Abstract
The 23-valent pneumococcal polysaccharide (Ps) vaccine offer protection against vaccine serotypes, but its cross-protection against vaccine-related serotypes is variable. We have demonstrated that the functional antibodies to serotype 15B are specific to the O-acetylated 15B-Ps and that they have low cross-reactivity with serotype 15C. Demonstration of functionally cross-reactive antibodies to vaccine-related serotypes is important for surveillance and vaccine development.
Pneumococcal (Pnc) vaccines offer protection against the serotypes of Streptococcus pneumoniae that are included in the vaccine (23). Potential for replacement disease with Pnc serotypes not included in the conjugate vaccine have prompted research on cross-reactive antibodies and their role in protection. Reports suggest that some pneumococcal vaccines offer protection against vaccine-related serotypes (5, 7, 15, 17, 21, 24). However, results from a vaccine trial in a population of children with high human immunodeficiency virus prevalence showed only a modest and nonsignificant (P = 0.25) level of cross-protection to vaccine-related serotypes 6A, 19A, and 19B (13). Recently, an increase in invasive disease caused by vaccine-related serotype 19A in children was reported regardless of the widespread vaccination with 7-valent pneumococcal conjugate vaccine (PCV7), which includes serotype 19F (16). In other reports, invasive disease due to Pnc serotypes not included in the PCV7 vaccine, such as 3, 15, and 33, were reported to have increased somewhat among children after PCV7 introduction (4, 12, 22). Although some level of cross-protection may be observed due to improved antigenic presentation (24), the role of cross-protective antibodies needs to be investigated further.
A 23-valent polysaccharide (Ps) vaccine was licensed to prevent invasive disease in persons >2 years of age who are at high risk for developing pneumococcal disease. This vaccine contains the capsular Ps of 23 different Pnc serotypes, including 15B. A certain level (11 to 53%) of cross-protection among related serotypes such as 9A and 18B has been reported with this vaccine (3). Before licensure of the 23-valent vaccine, about 90% of disease caused by serogroup 15 was due to 15A (31%), 15B (22%), and 15C (39%) (18). Serotypes 15B and 15C are undistinguishable by genetic typing techniques since they belong to the ST199 cluster (9). They have similar capsular Ps composition (1), except that the 15B-Ps is the O-acetylated variant of 15C-Ps (11). The purpose of the present study was to determine whether anti-capsular Ps antibodies for serotype 15B are functionally cross-reacting with serotype 15C and the effect of O acetylation on the functional antibody activity.
Three strains each for serotype 15B (DS4304-03, DS4063-03, and DS4160-02) and 15C (DS1615-95, DS3594-02, and DS5453-02) were selected from the Streptococcal Reference Laboratory (Centers for Disease Control and Prevention [CDC], Atlanta, GA). All strains were confirmed to belong to the major 15B/C sequence type, ST199, but they were serotyped as either 15B or 15C by Quellung reaction with rabbit immune sera specific for each serotype (14). All bacterial strains were frozen at mid-log phase, and individual aliquots were used for each assay.
Quality control sera for opsonophagocytosis assays (prevaccination sera [n = 8] and postvaccination sera [n = 7]) from individuals vaccinated with the 23-valent Pnc Ps vaccine (Pneumovax II; Sanofi Pasteur MSD, United Kingdom) were collected at the Oxford Blood Transfusion Service, Oxford, United Kingdom. Sera were lyophilized and stored at −20°C, resuspended, and stored at −70°C prior to use. Purified immunoglobulin G (IG; 10% Gamunex; Bayer, Elkhart, IN) was used as a positive control.
Absorption of immunoglobulin with pneumococci.
IG with functional antibodies to both Pnc serotypes was preabsorbed with live bacteria belonging to serogroups 15B (DS4304-03) or 15C (DS1615-95). For this purpose, Pnc strains were grown in Todd-Hewitt yeast extract broth (Difco, Detroit, MI) to an optical density at 420 nm of ∼0.4, and 1.0-ml aliquots were centrifuged at 8,200 × g for 5 min. IG was prediluted 1:4 in Hanks balanced salt solution (purchased from Gibco-BRL, Carlsbad, CA) supplemented with 0.1% gelatin (Difco). The bacterial pellet was resuspended in 200 μl of IG. This suspension was incubated with rotation (130 rpm/min) for 1 h at room temperature. After incubation, the mixture was centrifuged twice at 8,200 × g for 5 min, the bacterial pellet was discarded, and the absorbed IG was stored at 4°C. The preabsorbed IG was spot plated (5 μl) on Todd-Hewitt yeast extract agar and then incubated at 37°C in 5% CO2 for 24 h to check for the sterility after preabsorption.
De-O acetylation of 15B capsular Ps.
Purified capsular Ps of Streptococcus pneumoniae serotype 15B (ATCC 241-X; American Type Culture Collection, Manassas, VA) was de-O acetylated according to the methodology described by Ellerbroek et al. (6). Briefly, lyophilized 15B-Ps (5 mg) was suspended in 0.5 M NaOH (5 mg/ml) at pH 11, followed by incubation at 4°C in a rotary shaker for 24 h. After neutralization with 0.5 M glacial acetic acid, the solution was dialyzed against bi-distilled water and stored at −70°C till use. The extent of de-O acetylation of the 15B-Ps was quantified by using proton nuclear magnetic resonance (NMR) spectroscopy at the Complex Carbohydrate Research Center, University of Georgia, Athens. For this, native and de-O-acetylated 15B-Ps were dissolved in 0.7 ml of D2O. A one-dimensional proton NMR spectrum was acquired on a Varian Inova-500 MHz spectrometer at 298K (25°C) using a Varian pulse sequence. Proton chemical shifts were measured relative to the HDO signal (δ = 4.77 ppm).
Absorption of IG with native and deacetylated 15B-Ps.
IG (100 μl) was mixed with 100 μl of 15B-Ps solution (500 μg; native or de-O acetylated) and incubated in a rotary shaker (130 rpm) at 4°C for 12 to 16 h. This suspension was centrifuged at 8,200 × g for 5 min to remove immune complexes, and the supernatant was stored at −70°C until use.
Opsonophagocytic killing assay.
Serum samples were tested for opsonophagocytic antibodies to the Pnc serotypes 15B and 15C (n = 3 strains for each serotype) by using a reference opsonophagocytic killing assay (OPA) as previously described by Romero-Steiner et al. (20). IG was used as the positive control for both serotypes 15B and 15C. Also, IG preabsorbed with pneumococci or 15B-Ps (native or de-O acetylated) was evaluated for changes in opsonophagocytic titer against Pnc serotypes 15B (DS4304-03) and 15C (DS1615-95).
Serum opsonophagocytic activities in response to three strains each of serotypes 15B and 15C strains are given in Table 1. Prevaccination sera (n = 8) had opsonophagocytic activities below the limit of detection (OPA titer of 4) to both 15B and 15C. Postvaccination sera had either high titers (titers of 128 to 2,048) or were below the level of detection (titer of 4) for serotype 15B strains; however, most sera were less than a titer of 4 for the 15C strains. Among the postvaccination sera (n = 7), four had high OPA titers (≥128) to serotype 15B strains. Only one postvaccination serum sample (756; Table 1) demonstrated opsonic activity to two of the three serotype 15C strains tested. Comparison of OPA titers to 15B with the OPA titers to 15C in postvaccination sera showed a poor correlation among titers, with an r value of 0.139.
TABLE 1.
Median OPA titer of pre- and postvaccination sera to Pnc serotypes 15B and 15C
| Serum sample | Median OPA titera
|
|||||
|---|---|---|---|---|---|---|
| Serotype 15B (PPV-23)
|
Serotype 15C (non-PPV-23)
|
|||||
| DS4304-03 | DS4063-03 | DS4160-02 | DS1615-95 | DS3594-02 | DS5453-02 | |
| Prevaccination | ||||||
| 708 | 4 | 4 | 4 | 4 | 4 | 4 |
| 712 | 4 | 4 | 4 | 4 | 4 | 4 |
| 714 | 4 | 4 | 4 | 4 | 4 | 4 |
| 722 | 4 | 4 | 4 | 4 | 4 | 4 |
| 760 | 4 | 4 | 4 | 4 | 4 | 4 |
| 780 | 4 | 4 | 32 | 4 | 4 | 4 |
| 762 | 4 | 4 | 4 | 4 | 4 | 4 |
| 766 | 4 | 4 | 4 | 4 | 4 | 4 |
| Postvaccination | ||||||
| 756 | 512 | 256 | 512 | 32 | 4 | 64 |
| 758 | 128 | 512 | 4 | 4 | 64 | 4 |
| 768 | 2,048 | 2,048 | 2,048 | 32 | 4 | 4 |
| 770 | 1,024 | 2,048 | 2,048 | 4 | 4 | 4 |
| 772 | 4 | 4 | 4 | 4 | 4 | 4 |
| 774 | 4 | 4 | 4 | 4 | 4 | 4 |
| 776 | 4 | 4 | 4 | 4 | 4 | 4 |
| IG | 256 | 256 | 256 | 64 | 64 | 64 |
An OPA titer of <8 was reported as a titer of 4. Experiments were performed in duplicate on two to three separate assay days. All strains of serotypes 15B and 15C were genotyped to ST199.
The functional antibody activity measured for Pnc 15B and 15C was very specific. Whereas preabsorption of IG with live bacteria of Pnc serotype 15B resulted in the loss of opsonophagocytic activity (a 94% reduction in titer) for serotype 15B (homologous competition), heterologous absorption of IG with live bacteria of Pnc serotype 15C showed no reduction in the OPA titer for serotype 15B (Table 2). Similar results were recorded in IG preabsorbed with live bacteria of Pnc serotype 15C. Whereas homologous absorption of IG with Pnc serotype 15C resulted in 75% reduction in the OPA titer for serotype 15C, heterologous absorption with Pnc serotype 15B resulted in a 50% increase in OPA titer for serotype 15C (Table 2). When these experiments were performed with a postvaccination serum (i.e., serum 758) preabsorbed with Pnc serotype 15B, there was a 94% reduction in the OPA titer for Pnc serotype 15B, with no change in OPA titer for serotype 15C and vice versa (data not shown).
TABLE 2.
OPA titer of absorbed and nonabsorbed IG to Pnc serotypes 15B and 15Ca
| Target bacterium for OPA | Median OPA titer
|
|||||
|---|---|---|---|---|---|---|
| IG absorbed with live bacteriab
|
IG absorbed with Psc
|
|||||
| Nonabsorbed | Pnc 15B (DS4304-03) | Pnc 15C (DS1615-95) | Nonabsorbed | Pnc 15B-Ps (native) | Pnc 15B-Ps (de-O acetylated) | |
| 15B DS4304-03 | 128 | 4d | 128 | 128 | 8e | 64 |
| 15C DS1615-95 | 32 | 64 | 4 | 64 | 128 | 8 |
Experiments were performed in duplicate on two to three separate assay days.
The strain designation is in parentheses.
Pnc serotype 15B PS (native or deacetylated) concentration, 5 mg/ml.
An OPA titer of <8 was reported as a titer of 4.
An OPA titer of <16 was reported as a titer of 8. A dilution of 1:16 was the lowest dilution that could be tested under the assay conditions.
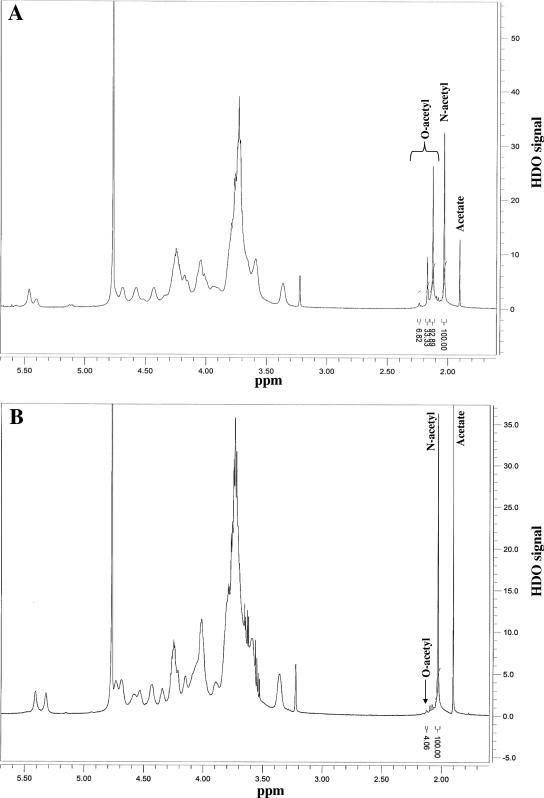
When similar experiments were performed with purified 15B-Ps for absorption, there was no change in the OPA titer for serotype 15C (Table 2). However, the 15B OPA titer was reduced by 94%. Conversely, preabsorbing IG with de-O-acetylated 15B-Ps (chemically the same as 15C-Ps) had little effect on the OPA titer for serotype 15B with the loss of OPA titer to serotype 15C. NMR spectral analysis confirmed 97% de-O acetylation in de-O-acetylated 15B-Ps (Fig. 1B).
FIG. 1.
NMR spectrum for native and de-O-acetylated 15B Ps. (A) Native 15B-Ps showed O-acetyl signals at 2.24, 2.17, and 2.13 ppm and an N-acetyl signal at 2.03 ppm. The areas of these peaks were 6.82, 33.33, 92.89, and 100.00, respectively. (B) De-O-acetylated 15B-Ps showed a very small O-acetyl peak at 2.13 ppm and an N-acetyl signal at 2.03 ppm. The areas of these peaks were 4.06 and 100.00.
Opsonophagocytosis is a correlate of protection for vaccine-induced immunity (19, 20). Therefore, low OPA activity or cross-reactivity to a highly related serotype such as 15C is relevant in terms of the evaluation of vaccine induced cross-protection. Yu et al. have reported different levels of cross-reactive opsonic antibodies for serotype 19A depending on the type of protein carrier used for conjugation of 19F Ps (21). However, all vaccines evaluated elicited opsonic titers to serotype 6A in at least 50% of infants and adults. Recent surveillance studies also show that PCV7 provides cross-protection against invasive disease and otitis media caused by serotype 6A (7, 23).
Despite of their genotypic homology (ST199), Pnc serotypes 15B and 15C have a clear and measurable antigenic difference that is demonstrated by the difference in functional antibody activity. We have shown here that the primary functional epitope of 15B-Ps is linked to the O acetylation of the monosaccharide residues. Removal of this O-acetyl group results in loss of the functional antibody activity. This was further confirmed by the absence of cross-reactive functional antibodies in postvaccination sera to serotype 15C, whose capsular Ps is naturally de-O acetylated. The lack of O acetylation can contribute to a change in the antigenic structure of the capsular polymer, making it nonreactive with the anti-15B antibodies. O acetylation has been shown to be an important parameter when measuring functional antibody activity in meningococcus (2, 10). With meningococcus groups C and Y, the O acetylation masks the epitopes on the Ps backbone to which functional antibody is directed (8). Although some investigators (21) have suggested that conjugation of Ps to different carriers can potentially result in the generation of cross-reactive antibodies, in the case of unconjugated Ps there is no aid in the presentation of the antigen to the host. Hence, the lack of antibodies that are functionally cross-reactive under unconjugated conditions should be considered for future vaccine development and in the reporting prevalence data of pneumococci.
Acknowledgments
We acknowledge members of the Streptococcal Reference Laboratory, CDC, Atlanta, GA; Delois Jackson for providing the Pnc strains used in this study; and Bob Gertz for PFGE support. We thank Cynthia Whitney, CDC, for critical review of the manuscript.
This project was also supported in part by the U.S. Department of Energy-funded (DE-FG09-83ER-20097) Center for Plant and Microbial Complex Carbohydrates.
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Barker, S. A., M. Stacey, and J. M. Williams. 1960. New data about the structure of antigenic polysaccharides of pneumococcus. Bull. Soc. Chim. Biol. 421611-1618. [PubMed] [Google Scholar]
- 2.Berry, D. S., F. Lynn, C. H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 703707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler, J. C., R. F. Breiman, J. F. Campbell, H. B. Lipman, C. V. Broome, and R. R. Facklam. 1993. Pneumococcal polysaccharide vaccine efficacy: an evaluation of current recommendations. JAMA 2701826-1831. [PubMed] [Google Scholar]
- 4.Byington, C. L., K. Korgenski, J. Daly, K. Ampofo, A. Pavia, and E. O. Mason. 2006. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr. Infect. Dis. J. 25250-254. [DOI] [PubMed] [Google Scholar]
- 5.Cutts, F. T., S. M. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 3651139-1146. [DOI] [PubMed] [Google Scholar]
- 6.Ellerbroek, P. M., D. J. Lefeber, R. van Veghel, J. Scharringa, E. Brouwer, G. J. Gerwig, G. Janbon, A. I. Hoepelman, and F. E. Coenjaerts. 2004. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. J. Immunol. 1737513-7520. [DOI] [PubMed] [Google Scholar]
- 7.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344403-409. [DOI] [PubMed] [Google Scholar]
- 8.Fusco, P. C., E. K. Farley, C. H. Huang, S. Moore, and F. Michon. 2007. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 14577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gertz, R. E., Jr., M. C. McEllistrem, D. J. Boxrud, Z. Li, V. Sakota, T. A. Thompson, R. R. Facklam, J. M. Besser, L. H. Harrison, C. G. Whitney, and B. Beall. 2003. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J. Clin. Microbiol. 414194-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giardina, P. C., E. Longworth, R. E. Evans-Johnson, M. L. Bessette, H. Zhang, R. Borrow, D. Madore, and P. Fernsten. 2005. Analysis of human serum immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin. Diagn. Lab. Immunol. 12586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansson, P. E., B. Lindberg, U. Lindquist, and J. Ljungberg. 1987. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae types 15B and 15C. Carbohydr. Res. 162111-116. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan, S. L., E. O. Mason, Jr., E. R. Wald, G. E. Schutze, J. S. Bradley, T. Q. Tan, J. A. Hoffman, L. B. Givner, R. Yogev, and W. J. Barson. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113443-449. [DOI] [PubMed] [Google Scholar]
- 13.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 3491341-1348. [DOI] [PubMed] [Google Scholar]
- 14.Neufeld, F. 1902. Uber die agglutina der Pneumokokken und uber die theorien der Agglutination. Z. Hyg. Infeckt-Kr. 4054-72. [Google Scholar]
- 15.Ortqvist, A. 2001. Pneumococcal vaccination: current and future issues. Eur. Respir. J. 18184-195. [DOI] [PubMed] [Google Scholar]
- 16.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 1921988-1995. [DOI] [PubMed] [Google Scholar]
- 17.Park, I., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 451225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins, J. J. B., R. R. Austrian, C. C. J. Lee, S. S. C. Rastogi, G. G. Schiffman, J. J. Henrichsen, P. P. H. Mäkelä, C. C. V. Broome, R. R. R. Facklam, and R. R. H. Tiesjema. 1983. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 1481136-1159. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Steiner, S., C. E. Frasch, G. Carlone, R. A. Fleck, D. Goldblatt, and M. H. Nahm. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeland, E., H. Jakobsen, G. Ingolfsdottir, S. T. Sigurdardottir, and I. Jonsdottir. 2001. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J. Infect. Dis. 183253-260. [DOI] [PubMed] [Google Scholar]
- 22.Singleton, R. J., T. W. Hennessy, L. R. Bulkow, L. L. Hammitt, T. Zulz, D. A. Hurlburt, J. C. Butler, K. Rudolph, and A. Parkinson. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2971784-1792. [DOI] [PubMed] [Google Scholar]
- 23.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 3481737-1746. [DOI] [PubMed] [Google Scholar]
- 24.Yu, X., B. Gray, S. Chang, J. I. Ward, K. M. Edwards, and M. H. Nahm. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 1801569-1576. [DOI] [PubMed] [Google Scholar]