Abstract
Sporotrichosis is an important subcutaneous mycosis, with an increasing worldwide incidence. However, few data are available regarding the immunological aspects of Sporothrix schenckii infection, particularly the humoral responses to the fungus. In this study we measured immunoglobulin G (IgG), IgM, and IgA in sera from 41 patients with sporotrichosis before antifungal treatment and from another 35 patients with sporotrichosis during itraconazole treatment by using a recently described S. schenckii exoantigen enzyme-linked immunosorbent assay (ELISA). More than 95% of patients had detectable IgA antibodies, and more than 85% had IgM and IgG antibodies before treatment. The number of patients with IgG antibodies increased to 91% during treatment. Conversely, significantly fewer samples from treated patients were positive for IgM (71%) and IgA (89%). Overall, 78% of patients had detectable levels of all isotypes tested at diagnosis, and this percentage dropped to 62.9% in patients receiving itraconazole. Testing of all three isotypes improved the sensitivity; at least two isotypes were detected in 93% of patients before and 89% after treatment. The reactivity of 94 sera from patients with other diseases and healthy individuals was also tested. Cross-reactivity occurred in 33% of the heterologous sera. Most of them were positive only in one isotype, 8.5% were positive for at least two isotypes, and only one serum (1.1%) was positive for the three isotypes. Antibodies produced during S. schenckii infection are diverse, and we demonstrate that an exoantigen ELISA for the detection of combinations of IgA, IgG, and IgM antibodies is a highly sensitive and specific diagnostic assay for sporotrichosis.
The host response to infection is the culmination of intricate interactions between a microbe and the host's innate and adaptive immune system. In this context, whereas substantial information is available about the cellular immune response against endemic fungi, the role of antibody in these mycoses is relatively poorly understood (37). Some mechanisms by which antibodies can protect the host against fungal infections include the agglutination of fungal cells, interference with fungal attachment, enhancement of phagocytosis by host effector cells, neutralization of immunoregulatory molecules, and complement activation (13). In addition, antibodies are generated that do not confer protection or even enhance disease (13, 14, 40). Antibody isotypes and their role in the humoral immune response of patients have been studied in several types of mycosis (5, 11, 20, 24, 46, 50). For instance, in paracoccidioidomycosis, there is a differential expression of isotypes to the major Paracoccidioides brasiliensis antigen, a 43-kDa glycoprotein. Immunoglobulin G (IgG) is found predominantly in patients with the juvenile form of the disease, and IgA is found in patients with the adult form, which has been associated with mucosal protection in the adult form (5). In chromoblastomycosis, high levels of IgM are observed during the course of the disease, presumably due to constant antigen stimulation by continuous low background fungal degradation (20).
Sporotrichosis is a chronic granulomatous disease caused by Sporothrix schenckii, a saprophytic dimorphic fungus found on dead or senescent vegetation, such as thorns, hay, straw, sphagnum moss, and wood, and also in soil. For this reason, this subcutaneous mycosis is usually associated with puncture injuries in farmers, florists, leisure gardeners, nursery workers, landscapers, and greenhouse workers. S. schenckii exists as a mycelial form in the environment and as a yeast form in humans and other mammals, as well as when cultured at 35 to 37°C (34). No sexual stage has been observed, but the sexual or perfect stage of S. schenckii is thought to belong to the genus Ophiostoma (31).
Disease caused by S. schenckii is usually limited to cutaneous and subcutaneous tissues as a consequence of a traumatic implantation of the fungus into the skin (31, 34). Clinically, it may manifest as lymphocutaneous, fixed cutaneous, disseminated cutaneous, extracutaneous, and disseminated forms and very rarely as a primary pulmonary disease (4). The most common form of extracutaneous sporotrichosis is osteoarthritis (32). Disseminated sporotrichosis is rare, but the frequency of disseminated sporotrichosis is increased in human immunodeficiency virus (HIV)-infected individuals (26, 45).
The humoral immune response appears to have a role in prevention and control of sporotrichosis in experimentally infected mice (36); however, there is no information available about the antibody profile produced during human infection. Recently, our group described an enzyme-linked immunosorbent assay (ELISA) that is useful for detecting antibodies raised against mycelial exoantigens of S. schenckii (3). An outbreak of sporotrichosis has been occurring in Rio de Janeiro, Brazil, since 1998 (8). Sera from patients in this region have been collected either before treatment or during treatment with itraconazole. In this report, we describe the presence of IgG, IgM, and IgA antibodies to S. schenckii mycelial exoantigens in sera from patients with sporotrichosis and their application in the serodiagnosis of this mycotic infection.
MATERIALS AND METHODS
Study populations.
Sera from two groups of sporotrichosis patients cared for in the Instituto de Pesquisa Clínica Evandro Chagas (IPEC), Fiocruz, Brazil, were subjected to the exoantigen ELISA. The first group consisted of individuals from whom serum was collected prior to antifungal therapy (41 patients, 15 male and 26 female), and the patients had different clinical forms of sporotrichosis (8 fixed cutaneous, 11 lymphocutaneous, 17 disseminated cutaneous, and 5 extracutaneous). The second group consisted of 35 different patients (7 male and 28 female) whose sera were collected while they were receiving itraconazole for diagnosed sporotrichosis (12 fixed cutaneous, 16 lymphocutaneous, 5 disseminated cutaneous, and 2 extracutaneous). The duration of treatment of these patients ranged from 2 weeks to 13 months. The patients in both groups had sporotrichosis diagnosed based on isolation of S. schenckii in culture. Importantly, no immunological abnormalities, including HIV infection, were found in any of the patients. For cross-reactivity assays, serum samples from 68 patients with other culture-proven infectious diseases (13 with histoplasmosis, 12 with tuberculosis, 18 with leishmaniasis, 4 with aspergillosis, 9 with cryptococcosis, and 12 with paracoccidioidomycosis) collected before antifungal therapy and sera from 26 healthy blood donors (negative for antibodies to HIV, hepatitis B virus, Treponema pallidum, and Trypanosoma cruzi) were tested by the S. schenckii ELISA. None of the 170 subjects had received a sporotrichin skin test. The control samples were chosen randomly and were obtained from the Immunodiagnostic Section Serum Bank, Mycology Branch, IPEC.
S. schenckii strain and antigen production.
S. schenckii 23508, originally isolated from a dwelling of a patient with sporotrichosis, was used in the present study. This isolate was identified by biochemical testing, typical colony morphology, and microscopic appearance of growth on culture medium at 25 and 37°C (19). This strain is available in the culture collection from the Mycology Branch, IPEC-Fiocruz. The exoantigen used in the ELISA was prepared from the mycelial form of this strain according to the method of Mendoza et al. (33). In brief, Sabouraud dextrose broth (Difco Laboratories, Detroit, MI) was inoculated with S. schenckii mycelial phase and incubated at 28°C with shaking at 100 rpm for 14 days. Subsequently, culture supernatants were filtered through a 0.45-μm-pore-size mixed cellulose acetate membrane (Millipore Corp. Billerica, MA), concentrated 10-fold by pervaporation, and dialyzed for 3 days against distilled water at 4°C. Thimerosal (1:5,000) was added as a preservative. The antigen mixture was stored at 4°C until use.
ELISA.
Indirect ELISA was performed as described previously (3), with slight modifications to detect IgG, IgM, and IgA class antibodies against S. schenckii. Antigen was added (40 ng of protein in 100 μl of carbonate buffer [63 mM; pH 9.6] per well) to 96-well microtiter plates (Corning, Inc., Costar polystyrene EIA/RIA plates), followed by incubation for 90 min at 37°C and then overnight at 4°C. This concentration of S. schenckii protein was determined by checkerboard titration of twofold dilutions of antigen and high-titer human serum. Plates were washed three times with washing buffer (10 mM Tris-buffered saline [TBS], 0.1% Tween 20 [pH 7.3]) and blocked with Superblock blocking buffer in TBS (Pierce Biotechnology, Inc., Rockford, IL) according to the manufacturer's instructions. The plates were then washed three times with washing buffer, and serum samples were added in duplicate to wells at 1:4,000, 1:1,000, and 1:800 dilutions for IgG, IgM, and IgA detection, respectively, in incubation buffer (10 mM TBS, 0.1% Tween 20, 5% nonfat skimmed milk powder [pH 7.3]), followed by incubation at 37°C for 1 h. After three washes, the plates were incubated at 37°C for 1 h with goat anti-human IgG, IgM, or IgA alkaline phosphatase conjugate (Southern Biotech, Birmingham, AL) diluted 1:2,000 in incubation buffer at a final volume of 100 μl per well. Plates were washed three times, and then the enzymatic reaction was developed with the addition of 100 μl per well of 1.0 mg of p-nitrophenyl phosphate/ml in 0.1 M glycine buffer containing 1 mM MgCl2 and 1 mM ZnCl2 (pH 10.4) at 37°C for 30 min. The reaction was stopped by the addition of 25 μl of 3 M NaOH per well. Absorbances were measured on a microplate reader (Bio-Tek model μQuant) at 405 nm. This experiment was done twice on different dates under uniform laboratory conditions to avoid internal variations in order to ascertain the reproducibility of the assay. For each experiment two controls were made: secondary antibody alone to ensure that the reagent was not interacting with the antigen in the plate and a blank control to which no antigen, serum or conjugate was applied. The absorbance value reported for each patient was the mean of the values for each well where the patient's serum was applied.
Cutoff determination.
In order to choose the best cutoff value that gives high values of sensitivity and specificity, TG-ROC curves were constructed as previously described (23). The cutoff point was determined by the optical density (OD) value that corresponds to the intersection point between sensitivity and specificity plots. In order to determine sensitivities of the tests and the cutoff values, only sera from patients without treatment were used, since antifungal therapy can alter the humoral immune response in mycotic infections (20, 49).
Statistical analyses.
Comparisons of means were made by using the Student nonpaired t test using the GraphPad Prism 3.0 software. Analysis of correlations was made by analysis of variance using SigmaPlot 2000 software. A P value of ≤0.05 was considered statistically significant. To evaluate the discriminatory power of the described assays as diagnostic tests, we performed receiver operating characteristics (ROC) analysis of each ELISA using SPSS 14.0, in which sensitivity and specificity were calculated as a function of the cutoff value. For this, (1 − specificity) was plotted against sensitivity, and the area under the curve was calculated.
RESULTS
TG-ROC curves and cutoff determination.
The TG-ROC curves for the IgG, IgM, and IgA ELISAs were determined. The analysis of this curve showed cutoff values of 0.418, 0.284, and 0.223 for IgG, IgM, and IgA antibody detection, respectively.
Antibody detection in sera from patients with sporotrichosis.
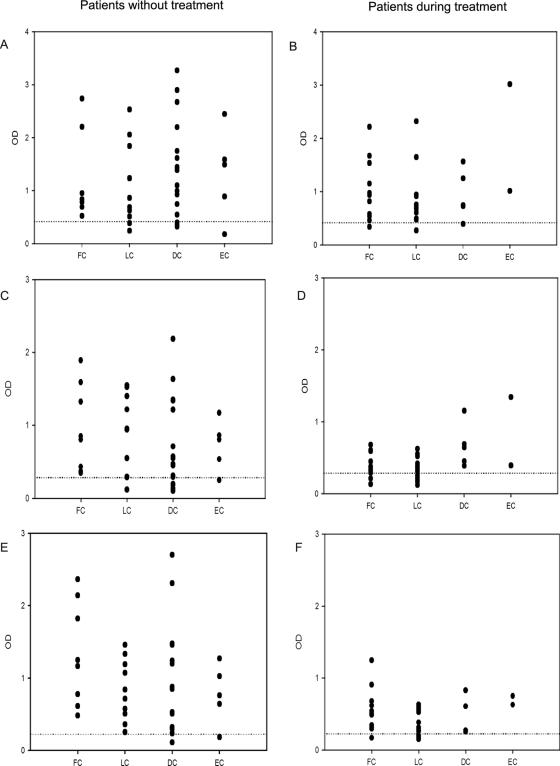
Fig. 1A shows the OD values for IgG in each unique serum from patients with different clinical forms of sporotrichosis. All sera from patients with the fixed cutaneous form of the disease were positive. Two patients with the lymphocutaneous form (18%), 3 patients with the disseminated cutaneous form (18%), and one patient with extracutaneous sporotrichosis (20%) were negative. No statistical differences were observed between the mean OD values of the different clinical forms. Figure 1B shows OD values for IgG in sera from patients with sporotrichosis receiving itraconazole. There were three patient serum samples that were negative by the IgG ELISA: one with the fixed cutaneous form of sporotrichosis (8%), one with lymphocutaneous sporotrichosis (6%) and one with the disseminated cutaneous disease (20%). The durations of the treatments for these patients were 10, 11, and 4 months, respectively.
FIG. 1.
Antibody levels in patients with several clinical forms of sporotrichosis against mycelial phase S. schenckii exoantigens. IgG (A and B), IgM (C and D), and IgA (E and F) ELISA results in patients with sporotrichosis who were not yet treated (A, C, and E) and in those receiving itraconazole (B, D, and F) are shown. Dashed lines indicate cutoff values for each single ELISA. FC, fixed cutaneous sporotrichosis; LC, lymphocutaneous sporotrichosis; DC, disseminated cutaneous sporotrichosis; EC, extracutaneous sporotrichosis.
Figure 1C shows the absorbance values for IgM reactivity in sera from patients with sporotrichosis before antifungal therapy. All of the patients with the fixed cutaneous form of the disease were positive. In contrast, six patients had negative results: one with lymphocutaneous disease (9%), four with the disseminated cutaneous form (24%), and one with extracutaneous disease (20%). No statistical difference was observed between the mean OD values in the IgM detection against mycelial S. schenckii antigens. The profile of IgM response in sera from patients receiving itraconazole is shown in Fig. 1D, where 10 patients (3 with the fixed cutaneous form and 7 with the lymphocutaneous form [25 and 44%, respectively]) were found to be negative by the ELISA.
The IgA reactivity profiles of patients with sporotrichosis before and during treatment are shown in Fig. 1E and F, respectively. Two patients were negative when tested before antifungal therapy: one with disseminated cutaneous disease and the other with the extracutaneous form. These two patients were also negative for IgG and IgM antibodies. As described above, no immune defects were identified in these patients. Four patients on itraconazole—one with fixed cutaneous disease (8%) and three with lymphocutaneous sporotrichosis (19%)—were determined to be negative by the ELISA. No statistical differences in absorbance were seen between the different clinical forms.
Analysis of antifungal therapy on antibody profile.
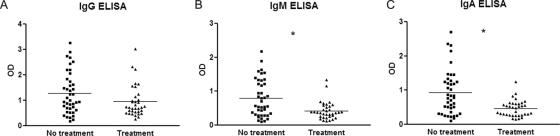
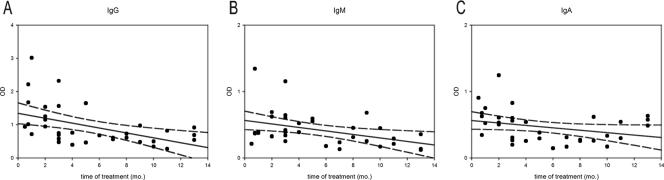
The 35 patients with sporotrichosis receiving itraconazole produced antibody at sufficient levels to be detected by at least one of the isotype ELISAs. Twenty-two (62.9%) were positive for IgG, IgM, and IgA, nine (25.7%) had two detectable isotypes, and four (11.4%) had one positive isotype. Figure 2 compares the OD values of sporotrichosis patients before and during antifungal therapy. There were significant differences (P < 0.001) between the mean ODs of IgM and IgA responses between patients not yet treated (0.79 ± 0.09 and 0.926 ± 0.102, respectively) and those receiving itraconazole (0.42 ± 0.04 and 0.47 ± 0.04, respectively). Although there was a decrease in the OD mean for IgG antibody detection between the two groups (1.28 ± 0.13 prior to treatment and 0.95 ± 0.10 on itraconazole), this difference was not statistically different. Analysis of antifungal therapy on antibody isotype responses in each of the clinical forms of sporotrichosis studied is shown in Table 1. Significant decreases in IgM and IgA levels were observed for the fixed cutaneous and lymphocutaneous forms of sporotrichosis between the patients prior to treatment compared to those on therapy. No statistical differences were observed in the clinical forms by IgG antibody levels or for IgM and IgA responses in disseminated cutaneous and extracutaneous sporotrichosis. In order to assess the relationship between length of antifungal therapy and antibody levels, we studied the correlation between these two variables (Fig. 3). A general decrease in levels during treatment time could be observed with each isotype, but the correlation was only significant for IgG and IgM, with P values of 0.004, 0.0160, and 0.074 for IgG, IgM, and IgA, respectively.
FIG. 2.
Comparison between IgG (A), IgM (B), and IgA (C) antibody levels in patients with sporotrichosis in the group not yet treated and in the group receiving itraconazole. *, P < 0.05.
TABLE 1.
ELISA results of IgG, IgM, and IgA responses against mycelial-phase S. schenckii exoantigens in patients with different clinical forms of sporotrichosis
| Antibody isotype | Clinical forma | Absorbance (mean ± SD)
|
Pb | |
|---|---|---|---|---|
| Before treatment | During treatment | |||
| IgG | FC | 1.212 ± 0.2839 | 0.9828 ± 0.1647 | 0.4645 |
| LC | 1.112 ± 0.2255 | 0.7996 ± 0.1269 | 0.2071 | |
| DC | 1.416 ± 0.2193 | 0.9376 ± 0.2080 | 0.2739 | |
| EC | 1.319 ± 0.3780 | 2.015 ± 1.001 | 0.4375 | |
| IgM | FC | 0.9507 ± 0.2087 | 0.3742 ± 0.05177 | 0.0049 |
| LC | 0.8296 ± 0.1639 | 0.3317 ± 0.03616 | 0.0017 | |
| DC | 0.7190 ± 0.1469 | 0.6656 ± 0.1345 | 0.8529 | |
| EC | 0.7243 ± 0.1560 | 0.8685 ± 0.4750 | 0.7071 | |
| IgA | FC | 1.327 ± 0.2516 | 0.5356 ± 0.08671 | 0.0028 |
| LC | 0.7783 ± 0.1305 | 0.3933 ± 0.04230 | 0.0034 | |
| DC | 0.8770 ± 0.1853 | 0.4459 ± 0.1165 | 0.2358 | |
| EC | 0.7769 ± 0.1836 | 0.6885 ± 0.06200 | 0.7863 | |
FC, fixed cutaneous; LC, lymphocutaneous; DC, disseminated cutaneous; EC, extracutaneous.
The P value was calculated by using the unpaired Student t test.
FIG. 3.
Correlation between time of treatment and levels of IgG (A), IgM (B), and IgA (C) in sera from patients with sporotrichosis. Dashed lines indicate the 95% confidence interval for the linear regression (continuous line) of values.
Cross-reactions.
Cross-reactions between IgG, IgM, and IgA antibodies present in sera from patients with other infectious diseases and in sera from normal subjects are shown in Fig. 4. There were more cross-reactions with the mycelial-phase S. schenckii exoantigens with IgM than the other isotypes. A total of 21 sera (22% of total heterologous sera) had detectable IgM in our assay. Of these, 2 were collected from patients with histoplasmosis (2 of 13 [15%]), 3 were from patients with tuberculosis (3 of 12 [25%]), 9 were from patients with leishmaniasis (9 of 18 [50%]), 1 was from a patient with aspergillosis (1 of 4 [25%]), 1 was from a patient with paracoccidioidomycosis (1 of 12 [8%]), and 5 were from normal subjects (5 of 26 [19%]). None of the tested patients infected with Cryptococcus neoformans had positive IgM or IgG tests. Cross-reactions were observed in 11 heterologous sera tested for IgG response against S. schenckii exoantigens. These positive reactions were observed in three sera from patients with histoplasmosis (3 of 13 [23%]), 2 were from patients with tuberculosis (2 of 12 [17%]), 3 were from patients with leishmaniasis (3 of 18 [17%]), 1 was from a patient with aspergillosis (1 of 4 [25%]), another was from a patient with paracoccidioidomycosis (1 of 12 [8%]), and one was a normal subject (1 of 26, 4%). IgA was the isotype with the least cross-reactions, which occurred in 8 sera from individuals with histoplasmosis (3 of 13 sera [23%]), leishmaniasis (2 of 18 sera [11%]), paracoccidioidomycosis (2 of 12 sera [17%]), and cryptococcosis (1 of 9 sera [11%]). Despite these cross-reactive test results for IgA, the mean OD value for each disease studied and for the normal subject group was statistically lower than the mean OD from patients with sporotrichosis before antifungal therapy. All sera from normal subjects and patients with tuberculosis or aspergillosis were determined to be negative by the IgA assay.
FIG. 4.

Cross-reactions observed in IgG (A), IgM (B), and IgA (C) ELISAs using mycelial-phase S. schenckii exoantigens. Cross-reactions were studied in 94 sera from patients with histoplasmosis (Histo), tuberculosis (TB), leishmaniasis (Leish), aspergillosis (Asp), paracoccidioidomycosis (PCM), and cryptococcosis (Crypto) and with sera from normal human subjects (NHS). Dashed lines indicate the cutoff values for each ELISA. Samples above the cutoff were considered positive.
Comparison between ELISA tests.
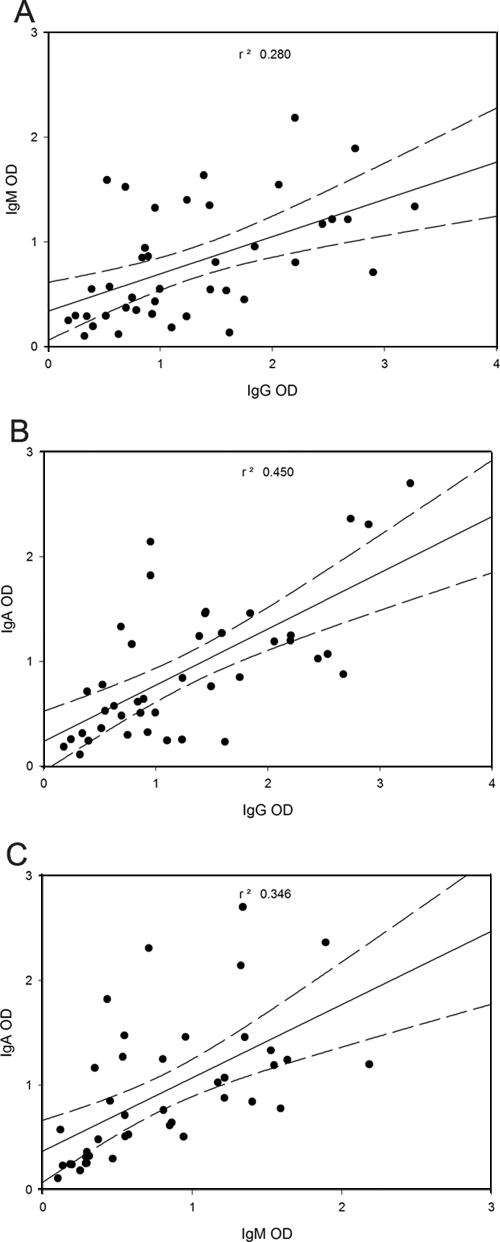
In order to compare the three immunoassays described, ROC curves were constructed by using the OD values of patients without treatment and the area under each curve was compared. Table 2 reveals that the area under the curve results were IgA > IgG > IgM. According to Greiner et al. (22), the values obtained for the areas under the curves shown in Table 2 classify the IgG and IgA ELISAs as highly accurate tests and the IgM ELISA as a moderately accurate test for the serodiagnosis of sporotrichosis. In addition, we also compared absorbance readings with the three ELISAs as a measure of specific antibody titers for each patient with sporotrichosis without treatment to test for correlation between the levels of different isotypes (Fig. 5). We found positive correlations for all comparisons (IgG and IgM, r = 0.53; IgG and IgA, r = 0.67; IgM and IgA, r = 0.59), and analysis of variance showed a positive correlation in each instance (P < 0.05 for all analyses).
TABLE 2.
Area under ROC curves for IgG, IgM, and IgA antibody detection in sporotrichosis
| Antibody class | Area | SE | Asymptotic 95% confidence interval
|
|
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| IgG | 0.936 | 0.023 | 0.890 | 0.982 |
| IgM | 0.856 | 0.042 | 0.774 | 0.939 |
| IgA | 0.957 | 0.022 | 0.913 | 1.001 |
FIG. 5.
Correlation between the antibody levels of different isotypes in each patient with sporotrichosis. The OD values for one ELISA were plotted versus the OD values of another ELISA to detect another isotype. The linear regression line in the middle is bracketed by two dashed lines, which indicate the 95% confidence interval. R2 values were calculated for each comparison by using SigmaPlot 2000. (A) Comparison between IgG and IgM levels; (B) comparison between IgG and IgA levels; (C) comparison between IgM and IgA levels.
Application of specific IgG, IgM, and IgA detection in the serodiagnosis of sporotrichosis.
Table 3 shows the parameters obtained for each single ELISA. As suggested by the ROC curve analysis, the best results were obtained for IgA isotype detection, with a sensitivity of 95.1%, a specificity of 91.5%, and an efficiency of 92.6%. In order to check whether the combination of results from two or three assays could improve diagnosis, we repeated the analysis considering as “positive” a serum sample with positive results in two specific isotypes, in all three isotypes tested, and in at least two isotypes. The results of this analysis are shown in Table 4. We observed a general increase in specificity in all cases due to the fact that heterologous sera typically cross-reacted in only one of the S. schenckii exoantigen ELISA (23 of 31 cross-reactive sera [74.2%]). When a serum sample was categorized as “positive” upon reactivity against the S. schenckii mycelial exoantigens in two or three isotype ELISAs, the combined assay achieved 92.7% sensitivity and 91.5% specificity, with an efficiency of 91.9%.
TABLE 3.
Serological parameters of ELISA tests to detect IgG, IgM, and IgA against S. schenckii exoantigens in the serodiagnosis of sporotrichosisa
| Immunoglobulin | Sensitivity (%) | Specificity (%) | Efficiency (%) | PPV (%) | NPV (%) | Likelihood ratio
|
|
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| IgG | 85.4 | 87.2 | 87.4 | 76.1 | 93.3 | 7.2949 | 0.1657 |
| IgM | 85.4 | 77.7 | 80.0 | 62.5 | 92.4 | 3.8211 | 0.1884 |
| IgA | 95.1 | 91.5 | 92.6 | 83.0 | 97.7 | 11.1768 | 0.0533 |
PPV, positive predictive value; NPV, negative predictive value.
TABLE 4.
Combination of ELISA test results in the serodiagnosis of sporotrichosisa
| Isotypes | Sensitivity (%) | Specificity (%) | Efficiency (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| IgG and IgM positive | 78.0 | 95.7 | 90.4 | 88.9 | 90.9 |
| IgG and IgA positive | 85.4 | 97.9 | 94.1 | 94.6 | 93.9 |
| IgM and IgA positive | 85.4 | 95.7 | 92.6 | 89.7 | 93.8 |
| At least two positive isotypesb | 92.7 | 91.5 | 91.9 | 82.6 | 96.6 |
| IgG, IgM, and IgA positive | 78.0 | 98.9 | 92.6 | 97.0 | 91.2 |
PPV, positive predictive value; NPV, negative predictive value.
In this case, a positive sample was considered when the OD values for two isotypes were above the cutoff value, not regarding which isotype was positive.
DISCUSSION
Sporotrichosis is a cosmopolitan disease and is considered the most prevalent subcutaneous mycosis in Latin America (18). The gold standard method for the diagnosis of sporotrichosis is culture, but the time necessary for fungal growth and identification can be up to 3 weeks. In addition, in cases of extracutaneous disease, collection of clinical specimens may be difficult, requiring invasive procedures. Obtaining clinical specimens is also not possible in cases of spontaneous regression of lesions. As a consequence, the establishment of serological methods for diagnosis and follow-up of therapy in sporotrichosis is urgently needed.
Historically, antibody response against S. schenckii antigens has been detected by using agglutination or immunoprecipitation assays (2, 10, 15, 28, 29, 33, 47); these assays do not allow differentiation among different antibody isotypes in the humoral immune response against this dimorphic fungus. More recently, ELISA-based tests have been used to study antibodies produced in sporotrichosis (9, 39, 43). In contrast to the present study, prior investigations only assayed for IgG isotype responses. However, antibodies of different isotypes can participate in the defense of a host against pathogenic fungi (17, 38, 41). Although murine data suggest that there are differences in isotype responses to sporotrichosis (36), there is no detailed information about humoral immune response in human disease. As with many microorganisms, not all individuals in areas of endemicity exposed to the fungus will develop disease, but they may develop a specific humoral response (5, 21). Because of the lack of knowledge about antibodies produced in sporotrichosis we decided to study IgG, IgM, and IgA responses in the sera of patients with sporotrichosis against the exoantigens produced by this fungus in its infective form.
The majority of our patients with sporotrichosis had antibodies of IgG, IgM, and IgA classes against mycelial-phase exoantigens of S. schenckii on presentation to our clinic, and reactivity persisted during the treatment of the disease, with a trend toward lower reactivity with increasing length of time on therapy. To our knowledge, this is the first report of IgM and IgA antibodies in human sporotrichosis. These two classes of antibodies potentially have important roles in the pathogenesis of the disease. It has been shown that S. schenckii can activate complement by the alternate pathway and that classical activation cannot be excluded (44). IgM antibodies especially can have a role in the activation of complement by the classical pathway and have been shown to activate complement in several mycotic diseases (30). Mucosal involvement can occur in sporotrichosis (7, 16, 25, 31, 42) and, in this context, IgA might represent an important mechanism of defense. It is interesting that IgM and IgA antibodies remain at detectable levels in the majority of patients undergoing itraconazole treatment, although the levels differ depending on the clinical form of the patient. Patients with fixed cutaneous and lymphocutaneous sporotrichosis had lower OD values while receiving itraconazole. However, no difference was seen in the mean OD values of IgM and IgA in patients with disseminated cutaneous and extracutaneous sporotrichosis, before and during therapy. This could be due to a higher fungal burden in these patients, leading to continuous antigen presentation and antibody production, especially for IgM antibodies. Most S. schenckii antigens are glycosylated (31), and the glycosidic moieties could evoke an IgM response. Although the percentage of patients with detectable levels of IgG antibodies increased in the group of patients receiving itraconazole, their mean OD levels did not show a significant decrease for any of the clinical forms studied, which could be due to the long half-life of IgG antibodies. However, this isotype, as well as IgM, appears to decrease in sera during treatment, and they can be used as a marker to study the clinical efficacy of antifungal therapy. Since disseminated cutaneous and extracutaneous sporotrichosis are not common forms of the disease, we did not have a large number of sera from such patients, especially during itraconazole therapy. Hence, it is important to study a larger number of sera from patients with these clinical forms to validate our hypothesis. In fact, we are now studying the antibody response of patients from diagnosis through treatment to better validate the use of these ELISAs in the follow-up of patients after treatment with itraconazole and other drugs.
Cross-reactions between fungal antigens have been described in several different studies (9, 12, 27, 28, 35, 49, 51), and some of them show that S. schenckii cross-reacts against several fungi and some bacteria (27, 28, 35, 48). We observed that 33% of sera from individuals without sporotrichosis were positive by at least one of our isotype ELISAs. Of the cross-reactive sera, 74.2% of them had only one positive antibody isotype, which we postulate occurred because of similarities between fungal or other microbial antigens instead of previous contact with S. schenckii, since only 2.6% of seroreactive patients with sporotrichosis had only one positive isotype. Even during treatment, patients with sporotrichosis typically were positive by at least two antibody class ELISAs. However, for the other 25.8% of individuals without sporotrichosis and with serological reactivity against S. schenckii exoantigens, especially one patient with histoplasmosis that was positive in all three assays, the possibility of previous contact with S. schenckii could not be discarded, since our patients live in the metropolitan area of Rio de Janeiro, a region where sporotrichosis is endemic (7, 31).
American tegumentary leishmaniasis is a vector-borne disease caused in Brazil mainly by Leishmania (Viannia) braziliensis, an intracellular pathogen. This unique Leishmania species can cause a spectrum of clinical presentation ranging from self-healing or benign cutaneous lesions to more severe forms, such as mucosal leishmaniasis. This disease had a higher level of cross-reactivity with the S. schenckii antigens. Although Leishmania-S. schenckii coinfection can occur (1), it is an unusual finding, since the routes of transmission for these two diseases are distinct. Cross-reactivity is seen in patients with sporotrichosis when subjected to the Montenegro skin test (6), and our results show a broad cross-reaction range in patients with leishmaniasis and S. schenckii exoantigens. For this reason, it is necessary to study more specific antigens and/or identify specific epitopes on available antigens to improve the differential serodiagnosis of these diseases that share some clinical characteristics but that have distinct therapeutic conducts.
Serodiagnosis of sporotrichosis by ELISA is a challenging problem, especially in terms of specificity (9). The increasing awareness of this disease, along with life-threatening disease that may occur in immunocompromised patients (26) and diagnostic challenges, in particular with this latter group of patients, provides a strong rationale for pursuing improved methods of diagnosis. Our previous work on IgG detection in sera from patients with sporotrichosis showed a higher sensitivity value than the one found in the present study. This difference can be explained by some alterations such as different 96-well microtiter plates and conjugate that we introduced in the ELISAs described here in order to allow for IgM and IgA detection. Another factor for the observed disparity can be the different sera used in these studies. Since all sera were randomly selected, some of our sera were collected in the very beginning of the disease, potentially before the immune system could effectively produce specific immunoglobulins. One observation that supports this thesis is that three patients with a negative IgG serology had detectable levels of IgM antibodies. However, ROC curves in both studies present similar areas under the curve (higher than 0.9), indicating that the assays are very accurate tests for sporotrichosis serodiagnosis. In the present study, we noticed specificity values ranging from 77.7 to 91.5%, depending on which isotype was being detected. However, when we combined the results from the three ELISAs, an increase in specificity was observed, particularly when we considered serum samples that had positive results in at least two different isotypes to represent a positive test result. This is significant, since no specific immunological test is commercially available. In conclusion, we strongly suggest that in the serodiagnosis of sporotrichosis that IgG, IgM, and IgA isotypes have to be tested and a combination of results be utilized in order to provide the most accurate result. Finally, it is important to note that serodiagnosis tests in sporotrichosis do not provide a definitive diagnosis; they are conjunctive tools for the diagnosis of this infection. The results must be interpreted according to the clinical findings and the eco-epidemiologic history of the patient.
Acknowledgments
R.A.-P. was supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129). J.D.N. is supported in part by NIH AI52733 and AI056070-01A2, a Wyeth Vaccine Young Investigator Research Award from the Infectious Disease Society of America, and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519). R.M.Z.-O. is in part supported by CNPq 306288/2006-0.
We thank Armando de Oliveira Schubach and Maria Clara Gutierrez Galhardo for providing clinical information about the patients included in this study.
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Agudelo, S. P., S. Restrepo, and I. D. Velez. 1999. Cutaneous New World leishmaniasis-sporotrichosis coinfection: report of 3 cases. J. Am. Acad. Dermatol. 401002-1004. [DOI] [PubMed] [Google Scholar]
- 2.Albornoz, M. B., E. Villanueva, and E. D. Torres. 1984. Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis. Mycopathologia 85177-183. [DOI] [PubMed] [Google Scholar]
- 3.Almeida-Paes, R., M. A. Pimenta, C. V. Pizzini, P. C. Monteiro, J. M. Peralta, J. D. Nosanchuk, and R. M. Zancope-Oliveira. 2007. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin. Vaccine Immunol. 14244-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo, T., A. C. Marques, and F. Kerdel. 2001. Sporotrichosis. Int. J. Dermatol. 40737-742. [DOI] [PubMed] [Google Scholar]
- 5.Baida, H., P. J. Biselli, M. Juvenale, G. M. Del Negro, M. J. Mendes-Giannini, A. J. Duarte, and G. Benard. 1999. Differential antibody isotype expression to the major Paracoccidioides brasiliensis antigen in juvenile and adult form paracoccidioidomycosis. Microbes Infect. 1273-278. [DOI] [PubMed] [Google Scholar]
- 6.Barros, M. B. L., A. Schubach, A. C. Francesconi-do-Valle, M. C. Gutierrez-Galhardo, T. M. Schubach, F. Conceicao-Silva, M. de Matos Salgueiro, E. Mouta-Confort, R. S. Reis, M. Fatima Madeira, T. Cuzzi, L. P. Quintella, J. P. Silva Passos, M. J. Conceicao, and M. C. A. Marzochi. 2005. Positive Montenegro skin test among patients with sporotrichosis in Rio de Janeiro. Acta Trop. 9341-47. [DOI] [PubMed] [Google Scholar]
- 7.Barros, M. B. L., A. O. Schubach, M. C. Galhardo, T. M. Schubach, R. S. dos Reis, M. J. Conceicao, and A. C. F. Valle. 2003. Sporotrichosis with widespread cutaneous lesions: report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. Int. J. Dermatol. 42677-681. [DOI] [PubMed] [Google Scholar]
- 8.Barros, M. B. L., T. M. Schubach, M. C. Galhardo, A. O. Schubach, P. C. Monteiro, R. S. Reis, R. M. Zancope-Oliveira, M. S. Lazera, T. Cuzzi-Maya, T. C. Blanco, K. B. Marzochi, B. Wanke, and A. C. F. Valle. 2001. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem. Inst. Oswaldo Cruz. 96777-779. [DOI] [PubMed] [Google Scholar]
- 9.Bernardes-Engemann, A. R., R. C. Costa, B. R. Miguens, C. V. Penha, E. Neves, B. A. Pereira, C. M. Dias, M. Mattos, M. C. Gutierrez, A. Schubach, M. P. Oliveira Neto, M. Lazera, and L. M. Lopes-Bezerra. 2005. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 43487-493. [DOI] [PubMed] [Google Scholar]
- 10.Blumer, S. O., L. Kaufman, W. Kaplan, D. W. McLaughlin, and D. E. Kraft. 1973. Comparative evaluation of five serological methods for the diagnosis of sporotrichosis. Appl. Microbiol. 264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno, J. P., M. J. Mendes-Giannini, G. M. Del Negro, C. M. Assis, C. K. Takiguti, and M. A. Shikanai-Yasuda. 1997. IgG, IgM and IgA antibody response for the diagnosis and follow-up of paracoccidioidomycosis: comparison of counterimmunoelectrophoresis and complement fixation. J. Med. Vet. Mycol. 35213-217. [DOI] [PubMed] [Google Scholar]
- 12.Camargo, Z. P., R. G. Baruzzi, S. M. Maeda, and M. C. Floriano. 1998. Antigenic relationship between Loboa loboi and Paracoccidioides brasiliensis as shown by serological methods. Med. Mycol. 36413-417. [PubMed] [Google Scholar]
- 13.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 634211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadevall, A., and L. A. Pirofski. 2006. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv. Immunol. 911-44. [DOI] [PubMed] [Google Scholar]
- 15.Casserone, S., I. A. Conti-Diaz, E. Zanetta, and M. E. P. Pereira. 1983. Serologia de la esporotricosis cutânea. Sabouraudia 21317-321. [PubMed] [Google Scholar]
- 16.Castro, R. M., M. F. Sabogal, L. C. Cuce, and A. Salebian. 1981. Disseminate sporotrichosis: report of a clinical case with mucocutaneous, osteo-articular, and ocular lesions. Mykosen 2492-96. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi, A. K., A. Kavishwar, G. B. Shiva Keshava, and P. K. Shukla. 2005. Monoclonal immunoglobulin G1 directed against Aspergillus fumigatus cell wall glycoprotein protects against experimental murine aspergillosis. Clin. Diagn. Lab. Immunol. 121063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti Diaz, I. A. 1989. Epidemiology of sporotrichosis in Latin America. Mycopathologia 108113-116. [DOI] [PubMed] [Google Scholar]
- 19.Dixon, D. M., I. F. Salkin, R. A. Duncan, N. J. Hurd, J. H. Haines, M. E. Kemna, and F. B. Coles. 1991. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 291106-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterre, P., M. Jahevitra, and A. Andriantsimahavandy. 2000. Humoral immune response in chromoblastomycosis during and after therapy. Clin. Diagn. Lab. Immunol. 7497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esterre, P., M. Jahevitra, E. Ramarcel, and A. Adriantsimahavandy. 1997. Evaluation of the ELISA technique for the diagnosis and the seroepidemiology of chromoblastomycosis. J. Mycol. Med. 7137-141. [Google Scholar]
- 22.Greiner, M., D. Pfeiffer, and R. D. Smith. 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 4523-41. [DOI] [PubMed] [Google Scholar]
- 23.Greiner, M., D. Sohr, and P. Gobel. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185123-132. [DOI] [PubMed] [Google Scholar]
- 24.Hamouda, T., C. D. Jeffries, E. M. Ekladios, A. M. el-Mishad, M. el-Koomy, and N. Saleh. 1994. Class-specific antibody in human dermatophytosis reactive with Trichophyton rubrum derived antigen. Mycopathologia 12783-88. [DOI] [PubMed] [Google Scholar]
- 25.Hampton, D. E., A. Adesina, and J. Chodosh. 2002. Conjunctival sporotrichosis in the absence of antecedent trauma. Cornea 21831-833. [DOI] [PubMed] [Google Scholar]
- 26.Hardman, S., I. Stephenson, D. R. Jenkins, M. J. Wiselka, and E. M. Johnson. 2005. Disseminated Sporothrix schenckii in a patient with AIDS. J. Infect. 5173-77. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki, H., Y. Nakamura, and R. W. Wheat. 1981. Serological cross-reactivity between Sporothrix schenckii and various unrelated fungi. Mycopathologia 7365-68. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaki, H., R. W. Wheat, D. P. Kiel, and N. F. Conant. 1978. Serological cross-reactivity among Sporothrix schenckii, Ceratocystis, Europhium, and Graphium species. Infect. Immun. 21585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlin, J. V., and H. S. Nielsen, Jr. 1970. Serologic aspects of sporotrichosis. J. Infect. Dis. 121316-327. [DOI] [PubMed] [Google Scholar]
- 30.Kozel, T. R. 1996. Activation of the complement system by pathogenic fungi. Clin. Microbiol. Rev. 934-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes-Bezerra, L. M., A. O. Schubach, and R. O. Costa. 2006. Sporothrix schenckii and sporotrichosis. An. Acad. Bras. Cienc. 78293-308. [DOI] [PubMed] [Google Scholar]
- 32.Manhart, J. W., J. A. Wilson, and B. C. Korbitz. 1970. Articular and cutaneous sporotrichosis. JAMA 214365-367. [PubMed] [Google Scholar]
- 33.Mendoza, M., A. M. Diaz, M. B. Hung, E. A. Zambrano, E. Diaz, and M. C. Albornoz. 2002. Production of culture filtrates of Sporothrix schenckii in diverse culture media. Med. Mycol. 40447-454. [DOI] [PubMed] [Google Scholar]
- 34.Morris-Jones, R. 2002. Sporotrichosis. Clin. Exp. Dermatol. 27427-431. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura, Y., H. Ishizaki, and R. W. Wheat. 1977. Serological cross-reactivity between group B Streptococcus and Sporothrix schenckii, Ceratocystis species, and Graphium species. Infect. Immun. 16547-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nascimento, R. C., and S. R. Almeida. 2005. Humoral immune response against soluble and fractionate antigens in experimental sporotrichosis. FEMS Immunol. Med. Microbiol. 43241-247. [DOI] [PubMed] [Google Scholar]
- 37.Nosanchuk, J. D. 2005. Protective antibodies and endemic dimorphic fungi. Curr. Mol. Med. 5435-442. [DOI] [PubMed] [Google Scholar]
- 38.Nosanchuk, J. D., J. N. Steenbergen, L. Shi, G. S. Deepe, Jr., and A. Casadevall. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Investig. 1121164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penha, C. V., and L. M. Bezerra. 2000. Concanavalin A-binding cell wall antigens of Sporothrix schenckii: a serological study. Med. Mycol. 381-7. [PubMed] [Google Scholar]
- 40.Pirofski, L. A., and A. Casadevall. 1996. Cryptococcus neoformans: paradigm for the role of antibody immunity against fungi? Zentbl. Bakteriol. 284475-495. [DOI] [PubMed] [Google Scholar]
- 41.Rivera, J., and A. Casadevall. 2005. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J. Immunol. 1748017-8026. [DOI] [PubMed] [Google Scholar]
- 42.Schubach, A., M. B. de Lima Barros, T. M. Schubach, A. C. Francesconi-do-Valle, M. C. Gutierrez-Galhardo, M. Sued, M. de Matos Salgueiro, P. C. Fialho-Monteiro, R. S. Reis, K. B. Marzochi, B. Wanke, and F. Conceicao-Silva. 2005. Primary conjunctival sporotrichosis: two cases from a zoonotic epidemic in Rio de Janeiro, Brazil. Cornea 24491-493. [DOI] [PubMed] [Google Scholar]
- 43.Scott, E. N., and H. G. Muchmore. 1989. Immunoblot analysis of antibody responses to Sporothrix schenckii. J. Clin. Microbiol. 27300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott, E. N., H. G. Muchmore, and D. P. Fine. 1986. Activation of the alternative complement pathway by Sporothrix schenckii. Infect. Immun. 516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva-Vergara, M. L., F. R. Maneira, R. M. Oliveira, C. T. Santos, R. M. Etchebehere, and S. J. Adad. 2005. Multifocal sporotrichosis with meningeal involvement in a patient with AIDS. Med. Mycol. 43187-190. [DOI] [PubMed] [Google Scholar]
- 46.Silva, V., O. Fischman, and Z. P. Camargo. 1997. Humoral immune response to Malassezia furfur in patients with pityriasis versicolor and seborrheic dermatitis. Mycopathologia 13979-85. [DOI] [PubMed] [Google Scholar]
- 47.Smith, P. W., G. W. Loomis, J. L. Luckasen, and R. K. Osterholm. 1981. Disseminated cutaneous sporotrichosis: three illustrative cases. Arch. Dermatol. 117143-144. [PubMed] [Google Scholar]
- 48.Takata, M., and H. Ishizaki. 1983. Correlations among culture times, sugar composition and biological activities of Sporothrix schenckii antigens. Mycopathologia 8431-39. [DOI] [PubMed] [Google Scholar]
- 49.Valle, A. C. F., R. L. Costa, P. C. Fialho Monteiro, J. Von Helder, M. M. Muniz, and R. M. Zancope-Oliveira. 2001. Interpretation and clinical correlation of serological tests in paracoccidioidomycosis. Med. Mycol. 39373-377. [DOI] [PubMed] [Google Scholar]
- 50.van de Sande, W. W., D. J. Janse, V. Hira, H. Goedhart, R. van der Zee, A. O. Ahmed, A. Ott, H. Verbrugh, and A. van Belkum. 2006. Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. J. Immunol. 1771997-2005. [DOI] [PubMed] [Google Scholar]
- 51.Zancope-Oliveira, R. M., S. L. Bragg, E. Reiss, B. Wanke, and J. M. Peralta. 1994. Effects of histoplasmin M antigen chemical and enzymatic deglycosylation on cross-reactivity in the enzyme-linked immunoelectrotransfer blot method. Clin. Diagn. Lab. Immunol. 1390-393. [DOI] [PMC free article] [PubMed] [Google Scholar]