Abstract
We report a follow-up study of 29 subjects with negative tuberculin skin test (TST) results in association with positive gamma interferon release assay (IGRA) results, mainly due to responses to CFP-10 in the T-SPOT.TB assay, during a contact investigation. One year later, 12/29 subjects (41%) had converted to positive TST results in association with negative IGRA results.
In 2004, a Dutch supermarket employee was diagnosed with extensive smear-positive pulmonary tuberculosis (TB). When the contact investigation revealed that the employee had infected the majority of his close contacts and also a significant proportion of casual contacts, a large-scale investigation of all supermarket customers (>20,000) was performed in February 2005. This initial report identified more than 400 additional persons with positive tuberculin skin test (TST) results (2; unpublished data). Using a subset of these contacts, the TST was directly compared with two Mycobacterium tuberculosis-specific gamma interferon (IFN-γ) release assays (IGRAs)—the QuantiFERON-TB Gold In-Tube (QFT-GIT) assay (Cellestis, Carnegie, Australia) and the T-SPOT.TB assay (Oxford Immunotec, Abingdon, United Kingdom)—the results of which have been published previously (2). The advantage of the IGRA over the TST is the use of the M. tuberculosis-specific antigens ESAT-6 and CFP-10 (and additionally TB7.7 in the QFT-GIT test), as has been described extensively elsewhere (1, 12, 15, 17, 20). In contrast, the TST is based on purified protein derivative, which consists of a crude mixture of antigens with broad cross-reactivity and therefore leads to the occurrence of false-positive responses after vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) or exposure to nontuberculous mycobacteria. Among the 505 individuals with negative TST results (defined as an induration of <10 mm) in the comparative study, 42 individuals (8.3%) had positive IGRA results. The significance of this finding was not clear and prompted further investigation. In order to study the subgroup with negative TST and positive IGRA results, we repeated all three tests 1 year after the initial contact investigation.
The participants in the contact investigation in February 2005 who had negative TST results and positive IGRA results (n = 42) were invited in January 2006 to participate in the study presented here (2). The participants with positive TST results in the 2006 study were offered chest radiography at the Municipal Health Authority. The ethical review board of the Leiden University Medical Center approved the study protocol (protocol number P05.53), and all participants provided informed consent. The TST, T-SPOT.TB test, and QFT-GIT test were performed in accordance with the respective manufacturers’ instructions as described in the previous study (2). In the present study, data for the TSTs and IGRAs obtained at both time points (February 2005 and January 2006) were obtained for 29 participants.
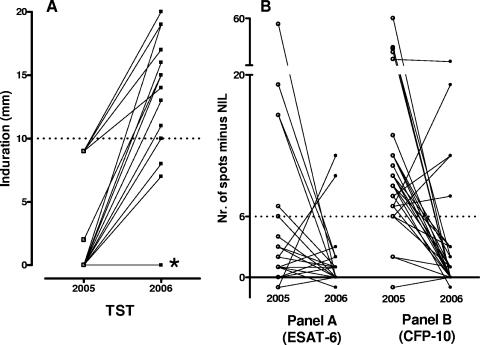
Using a ≥10-mm cutoff to define a positive TST result, we determined that 17 of 29 participants (58.6%) had converted to TST positivity 1 year after the initial contact investigation, while 12 of 29 individuals (41.4%) remained TST negative (Table 1 and Fig. 1A). When TST conversion was defined by an induration of ≥10 mm with an increase of at least 6 mm, 17/29 participants (59%) had TST conversion.
TABLE 1.
Characteristics of the study population and results of the assays at both time pointsa
| Group (no. of subjects) | Age, yr (avg ± SD) | TST
|
T-SPOT.TBb
|
QFT-GIT resultc
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005
|
2006
|
2005
|
2006
|
2005 | 2006 | ||||||
| Induration diam, mm (avg ± SD) | Result | Induration diam, mm (avg ± SD) | Result | Median no. of spots (range) | Result | Median no. of spots (range) | Result | ||||
| 1 (9) | 41 ± 13 | 0 | Neg | 0 | Neg | 10 (8-35) | Pos | 1 (0-3) | Neg | Neg | Neg |
| 2 (1) | 51 | 0 | Neg | 0 | Neg | 7 | Pos | 8 | Pos | Neg | Neg |
| 3 (2) | 47 ± 18 | 0 | Neg | 8 ± 0.7 | Neg | 13 (12-14) | Pos | 0 (0-0) | Neg | Neg | Neg |
| 4 (11) | 42 ± 11 | 3 ± 4 | Neg | 15 ± 3.4 | Pos | 10 (6-60) | Pos | 1 (0-3) | Neg | Neg | Neg |
| 5 (4) | 40 ± 13 | 9 ± 0 | Neg | 18 ± 2.5 | Pos | 8 (6-25) | Pos | 15 (12-23) | Pos | Neg | Neg |
| 6 (1) | 42 | 9 | Neg | 19 | Pos | 12 | Pos | 2 | Neg | Pos (1.25) | Pos (0.58) |
| 7 (1) | 58 | 0 | Neg | 19 | Pos | −1 | Neg | 0 | Neg | Pos (0.56) | Neg |
Pos, positive; Neg, negative.
The cutoff point for the T-SPOT.TB assay is six spots above nil (spots obtained in the medium-stimulated well).
The values in parentheses are given as IU/ml. The cutoff point for the QFT-GIT assay is 0.35 IU/ml above nil (IU/ml IFN-γ produced in the unstimulated tube).
FIG. 1.
(A) TST responses in 2005 and 2006. *, 10 subjects with TST results of 0 mm at both time points; dotted line, cutoff value for a positive test. (B) Time course of individual spot counts for ESAT-6 (test panel A) and CFP-10 (test panel B) of the TSPOT.TB assays in 2005 and 2006. Dotted line, the cutoff value for a positive test. Nil, spots obtained in the medium-stimulated well.
Of the 28 persons with positive T-SPOT.TB results during the contact investigation in 2005, 23 persons (82%) had reverted to T-SPOT.TB negativity (Table 1). One person (3.4%) remained negative by the T-SPOT.TB assay.
Consistently negative QFT-GIT results were found in 27/29 (93.1%) participants. One person (3.4%) reverted from a positive to a negative result, and one person (3.4%) remained positive (Table 1). For the group with conflicting results, the cumulative shopping time, a measure of exposure to the index patient in the supermarket, was not significantly different from that of the groups with concordant positive or negative TST and IGRA results (2).
Of the 12 persons without TST conversion (Table 1), all had negative QFT-GIT results at both time points, while the T-SPOT.TB results of 11/12 subjects had changed from positive to negative. For 10/12 persons, positive T-SPOT.TB results in 2005 were caused only by responses to CFP-10 (Fig. 1B, test panel B in the T-SPOT.TB assay).
Of the 17 individuals with TST conversion (Table 1), 15 had consistently negative QFT-GIT results, 1 person's QFT-GIT results converted from positive to negative, and 1 person's QFT-GIT results remained positive. T-SPOT.TB results reverted from positive to negative in 12/17 persons, 4 persons remained positive, and 1 person remained negative. In 2005, 14/16 persons showed maximum spot counts in response to CFP-10 and 2/16 in response to ESAT-6 (Fig. 1B, test panels B and A, respectively).
In this paper, we report on individuals who presented negative TST results in association with positive IGRA results (predominantly T-SPOT.TB positive) following an outbreak in February 2005 (2). These subjects had not received treatment for latent TB infection. One year later (January 2006), more than half of these persons converted to TST positivity, while the majority became IGRA negative. Given the epidemiological settings in The Netherlands, it was unlikely that the observed TST conversions were due to reexposure to M. tuberculosis, the boosting of Mantoux reactivity by past TB infection, or reactivity to nontuberculous mycobacteria. None of the subjects were BCG vaccinated. Our study is the first to report a follow-up of both IGRA formats and the TST for subjects with discrepant test results during a contact investigation. The association of a negative TST result with a positive IGRA result has been reported in several studies, with various percentages in different settings (7, 11, 16, 19, 21). In two of the studies (16, 21), the TST and one of the two IGRAs were repeated, showing reversion to negative IGRA results for some subjects. A novel finding in our study is that more than half of the subjects had TST conversion but IGRA reversion. Of note, this phenomenon was observed in only 2 to 3% of the original study population, but the biological and clinical significance of this novel finding may be of high importance. Treatment based on IGRA results would identify this group as eligible for treatment, whereas treatment based on TST results would not. The actual risk of progression to TB disease in this subgroup is unknown and may not be the same for individuals with initial positive TST results.
We think that it is unlikely that all or most of the positive T-SPOT.TB results obtained in 2005 were false-positive results, since we documented TST conversion 1 year later in more than half of the subjects. An alternative explanation for the discordant results could be transient TB infection without clinical relevance in this group, a concept that has been mentioned earlier (7, 14, 19). If the risk of progression to TB disease were indeed extremely low, as suggested by the hypothesis of transient TB infection, no treatment would be indicated.
A striking observation was that most positive T-SPOT.TB results during the initial screening in 2005 were in response to CFP-10 (Fig. 1B, test panel B) and not to ESAT-6 (Fig. 1B, test panel A). Thus, the reactivity to CFP-10 reverted to negative in most of the participants testing negative 1 year later. In previous studies, it has been postulated that the response to the CFP-10 antigen might be indicative of active replication of the bacteria, whereas responses to the ESAT-6 antigen may persist as an immunological “scar” (4, 7, 9).
In contrast to the initially positive T-SPOT.TB results, the QFT-GIT results were almost uniformly negative for our subjects. The QFT-GIT assay also includes CFP-10, but differences in assay formats might be a relevant factor. The QFT-GIT assay is based on whole blood and thus is dependent on the number of antigen-specific T cells that are present per volume of blood as well as on the amount of IFN-γ that is produced by each cell upon antigenic stimulation, whereas the enzyme-linked immunospot assay uses a defined number of cells. A transient TB infection may induce a relatively low number of antigen-responsive T cells and/or cells producing only a limited amount of IFN-γ, which may explain the observed interassay difference. It has repeatedly been suggested that the T-SPOT.TB assay might be more sensitive than the QFT-GIT assay for detection of latent TB infection (8, 10, 13). This could be an advantage, especially in immunocompromised patients, children, and the elderly (5, 6, 8, 18). In immunocompetent persons, however, it could be argued that a higher sensitivity might be associated with detection of an infection without clinical relevance. Further studies are needed to address which assay has the highest relevance for therapeutic decision making, especially since there seems to be no direct correlation between IFN-γ production and protection against active disease (3).
In conclusion, this study reports on TB contacts with late TST conversion in association with reversion from positive to negative T-SPOT.TB results within a period of 1 year. Further studies are needed to get a better understanding of this phenomenon, which is of high practical importance when IGRA results will be used for therapeutic decision making.
Acknowledgments
We thank all the participants and the staff of the Municipal Health Authority in Utrecht. We also thank Nigel Savage for carefully reading the manuscript.
This study was funded by unrestricted grants from the Mr. Willem Bakhuys Roozeboom Foundation, Laren, The Netherlands, without any role of the sponsor in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 3561099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., S. F. Thijsen, E. M. Leyten, J. J. Bouwman, W. P. Franken, B. F. Koster, et al. 2007. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am. J. Respir. Crit. Care Med. 175618-627. [DOI] [PubMed] [Google Scholar]
- 3.Bennekov, T., J. Dietrich, I. Rosenkrands, A. Stryhn, T. M. Doherty, and P. Andersen. 2006. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. Eur. J. Immunol. 363346-3355. [DOI] [PubMed] [Google Scholar]
- 4.Chee, C. B., K. W. KhinMar, S. H. Gan, T. M. Barkham, M. Pushparani, and Y. T. Wang. 2007. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am. J. Respir. Crit. Care Med. 175282-287. [DOI] [PubMed] [Google Scholar]
- 5.Connell, T., N. Bar-Zeev, and N. Curtis. 2006. Early detection of perinatal tuberculosis using a whole blood interferon-gamma release assay. Clin. Infect. Dis. 42e82-e85. [DOI] [PubMed] [Google Scholar]
- 6.Dheda, K., A. Lalvani, R. F. Miller, G. Scott, H. Booth, M. A. Johnson, et al. 2005. Performance of a T-cell-based diagnostic test for tuberculosis infection in HIV-infected individuals is independent of CD4 cell count. AIDS 192038-2041. [DOI] [PubMed] [Google Scholar]
- 7.Ewer, K., K. A. Millington, J. J. Deeks, L. Alvarez, G. Bryant, and A. Lalvani. 2006. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 174831-839. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara, G., M. Losi, R. D'Amico, P. Roversi, R. Piro, M. Meacci, et al. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 3671328-1334. [DOI] [PubMed] [Google Scholar]
- 9.Fox, A., D. J. Jeffries, P. C. Hill, A. S. Hammond, M. D. Lugos, D. Jackson-Sillah, et al. 2007. ESAT-6 and CFP-10 can be combined to reduce the cost of testing for Mycobacterium tuberculosis infection, but CFP-10 responses associate with active disease. Trans. R. Soc. Trop. Med. Hyg. 101691-698. [DOI] [PubMed] [Google Scholar]
- 10.Granger, C. 2006. The specificity of interferon-γ-based blood tests in the identification of latent tuberculosis infection. Eur. Respir. J. 281283. [DOI] [PubMed] [Google Scholar]
- 11.Hill, P. C., R. H. Brookes, A. Fox, K. Fielding, D. J. Jeffries, D. Jackson-Sillah, et al. 2004. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin. Infect. Dis. 38966-973. [DOI] [PubMed] [Google Scholar]
- 12.Lalvani, A., L. Richeldi, and H. Kunst. 2005. Interferon gamma assays for tuberculosis. Lancet Infect. Dis. 5322-324. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J. Y, H. J. Choi, I.-N. Park, S.-B. Hong, Y.-M. Oh, C.-M. Lim, et al. 2006. Comparison of two commercial interferon gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur. Respir. J. 2824-30. [DOI] [PubMed] [Google Scholar]
- 14.Nardell, E. A., and R. S. Wallis. 2006. Here today-gone tomorrow: the case for transient acute tuberculosis infection. Am. J. Respir. Crit. Care Med. 174734-735. [DOI] [PubMed] [Google Scholar]
- 15.Pai, M. 2005. Alternatives to the tuberculin skin test: interferon-gamma assays in the diagnosis of Mycobacterium tuberculosis infection. Indian J. Med. Microbiol. 23151-158. [DOI] [PubMed] [Google Scholar]
- 16.Pai, M., R. Joshi, S. Dogra, D. K. Mendiratta, P. Narang, S. Kalantri, et al. 2006. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am. J. Respir. Crit. Care Med. 174349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai, M., L. W. Riley, and J. M. Colford, Jr. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4761-776. [DOI] [PubMed] [Google Scholar]
- 18.Piana, F., L. R. Codecasa, P. Cavallerio, M. Ferrarese, G. B. Migliori, L. Barbarano, et al. 2006. Use of a T-cell-based test for detection of tuberculosis infection among immunocompromised patients. Eur. Respir. J. 2831-34. [DOI] [PubMed] [Google Scholar]
- 19.Richeldi, L., K. Ewer, M. Losi, B. M. Bergamini, P. Roversi, J. Deeks, et al. 2004. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am. J. Respir. Crit. Care Med. 170288-295. [DOI] [PubMed] [Google Scholar]
- 20.Rothel, J. S., and P. Andersen. 2005. Diagnosis of latent Mycobacterium tuberculosis infection: is the demise of the Mantoux test imminent? Expert Rev. Anti-Infect. Ther. 3981-993. [DOI] [PubMed] [Google Scholar]
- 21.Shams, H., S. E. Weis, P. Klucar, A. Lalvani, P. K. Moonan, J. M. Pogoda, et al. 2005. Enzyme-linked immunospot and tuberculin skin testing to detect latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 1721161-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]