L.) donovani. The GenBank accession numbers for the leif gene are as follows: Q25225, L. (V.) braziliensis; and A81464, L. (L.) major. The GenBank accession numbers for the sti1 gene are as follows: AAB37318, L. (L.) major; and AAY88229.1, L. (L.) donovani.
RESULTS
Identification and characterization of the homolog genes lack, lmsti1, leif, and tsa from L. (V.) braziliensis.
The deduced amino acid sequences of the different genes isolated from L. (V.) braziliensis were compared to previously described genes of Leishmania. The deduced amino acid sequence of the lack ORF was almost identical to the published sequence of L. (V.) braziliensis, being different in only 11 amino acids, four of them with conservatives modifications (Table 1). The previously described immunodominant T-cell epitope recognized by CD4+ T cells from H-2d mice (amino acids 156 to 173) was invariant (19).
TABLE 1.
Comparison of the predicted amino acids sequences of the genes lack, lmsti1, leif, and tsa cloned from L. (V.) braziliensis or other species of Leishmania
| Gene | Predicted amino acidsa
|
||||
|---|---|---|---|---|---|
| L. (V.) braziliensisb | L. (L.) major | L. (L.) amazonensis | L. (L.) chagasi | L. (L.) donovani | |
| lack | 43, 58, 99, 115, 148, 183, 231, 237, 254, 256, 277 | 31, 43, 58, 99, 148, 183, 231, 237, 254, 256, 277 | 43, 58, 99, 148, 183, 231, 237, 254, 256, 277 | 43, 58, 99, 183, 231, 237, 254, 256, 277, 312 | 43, 48, 99, 183, 231, 237, 254, 256, 277 |
| tsa | NA | 8, 14, 18, 32, 56, 58, 63, 64, 70, 74, 75, 78, 91, 99, 101, 111, 113, 115, 120, 122, 123, 136, 138, 144, 152, 181, 182, 188, 189, 191, 192 | NA | 5, 8, 10, 18, 33, 58, 61, 62, 63, 64, 70, 74, 75, 78, 91, 99, 101, 111, 113, 120, 123, 136, 138, 139, 144, 181, 182, 188, 189, 191, 192 | 5, 8, 10, 18, 33, 58, 61, 62, 63, 64, 70, 74, 75,78, 91, 99, 101, 111, 113, 120, 123, 136, 138, 139, 144, 181, 182, 188, 189, 191, 192 |
| leif | 363 | 2, 4, 6, 7, 125, 149, 384 | NA | NA | NA |
| sti1 | NA | 60, 68, 98, 158-220*, 250, 254, 270, 310, 314, 530 | NA | NA | 61, 69, 100, 106, 158-220*, 251, 271, 287, 310, 312, 316, 379 |
The positions of amino acid residue substitutions are based on the predicted amino acid sequences obtained by CLUSTAL W alignments of the lack, tsa, lmsti1, and leif genes from different species of Leishmania. *, predicted amino acids between positions 158 and 220 of sti1 (for details, see Fig. 1).
The L. (V.) braziliensis lack and leif genes had already been cloned from different parasite strains. NA, gene sequence not available in GenBank.
The tsa gene cloned from L. (V.) braziliensis had not been previously reported. Comparing the complete deduced amino acid sequence from L. (V.) braziliensis to the L. (L.) major sequence (35), we observed 83% of identity with 32 amino acid substitutions, with 17 being conservative (Table 1).
The deduced amino acid sequences of the lbsti1 ORF cloned from L. (V.) braziliensis had the amino- and the carboxyl-terminal regions highly conserved compared to the same gene from L. (L.) major, with only a few substitutions (Table 1) (34, 36). Interestingly, within the region comprising the amino acids 158 to 220 there are several amino acid modifications. In this region, there are 30 different amino acids, with seven conservative modifications. Also, the loss of two amino acid residues was observed in the L. (V.) braziliensis sequence (Fig. 1).
FIG. 1.
Comparison of the predicted amino acids 158 to 220 of the sti1 genes from L. (V.) braziliensis, L. (L.) major, or L. (L.) donovani. Alignments were obtained by the CLUSTAL W method. Residues that are different in the predicted amino acid sequences of L. (V.) braziliensis are boxed with a black background. Positions with substitutions between the L. (L.) major and L. (L.) donovani are boxed with gray background.
The leif gene we cloned from L. (V.) braziliensis displayed 100 or 98% identity with the previously described ORFs from L. (V.) braziliensis or L. (L.) major, respectively (29). In the latter case, we found seven amino acid substitutions (five being conservative) over their entire sequence (Table 1). The deduced amino acid sequence retained a series of conserved motifs described previously as characteristic of the DEAD box family of RNA helicases (29).
Expression of lack-, tsa-, Lbsti-, and leif-coding ORFs during the L. (V.) braziliensis life cycle.
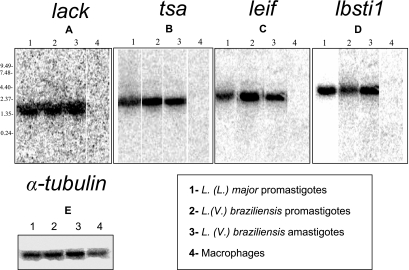
In order to characterize the transcripts expressed by the different forms of L. (V.) braziliensis, Northern blotting was performed with total RNA that was hybridized with the coding ORFs of lack, tsa, Lbsti, leif, and α-tubulin (positive control) (12). As shown in Fig. 2, both life stages of L. (V.) braziliensis and promastigotes of L. (L.) major (positive control) displayed bands corresponding to transcript sizes of approximately 1.5 to 2.0 kb (lack, Fig. 2A), 2.0 kb (tsa, Fig. 2B), 2.5 to 3.0 kb (leif, Fig. 2C), and 2.5 kb (lbsti1, Fig. 2D). The hybridization was specific for the parasite RNA, since the RNA from uninfected macrophages failed to hybridize with lack, tsa, Lbsti, and leif probes. All RNA samples from the different forms of the parasite and control uninfected macrophages hybridized with α-tubulin probe used as positive controls (Fig. 2E).
FIG. 2.
RNA expression of lack, tsa, leif, or Lbsti1 genes from L. (V.) braziliensis. Northern blot analysis was performed with lack (A), tsa (B), leif (C), lbsti (D), and α-tubulin (E) (12) ORFs as probes. Each lane contains 10 μg of total RNA of promastigotes of L. (L.) major (lane 1, positive control), promastigotes (lane 2) or amastigotes (lane 3) of L. (V.) braziliensis, or uninfected cultured macrophages (lane 4, negative control)/lane.
IIA for detection of LACK, TSA, LbSTI1, and LeIF antigens on amastigote and promastigote forms of L. (V.) braziliensis.
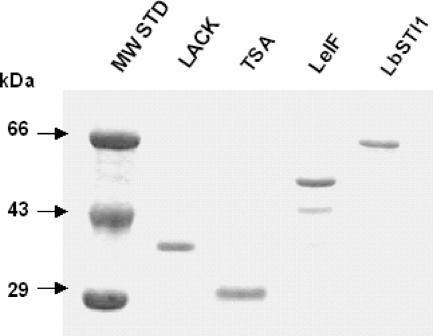
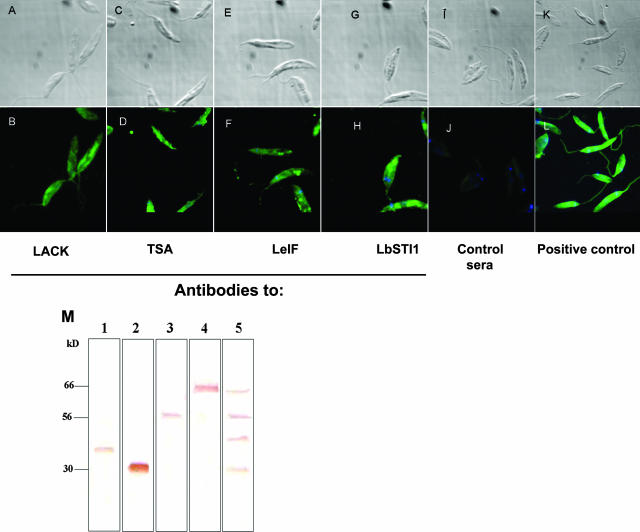
The coding ORFs of lack, tsa, Lbsti, or leif were subcloned in the pET22(+) vector, and the recombinant protein was expressed and purified as described in Materials and Methods. Figure 3 shows the SDS-PAGE analyses of the purified recombinant proteins. Each protein migrated as a single band (except the LeIF protein) and according to the expected molecular mass. To determine whether these four antigens were in fact expressed by promastigotes and amastigotes of L. (V.) braziliensis, we generated specific polyclonal antibodies to the different antigens. By IIA, it was possible to determine that promastigote forms of L. (V.) braziliensis (MHOM/BR/1975/M2903 strain) were specifically recognized by antibodies from mice immunized with each recombinant protein (Fig. 4). Antibody recognition was specific to the recombinant proteins because sera from mice immunized with CFA or IFA alone failed to react with promastigotes (Fig. 4J). Immunoblot analysis of the parasite extract confirmed that each antigen was recognized by the specific immune sera raised by mouse recombinant protein immunization (Fig. 4M).
FIG. 3.
SDS-PAGE analyses of purified bacterial recombinant proteins from L. (V.) braziliensis. Proteins were submitted to SDS-PAGE under reducing conditions and stained with Coomassie blue. MW, molecular mass standard. The amount of each recombinant protein was approximately 600 ng per lane.
FIG. 4.
IIA recognition of promastigotes of L. (V.) braziliensis by immune sera from mice immunized with recombinant proteins. Parasites were incubated with a pool of sera derived from mice immunized with the respective recombinant protein and imaged under Nomarski differential interference contrast (A, C, E, G, I, and K) or fluorescence (B, D, F, H, J, and L). Magnification, ×1,000. Control sera was obtained from mice immunized with CFA or IFA alone. (M) Immunoblot of promastigote extract (30 μg of protein/lane) stained with a pool of sera from BALB/c mice immunized with the different recombinant proteins. Lanes: 1, LACK-immunized mice; 2, TSA-immunized mice; 3, LeIF-immunized mice; 4, LbSTI1-immunized mice; 5, mice immunized with all four antigens.
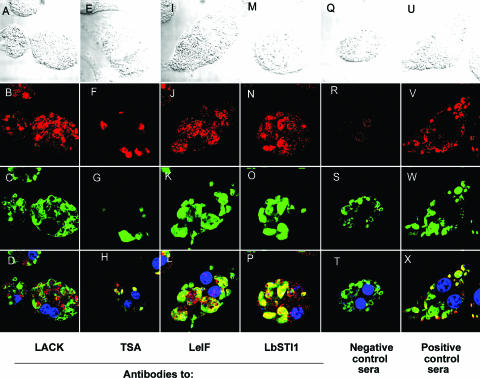
Subsequently, we performed IIA analysis with macrophages infected with L. (V.) braziliensis (MHOM/BR/1975/M2903 strain) as substrates. Amastigotes were specifically recognized by antibodies from mice immunized with each recombinant protein (Fig. 5). Antibody recognition was specific to the recombinant proteins because sera from mice immunized with CFA alone failed to react with control macrophages (data not shown) and infected macrophages (Fig. 5R). In order to visualize the amastigotes clearly, these infected macrophages were costained with sera from humans infected with L. (V.) braziliensis.
FIG. 5.
IIA recognition of amastigotes of L. (V.) braziliensis by immune sera from mice immunized with recombinant proteins. Mouse bone marrow macrophages infected with amastigotes of L. (V.) braziliensis were incubated with mouse antibodies specific to LACK (A to D), TSA (E to H), LeiF (I to L), or LbSTI1 (M to P). Additional slides were stained with negative (Q to T) or positive (U to X) control sera. All slides were costained with a pool of sera from humans infected with ML and with DAPI. Secondary antibodies to mouse IgG or human IgG were labeled with rhodamine (red fluorescence) or anti-FITC (green fluorescence), respectively. The images were captured by using Nomarski differential interference contrast (A, E, I, M, Q, and U) or fluorescence for rhodamine (B, F, J, N, R, and V) or FITC (C, G, R, O, S, and W). Fluorescence images for rhodamine, FITC, and DAPI (blue) were merged in panels D, H, L, P, T, and X. Magnification, ×1,000. Negative control sera were obtained from mice immunized with CFA or IFA alone.
Immune response generated by immunization with recombinant antigens from L. (V.) braziliensis.
To characterize the immune response generated by different immunization strategies using the L. (V.) braziliensis antigens, we immunized mice with plasmids encoding each antigen or the respective recombinant proteins. Recombinant proteins were administered in the presence of CpG ODN 1826 and alum.
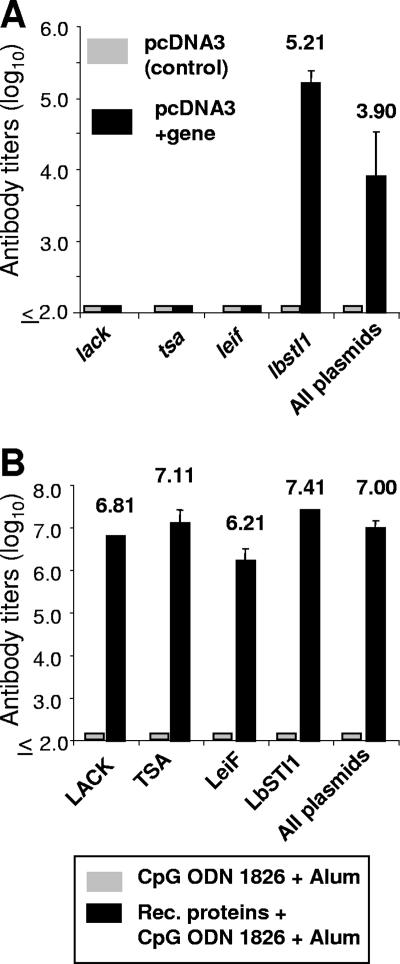
In BALB/c mice immunized with each plasmid DNA individually or a combination of all plasmids we only observed specific antibodies to the LbSTI1 antigen. This immune response was present in mice immunized with the lbsti1 gene or all of the genes together (Fig. 6A). In both cases, a predominance of IgG2a over IgG1 was observed. The ratios of IgG1 to IgG2a were 0.93 and 0.76 in mice immunized with the lbsti1 gene or all genes together, respectively. These immune sera were also tested by IIA. We observed that sera from DNA vaccinated mice failed to recognize promastigotes (data not shown).
FIG. 6.
Antibody immune response of mice immunized with eukaryotic expression plasmids or bacterial recombinant proteins expressing L. (V.) braziliensis antigens. (A) BALB/c mice (n = 6) were immunized with three doses of plasmids containing the indicated genes or pcDNA3 (control empty plasmid). Two weeks after the last immunization, blood samples were collected, and the sera were assayed by ELISA for the presence of antibodies to the respective recombinant proteins. Mice immunized with all plasmids were tested against the LbSTI1 recombinant protein only. (B) BALB/c mice (n = 7) were immunized with three doses of the indicated recombinant protein or adjuvant only (CpG ODN 1826 plus alum). Two weeks after the last immunization, blood samples were collected, and the sera were assayed by ELISA for the presence of antibodies to the respective recombinant proteins. Mice immunized with all proteins were tested in wells coated with all recombinant proteins. The results are presented as means ± the SD of reciprocal antibody dilutions (log10) providing an optical density at 492 nm greater than 0.1.
In BALB/c mice immunized with each recombinant protein individually or a combination of all of them we detected high titers of antibodies to the different antigens in each group of immunized mice (Fig. 6B). The ratios of IgG1 to IgG2a for each mouse group were as follows: 1.3, LACK; 0.92, TSA; 1.18, LeIf; 1.45, LbSTI1; and 1.48, all antigens. These immune sera were also tested by IIA. We observed that sera from recombinant protein-vaccinated mice strongly recognized promastigotes (data not shown). The antibody production was specific since the injection of pcDNA3 or adjuvant alone failed to elicit specific antibodies to any Leishmania antigen.
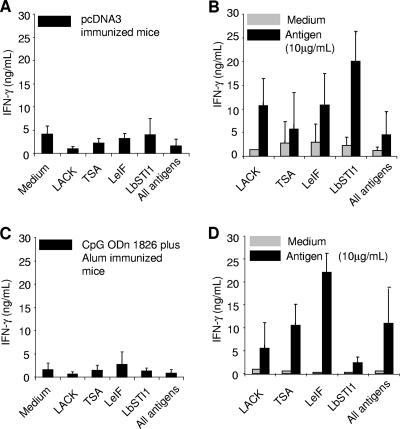
We also measured the IFN-γ produced by spleen cells from mice immunized with the different recombinant antigens as plasmid DNA or recombinant proteins. Upon in vitro restimulation with recombinant proteins, we detected antigen-specific IFN-γ production in cultured spleen cells from most groups of mice immunized with the distinct antigen either as plasmid DNA or recombinant protein (Fig. 7B and D). However, after antigenic stimulation in vitro, spleen cells from mice immunized with the tsa gene or the LbSTI1 recombinant protein failed to produce levels of IFN-γ that were significantly different from those of control cultures (Fig. 7A and C). The antigen-specific IFN-γ production was dependent on the immunization with the Leishmania antigens since spleen cells from control mice immunized with pcDNA3 (Fig. 7A) or CpG ODN 1826 and alum (Fig. 7C) failed to secrete this cytokine in the presence of the different recombinant antigens.
FIG. 7.
IFN-γ secretion by spleen cells from BALB/c mice immunized with different plasmids or recombinant antigens of L. (V.) braziliensis upon in vitro restimulation with the respective recombinant proteins. BALB/c mice were immunized with three doses of plasmids containing the indicated genes (B) or pcDNA3 (A). Alternatively, BALB/c mice were immunized with three doses of the indicated recombinant proteins (D) or alum plus CpG ODN (C). Two weeks after the last immunizing dose, spleen cells of each individual mouse were stimulated in vitro with 10 μg of recombinant protein/ml. The concentration of IFN-γ was estimated in cell culture supernatants. The results are expressed as average of three mice ± the SD. This experiment was reproduced twice with identical results. Asterisks denote statistically significant differences from values in the supernatants of spleen cells cultured in the absence of the recombinant protein (P < 0.05, one-way analysis of variance).
Immunization with L. (V.) braziliensis antigens failed to elicit protective immunity against experimental infection in BALB/c mice.
To determine whether immunization with any of these genes or antigens alone or in combination could elicit protective immunity against experimental infection, BALB/c mice were immunized twice with plasmid DNA, followed by one or two doses of recombinant protein in the presence CpG ODN 1826 and alum. The immune sera of these mice were tested by IIA. We observed that sera from vaccinated mice recognized promastigotes (data not shown).
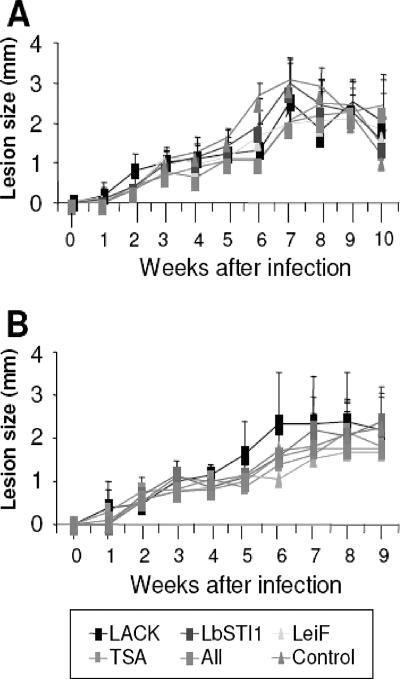
Two weeks after the last dose, mice were then challenged with infective promastigotes of L. (V.) braziliensis (MHOM/BR/01/BA788 strain) in the dermis in both ears. We monitored the nodule formation for 9 or 10 weeks. We did not observe a protective immune response as expressed by a significant reduction in the lesion size in immunized mice compared to the control group that received control plasmid followed by adjuvant (Fig. 8).
FIG. 8.
Lesion size caused by infective promastigotes of L. (V.) braziliensis in BALB/c mice immunized with eukaryotic expression plasmids, followed by bacterial recombinant proteins expressing Leishmania antigens. BALB/c mice were immunized twice intramuscularly with 100 μg per dose of the indicated plasmids or pcDNA3 (control). These animals were subsequently immunized intraperitoneally once (A) or twice (B) with the respective recombinant proteins (25 μg/dose) admixed with CpG ODN 1826 and alum or adjuvant alone (control). Two weeks after the last immunizing dose, mice were challenged i.d. in both ears with 105 promastigotes of L. (V.) braziliensis (strain MHOM/BR/01/BA788). The results are expressed as the average lesion size of three mice ± the SD. No statistically significant difference was observed when we compared the distinct mouse groups (P > 0.05, one-way analysis of variance).
DISCUSSION
The present study was designed to evaluate the vaccination potential of four L. (V.) braziliensis genes or antigens (LACK, LbSTI1, LeIF, and TSA) in a newly developed experimental model of infection (20). These four genes or antigens were selected based on previous successful vaccination against experimental infection in BALB/c mice with L. (L.) major (reviewed in references 9 and 21). Initially, we cloned these four genes from genomic DNA of L. (V.) braziliensis. Deduced amino acid sequence analysis of each one of these genes showed that they are quite conserved. Nevertheless, lbsti1 ORF presented a clear polymorphism compared to L. (L.) major and L. (L.) donovani ORFs in the region spanning amino acids 158 to 220 (Fig. 1). The significance of this polymorphism should be further evaluated in the future.
Subsequently, we characterized whether these genes or antigens were expressed on the different forms of L. (V.) braziliensis. We provided evidence that all four genes or antigens were expressed on promastigote and amastigote forms. Based on the fact that these antigens were expressed in the intracellular forms of L. (V.) braziliensis, we performed immunization studies with either recombinant plasmids or bacterial recombinant proteins. We found that plasmid immunization failed to induce an antibody response except after immunization with the gene lbsti1. Nevertheless, IFN-γ-producing spleen cells were generated after individual immunization with three of the four plasmids used containing the genes lack, leif, and lbsti1 or all plasmids together.
Immunization of BALB/c mice with recombinant proteins admixed with the adjuvants CpG ODN 1826 and alum induced potent antibody immune responses. IFN-γ-producing spleen cells were also generated in mice immunized individually with three of the antigens (LACK, TSA, and LeIF) or all of the antigens together. Finally, we immunized mice with a combined protocol consisting of priming with plasmid DNA followed by booster injections with recombinant proteins. This protocol was based on our previous study showing that the heterologous prime-boost immunization induced high levels of cell-mediated immunity against a Trypanosoma cruzi antigen (33). In spite of the immune responses induced by a heterologous prime-boost immunization with these genes or antigens after an i.d. challenge of immunized BALB/c mice with L. (V.) braziliensis infective promastigotes, we were unable to observe any significant reduction in the lesion size up to 10 weeks after challenge.
In addition to the BALB/c mouse model, we also immunized C57BL/6 mice with the different plasmids individually or all together. Similarly, we were unable to detect significant reduction of the primary lesion size after a subcutaneous footpad challenge with L. (V.) braziliensis (data not shown).
Thus far, we were unable to detect any reduction in the lesion sizes of vaccinated challenged animals. However, it will be important to establish more sensitive methods to determine the number of L. (V.) braziliensis in the lesion using real-time PCR. In general, our results contrast with previous findings from studies performed with L. (L.) major. Using distinct mouse models of infection, significant reduction in the primary lesion sizes were observed in mice vaccinated with these genes or antigens either as recombinant plasmids or proteins. The reduction in the lesion size was observed after a subcutaneous footpad challenge with large doses of promastigotes (5, 6, 10, 14, 15, and 30). Also, significant reductions in the lesion size were reported after i.d. challenge in the mouse ear (17, 18, 22). These results established unequivocally the protective potential of these recombinant genes or antigens provided as plasmid DNA or recombinant proteins.
Using our plasmid containing the lack gene of L. (V.) braziliensis, we confirmed that immunization of BALB/c mice elicited protective immunity capable of significantly reduce the lesion size after a footpad challenge with L. (L.) major (data not shown). Similarly, Melby et al. (16) showed that vaccination with the lack gene induced potent immune responses but failed to provide protective immunity against an experimental challenge with L. (L.) donovani (visceral leishmaniasis). Based on that finding, we considered that the biological differences between L. (L.) major, L. (V.) braziliensis, and L. (L.) donovani may account for the inability to elicit protective immune responses against a challenge with the last two.
In summary, although our results are essentially negative in terms of the induction of protective immunity, we consider it important because it suggests that the research efforts aimed at the development of a recombinant vaccine against L. (V.) braziliensis should be redirected toward different parasite antigens or different vaccination strategies.
Acknowledgments
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo and The Millennium Institute for Vaccine Development and Technology (CNPq; 420067/2005-1). G.S. was supported by fellowships from FAPESP and CAPES. M.L.D., C.I.O., C.L.B., R.A.M., and M.M.R. were supported by fellowships from CNPq.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Alfieri, S. C., V. Zilberfarb, and M. Rabinovitch. 1987. Destruction of Leishmania mexicana amazonensis amastigotes by leucine methyl ester: protection by other amino acid esters. Parasitology 9531-41. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri, C. L., A. I. Doine, and E. Freymuller. 1990. Lysosomal depletion in macrophages from spleen and foot lesions of Leishmania-infected hamster. Exp. Parasitol. 71218-228. [DOI] [PubMed] [Google Scholar]
- 3.Berman, J. D. 1988. Chemotherapy for leishmaniasis: biochemical-mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 10560-586. [DOI] [PubMed] [Google Scholar]
- 4.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 712744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos-Neto, A., J. R. Webb, K. Greeson, R. N. Coler, Y. A. Skeiky, and S. G. Reed. 2002. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 702828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos-Neto, A., R. Porrozzi, K. Greeson, R. N. Coler, J. R. Webb, Y. A. Skeiky, S. G. Reed, and G. Grimaldi, Jr. 2001. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 694103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CENEPI. 2007. Manual for surveillance of American integumentary leishmaniasis, 2nd ed. Brazilian Health Ministry, Brasília, Brazil. http://portal.saude.gov.br/portal/arquivos/pdf/manual_lta_2ed.pdf.
- 8.Champsi, J., and D. McMahon-Pratt. 1988. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect. Immun. 563272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coler, R. N., and S. G. Reed. 2005. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21244-249. [DOI] [PubMed] [Google Scholar]
- 10.Coler, R. N., Y. A. Skeiky, K. Bernards, K. Greeson, D. Carter, C. D. Cornellison, F. Modabber, A. Campos-Neto, and S. G. Reed. 2002. Immunization with a polyprotein vaccine consisting of the T-cell antigen thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect. Immun. 704215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Convit, J., M. Ulrich, C. T. Fernandez, F. J. Tapia, G. Caceres-Dittmar, M. Castes, and A. J. Rondon. 1993. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 87444-448. [DOI] [PubMed] [Google Scholar]
- 12.Fong, D., M. Wallach, J. Keithly, P. Melera, and K. P. Chang. 1984. Differential expression of mRNAs for α- and β-tubulin during differentiation of the parasitic protozoan Leishmania mexicana. Proc. Natl. Acad. Sci. USA 787624-7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grogl, M., T. N. Thomaso, and E. D. Franke. 1992. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am. J. Trop. Med. Hyg. 47117-126. [DOI] [PubMed] [Google Scholar]
- 14.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 41409-1415. [DOI] [PubMed] [Google Scholar]
- 15.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 1861137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 694719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. Skeiky, A. Campos-Neto, S. G. Reed, R. A. Seder, and D. L. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J. Immunol. 1665122-5128. [DOI] [PubMed] [Google Scholar]
- 18.Mendez, S., Y. Belkaid, R. A. Seder, and D. L. Sacks. 2002. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 203702-3708. [DOI] [PubMed] [Google Scholar]
- 19.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z. E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268563-566. [DOI] [PubMed] [Google Scholar]
- 20.Moura, T. R., F. O. Novais, F. Oliveira, J. Clarencio, A. Noronha, A. Barral, C. Brodskyn, and C. I. Oliveira. 2005. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 735827-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed, S. G., R. N. Coler, and A. Campos-Neto. 2003. Development of a leishmaniasis vaccine: the importance of MPL. Expert. Ver. Vaccines 2239-252. [DOI] [PubMed] [Google Scholar]
- 22.Rhee, E. G., S. Mendez, J. A. Shah, C. Y. Wu, J. R. Kirman, T. N. Turon, D. F. Davey, H. Davis, D. M. Klinman, R. N. Coler, D. L. Sacks, and R. A. Seder. 2002. Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T-cell responses and protection against Leishmania major infection. J. Exp. Med. 1951565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues, M. M., M. Ribeirão, V. L. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 673855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas, R., L. Valderrama, M. Valderrama, M. X. Varona, M. Ouellette, and N. G. Saravia. 2006. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 1931375-1383. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, NY.
- 26.Shaw, J. J. 1999. The relationship of sand fly ecology to the transmission of leishmaniasis in South America with particular reference to Brazil, p. 503-517. In J. Burger (ed.), Memoirs on entomology, international, vol. 14. Associated Publishers, Gainesville, FL. [Google Scholar]
- 27.Sheffield, P., S. Garrard, and Z. Derewenda. 1999. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr. Purif. 1534-39. [DOI] [PubMed] [Google Scholar]
- 28.Skeiky, Y. A., J. A. Guderian, D. R. Benson, O. Bacelar, E. M. Carvalho, M. Kubin, R. Badaro, G. Trinchieri, and S. G. Reed. 1995. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin-12. J. Exp. Med. 1811527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skeiky, Y. A., M. Kennedy, D. Kaufman, M. M. Borges, J. A. Guderian, J. K. Scholler, P. J. Ovendale, K. S. Pich, P. J. Morrissey, K. H. Grabstein, A. Campos-Neto, and S. G. Reed. 1998. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated cytokine profile. J. Immunol. 1616171-6179. [PubMed] [Google Scholar]
- 30.Skeiky, Y. A., R. N. Coler, M. Brannon, E. Stromberg, K. Greenson, R. T. Crane, A. Campos-Neto, and S. G. Reed. 2002. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL® adjuvant. Vaccine 203292-3303. [DOI] [PubMed] [Google Scholar]
- 31.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1998. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 633559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullman, B. 1995. Multidrug resistance and P-glycoproteins in parasitic protozoa. J. Bioenerg. Biomembr. 2777-84. [DOI] [PubMed] [Google Scholar]
- 33.Vasconcelos, J. R., S. B. Boscardin, M. I. Hiyane, S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. A DNA-priming protein-boosting regimen significantly improves type 1 immune response but not protective immunity to Trypanosoma cruzi infection in a highly susceptible mouse strain. Immunol. Cell Biol. 81121-129. [DOI] [PubMed] [Google Scholar]
- 34.Webb, J. R., A. Campos-Neto, Y. A. Skeiky, and S. G. Reed. 1997. Molecular characterization of the heat-inducible LmSTI1 protein of Leishmania major. Mol. Biochem. Parasitol. 89179-193. [DOI] [PubMed] [Google Scholar]
- 35.Webb, J. R., A. Campos-Neto, P. J. Ovendale, T. I. Martin, E. J. Stromberg, R. Badaro, and S. G. Reed. 1998. Human and murine immune response to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect. Immun. 663279-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb, J. R., D. Kaufmann, A. Campos-Neto, and S. G. Reed. 1996. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J. Immunol. 1575034-5041. [PubMed] [Google Scholar]