Abstract
We evaluated the seroprevalence of varicella-zoster virus (VZV) in the Finnish population among various age groups and genetically characterized VZV strains from documented cases of varicella and zoster. VZV-specific immunoglobulin G was measured in 2,842 serum samples that had been submitted for virological studies to the Department of Virology, University of Helsinki, from 1995 to 1996. Specimens for VZV genotyping were obtained from vesicular lesions from two pediatric patients and 26 adult patients. Seroprevalence to VZV varied markedly by age: 45% in children aged ≤2 months, 12.5% in children aged 6 to 8 months, and >90% in children near 10 years of age, plateauing thereafter into advanced age. The seroprevalence rates indicate that in Finland, as in other countries with temperate climates, primary VZV infection usually occurs during the first decade of life. Twenty-eight VZV DNA-positive specimens were analyzed to identify VZV vaccine and wild-type genotypes. All analyzed specimens were wild type and the European (E) genotype.
Varicella zoster virus (VZV) is a common, globally distributed virus that causes chickenpox upon primary infection; it is normally associated with a generalized vesicular rash but may rarely occur without obvious dermal lesions. VZV establishes lifelong latency upon first infection and can reactivate, usually in the elderly, to cause shingles (herpes zoster) (2, 12, 13, 15, 34). Patient age is often related to disease severity, with the greatest risk occurring among the very young and very old; immunocompromised persons are also at elevated risk for severe disease (7, 11, 14). Varicella vaccine use in Finland is currently limited to children with recognized immunosuppression due to leukemia, bone marrow transplant, et cetera. The seroprevalence rate for VZV varies among different populations, and lower prevalence rates have generally been observed in tropical versus temperate climates (9, 21, 26, 27). Over the past 25 years, there has been an evident increase in VZV seroprevalence among children 1 to 4 years of age in the United Kingdom (21). In Finland, VZV has become the agent most frequently associated with central nervous system infections, particularly encephalitis, for all age groups (18, 22). Currently, several methods are being used to identify and genotype VZV strains. A variety of methods have been explored for genotyping VZV both to discern strain variation and to discriminate vaccine strains from wild-type isolates (3, 4, 6, 8, 10, 16, 18, 23, 24, 31, 36).
We developed a novel strategy for VZV genotyping based on the complete sequencing of a short region in open reading frame 22 (ORF22) using material obtained directly from clinical samples (29). By sequencing a collection of 321 contemporary VZV isolates representing multiple countries and six continents, we sorted strains into three discrete geographically associated genotypes: E (European), J (Japanese), and M (mosaic). M strains were dominant in tropical latitudes, and J and E VZV isolates prevail in temperate climate latitudes (29). Genotype M isolates have an assortment of E-associated and J-associated mutations.
The epidemiology of VZV cases in the tropics differs from that in countries with a temperate climate. Characteristically, seroprevalence is markedly lower in tropical countries, and the pronounced seasonality of varicella disease observed in temperate climates is generally absent in warmer climates. What relationship, if any, exists between these differences in disease epidemiology and genotype is currently unknown.
We report here the comparison of two genotyping methods for wild-type VZV strains (29, 31) and seroprevalence data in the Finnish population among different age groups. Data presented in this paper also demonstrate the usefulness of and correlations between genotyping strategies.
MATERIALS AND METHODS
Serum sample testing.
Serum samples from 2,842 patients with suspected viral infection treated at neurologic, pediatric, or infectious disease wards or intensive care units of 18 tertiary care hospitals covering 77% of the Finnish population were sent to the Department of Virology, University of Helsinki, for serologic testing between 1995 and 1996. Serum immunoglobulin G (IgG) antibodies to VZV were measured using an in-house enzyme immunoassay test as previously described (32) (commercial antigen; VirionR, Würzburg, Germany). Each test run included positive and negative controls. The result was given in enzyme immune units. The cutoff for VZV seropositivity was ≥20 enzyme immune units.
Genotypic analysis of specimens.
VZV identification and genotyping were performed on 28 representative skin specimens. These specimens were collected from vesicular lesions of ambulatory and hospitalized pediatric and adult patients with varicella or zoster during 2003. All specimens were from clinical cases seen in tertiary care hospitals from different parts of Finland; no personal identifiers or demographic information was provided with the specimens. A sterile swab was used to scrub the base of unroofed vesicular lesions. For each selected patient, a swab of the base of a single vesicular lesion was collected. Specimens were placed onto Whatman FTA filters to inactivate virus and sent to the CDC for genotyping. Isolates from the Russian Federation, Estonia, Latvia, Germany, and Congo were obtained by the CDC through its international program to establish a repository of globally circulating VZV strains. The previously published genomic sequence data for the Dumas strain (gi9625875) and the parental Oka strain (gi26665420) were used as a reference for this study. VZV detection and discrimination of Oka vaccine from wild-type virus were accomplished using fluorescent resonance energy transfer-based real-time PCR targeting VZV ORF62 as previously described (1, 28, 30). PstI and BglI sites in ORF38 and ORF54 were evaluated using the same technology (29).
For genotyping, the ORF22 target region was amplified using conventional PCR as previously described (29) by using 100 ng of viral DNA or a 1-mm punch from an FTA card as a source of template. Specimens were also genotyped using an alternative method based on an analysis of single-nucleotide polymorphism (SNP) variation in seven amplicons representing regions in ORF1, ORF21, ORF31, ORF37, ORF50, ORF54, and ORF68 (4, 5, 31, 35, 37).
RESULTS
Serology.
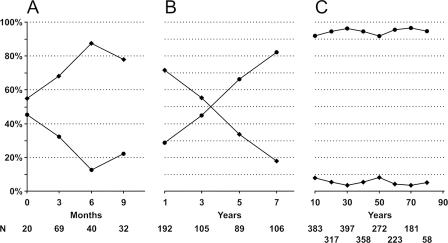
VZV IgG seroprevalence varied among age groups, with the lowest level observed in infants aged 6 to 8 months (Fig. 1A). With increasing age, seroprevalence steadily increased between the ages of 1 and 10 years and then plateaued at a high level throughout adulthood (Fig. 1B and C). VZV IgG was evident in most children <2 months old, reflecting the presence of maternal IgG antibodies, and all babies aged 7 months were seronegative. Among children 4 to 5 years old, seroprevalence exceeded 50%, and by 10 years of age, 91% of children were seropositive (only 3 of 33 children were negative). The sharpest rise in seroprevalence occurred in children aged 5 to 7 years, the age of school entry for the majority of children.
FIG. 1.
Seroprevalence of IgG antibody to VZV in the Finnish population. The total number of samples was 2,842 (482 negative and 2,360 positive). •, positive results; ⧫, negative results. (A) Infants <1 year of age. (B) Children 1 to 7 years of age. (C) Persons ≥10 years of age.
Genotypic analysis.
Of the two children aged 3 to 4 years and 26 adults from whom specimens were obtained, seven were ambulatory patients, and the rest were hospitalized for complications of the infection. All 28 specimens contained VZV DNA and were wild-type strains based on their vaccine SNP profile (Pst+ [ORF 38], Bgl− [ORF 54], and SmaI− [ORF 62]). All 28 samples tested were 100% identical to the European reference VZV Dumas strain in the 447-bp region of ORF22 (genotype E).
Ten samples contained sufficient DNA to permit additional genotyping by the multilocus (6, 31) method. This more extensive genotyping also revealed complete identity with the Dumas strain at all 35 SNP examined. All tested samples were classified as the C genotype with this VZV genotyping method, which is the equivalent to the E genotype using the ORF22 method. Only one difference, at position 33722, was detected (Table 1).
TABLE 1.
Nucleotide polymorphisms in representative isolates and laboratory reference strainsa
| ORF | Nucleotide position | Nucleotide identified in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dumas E strain (The Netherlands) | Finland varicella samples 1-4 | Finland zoster samples 5-10 | Russian Federation varicella samples 1-5 | Estonia varicella samples 1 and 2 | Latvia varicella samples 1 and 2 | Germany varicella samples 1 and 2 | Congo varicella M1 reference strain | Oka J reference strain | ||
| 1 | 560 | T | T | T | T | T | T | T | T | T |
| 561 | G | G | G | G | G | G | G | G | G | |
| 685 | G | G | G | G | G | G | G | G | A | |
| 703 | T | T | T | T | T | T | T | T | T | |
| 750 | G | G | G | G | G | G | G | G | G | |
| 763 | T | T | T | T | T | T | T | T | T | |
| 766 | A | A | A | A | A | A | A | A | A | |
| 789 | T | T | T | T | T | T | T | C | C | |
| 790 | T | T | T | T | T | T | T | C | C | |
| 791 | T | T | T | T | T | T | T | C | C | |
| 829 | T | T | T | T | T | T | T | T | T | |
| 892 | C | C | C | C | C | C | C | C | C | |
| 21 | 33646 | G | G | G | G | G | G | G | G | G |
| 33647 | A | A | A | A | A | A | A | A | A | |
| 33722 | T | C | C | C | C | C | C | C | C | |
| 33726 | T | T | T | T | T | T | T | C | C | |
| 33728 | T | T | T | T | T | T | T | C | C | |
| 22 | 37902 | A | A | A | A | A | A | A | A | G |
| 38055 | T | T | T | T | T | T | T | T | C | |
| 38081 | A | A | A | A | A | A | A | C | C | |
| 38177 | G | G | G | G | G | G | G | G | A | |
| 50 | 87841 | C | C | C | C | C | C | C | T | T |
| 54 | 95108 | C | C | C | C | C | C | C | C | C |
| 95118 | G | G | G | G | G | G | G | G | G | |
| 95241 | T | T | T | T | T | T | T | C | C | |
| 95262 | G | G | G | G | G | G | G | G | G | |
| 95300 | C | C | C | C | C | C | C | T | C | |
| 95333 | T | T | T | T | T | T | T | T | T | |
| 95339 | C | C | C | C | C | C | C | C | C | |
| 68 | 116255 | G | G | G | G | G | G | G | G | G |
| 116320 | C | C | C | C | C | C | C | C | C | |
| 116467 | T | T | T | T | T | T | T | C | T | |
| 116762 | T | T | T | T | T | T | T | T | T | |
Single-nucleotide differences from the Dumas E reference strain are shown in boldface type. The multisite and ORF22 genotyping methods determined that the genotypes of the Dumas E strain (The Netherlands), Finland varicella samples 1 to 4, Russian Federation varicella samples 1 to 5, Estonia varicella samples 1 and 2, Latvia varicella samples 1 and 2, and Germany varicella samples 1 and 2 were C and E, respectively. The multisite genotyping method determined that the genotype of the Congo varicella M1 reference strain was J1, and that of the Oka J reference strain was A2, while the ORF22 genotyping method determined that the genotype of the Congo varicella M1 reference strain was M1, and that of the Oka J reference strain was J.
DISCUSSION
This is the first study to report the genotypic analysis and seroprevalence of VZV in different areas of Finland. Serum samples from all over the country facilitated the calculation of representative seroprevalence rates for VZV. In our patient groups, the prevalence of VZV antibodies increased steadily from near zero at 6 to 7 months to over 90% by 10 years of age. The seroprevalence increased most rapidly over the school entry ages of 5 to7 years. However, a substantial fraction of persons remained VZV seronegative until after the age of 10 years, indicating a high level of susceptibility to VZV infection among older children.
Knowledge about these VZV seroprevalence data is important for understanding the dynamics underlying numerous complications associated with both primary and reactivated VZV infections in Finland (11, 12, 13, 14). Also, such seroprevalence data critically instruct appropriate immunization strategies for VZV (25). The impact of diseases associated with VZV infections, hospitalizations, and complications should be evaluated with respect to vaccination goals and strategies. Childhood immunization programs have dramatically changed the disease burden for many childhood infections (17, 33) and virtually changed the whole spectrum of childhood infections (20). Regarding VZV, a change in the epidemiology of primary infection appears to be evident (19); specifically, the highest incidence of varicella has apparently shifted from school-age children to small children aged 1 to 4 years (21).
To better comprehend the current VZV genotypes and geographic variation in Europe, we surveyed a variety of circulating strains from Finland and neighboring countries (Russian Federation, Lithuania, Estonia, and Germany) by performing genotypic analysis and SNP evaluation using two distinct and nonoverlapping methods (29). The VZV genome is one of the most highly conserved genomes among human viral pathogens, with only about 0.1% sequence variation between any two wild-type strains. Two independent groups established that VZV isolates from the same geographic region are quite similar (4, 29). Twenty-eight clinical varicella and zoster specimens representing strains circulating in Finland were identified being as genotype E isolates (genotype C using the multilocus method). No other genotypes were identified using either method. No vaccine strains were identified, and every strain had the Pst+ Bgl− SmaI− profile typical of most wild-type viruses (23, 24, 28, 30). E strains are the predominant genotype currently circulating in countries with temperate climates in Europe, North America, and eastern Australia, but often, countries on these continents also have other VZV genotypes in cocirculation. The detection of a single genotype in this study was not surprising, since about 98% of the Finnish population is of European origin, and travel between Finland and other European countries is more frequent than is travel to other parts of the world. Temperate Argentina revealed a similar uniformity for genotype E (9). Tropical countries such as Guinea Bissau, Zambia, Bangladesh, and Southern India predominately circulate M genotype strains, often with a BglI+ marker in ORF54 (4, 29, 31, 35, 36). In contrast to the Finnish specimens, M and J genotype isolates have often been identified in specimens from the United States, Brazil, the United Kingdom, and Australia. Significant genetic diversity has been observed among isolates from these countries, which has been attributed to recent patterns of human migration from other parts of the world (29, 31, 35). The significant rise in the prevalence of certain genotypes of varicella, together with the increase in the immigrant population found in the United Kingdom and the United States, is consistent with the genotypic analysis of circulating genotypes in different countries of origin for immigrants (31, 35). As such, the global distribution of circulating VZV genotypes is in flux, a process that appears to be driven by patterns of immigration in the world. Our findings in the current study, while characterizing a region that is stable with respect to VZV genotype, are consistent with this hypothesis.
Acknowledgments
We express our appreciation to the staff in all the hospitals, health care centers, and laboratories participating in serum and swab collection and Kirsti Räihä for excellent technical assistance in data entry. We also thank Marlene Deleon-Carnes for excellent technical assistance.
This work was supported in 2002 by an unmet-needs grant from the National Vaccine Program Office, Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Footnotes
Published ahead of print on 11 July 2007.
REFERENCES
- 1.Argaw, T., J. I. Cohen, M. Klutch, K. Lekstrom, T. Yoshikawa, Y. Asano, and P. R. Krause. 2000. Nucleotide sequences that distinguish Oka vaccine from parental Oka and other varicella-zoster virus isolates. J. Infect. Dis. 1811153-1157. [DOI] [PubMed] [Google Scholar]
- 2.Arvin, A. M. 1996. Varicella-zoster virus. Clin. Microbiol. Rev. 9361-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-Muir, W., K. Hawrami, J. Clarke, and J. Breuer. 2001. Investigation of varicella-zoster virus variation by heteroduplex mobility assay. Arch. Virol. Suppl. 1717-25. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Muir, W., F. T. Scott, P. Aaby, J. John, P. Matondo, Q. L. Chaudhry, M. Siqueira, A. Poulsen, K. Yaminishi, and J. Breuer. 2003. Genetic variation of varicella-zoster virus: evidence for geographical separation of strains. J. Med. Virol. 70(Suppl. 1)S42-S47. [DOI] [PubMed] [Google Scholar]
- 5.Campsall, P. A., N. H. Au, J. S. Prendiville, D. P. Speert, R. Tan, and E. E. Thomas. 2004. Detection and genotyping of varicella-zoster virus by TaqMan allelic discrimination real-time PCR. J. Clin. Microbiol. 421409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr, M. J., G. P. McCormack, and B. Crowley. 2004. Genetic variation in clinical varicella-zoster virus isolates collected in Ireland between 2002 and 2003. J. Med. Virol. 73131-136. [DOI] [PubMed] [Google Scholar]
- 7.Choo, P. W., J. G. Donahue, J. E. Manson, and R. Platt. 1995. The epidemiology of varicella and its complications. J. Infect. Dis. 172706-712. [DOI] [PubMed] [Google Scholar]
- 8.Chow, V. T., S. S. Wan, S. Doraisingham, and A. E. Ling. 1993. Comparative analysis of the restriction endonuclease profiles of the Dumas and Singapore strains of varicella-zoster virus. J. Med. Virol. 40339-342. [DOI] [PubMed] [Google Scholar]
- 9.Dayan, G. H., M. S. Panero, R. Debbag, A. Urquiza, M. Molina, S. Prieto, M. Del Carmen Perego, G. Scagliotti, D. Galimberti, G. Carroli, C. Wolff, D. S. Schmid, V. Loparev, D. Guris, and J. Seward. 2004. Varicella seroprevalence and molecular epidemiology of varicella-zoster virus in Argentina, 2002. J. Clin. Microbiol. 425698-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumas, A. M., J. L. Geelen, M. W. Weststrate, P. Wertheim, and J. van der Noordaa. 1981. XbaI, PstI, and BglII restriction enzyme maps of the two orientations of the varicella-zoster virus genome. J. Virol. 39390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echevarria, J. M., I. Casas, and P. Martinez-Martin. 1997. Infections of the nervous system caused by varicella-zoster virus: a review. Intervirology 4072-84. [DOI] [PubMed] [Google Scholar]
- 12.Fleisher, G., W. Henry, M. McSorley, A. Arbeter, and S. Plotkin. 1981. Life threatening complications of varicella. Am. J. Dis. Child. 135896-899. [DOI] [PubMed] [Google Scholar]
- 13.Gilden, D. H., B. R. Beinlich, E. M. Rubinstein, E. Stommel, R. Swenson, D. Rubinstein, and D. Mahalingam. 1994. Varicella-zoster virus myelitis: an expanding spectrum. Neurology 441818-1823. [DOI] [PubMed] [Google Scholar]
- 14.Gilden, D. H., B. K. Kleinschmidt-DeMasters, J. J. LaGuardia, R. Mahalingam, and R. J. Cohrs. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342635-645. [DOI] [PubMed] [Google Scholar]
- 15.Gilden, D. H., R. R. Wright, S. A. Schneck, J. M. Gwaltney, Jr., and R. Mahalingam. 1994. Zoster sine herpete, a clinical variant. Ann. Neurol. 35530-533. [DOI] [PubMed] [Google Scholar]
- 16.Hawrami, K., and J. Breuer. 1997. Analysis of United Kingdom wild-type strains of varicella-zoster virus: differentiation from the Oka vaccine strain. J. Med. Virol. 5360-62. [DOI] [PubMed] [Google Scholar]
- 17.Heisler, M. B., and J. B. Richmond. 1994. Lessons from Finland's successful immunization program. N. Engl. J. Med. 3311446-1447. [DOI] [PubMed] [Google Scholar]
- 18.Koskiniemi, M., T. Rantalaiho, H. Piiparinen, C.-H. von Bonsdorff, M. Färkkilä, A. Järvinen, E. Kinnunen, S. Koskiniemi, L. Mannonen, M. Muttilainen, K. Linnavuori, J. Porras, M. Puolakkainen, K. Räihä, E.-M. Salonen, P. Ukkonen, A. Vaheri, V. Valtonen, et al. 2001. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J. Neurovirol. 7400-408. [DOI] [PubMed] [Google Scholar]
- 19.Koskiniemi, M., J. Rautonen, E. Lehtokoski-Lehtiniemi, and A. Vaheri. 1991. Epidemiology of encephalitis in children: a 20-year survey. Ann. Neurol. 29492-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koskiniemi, M., and A. Vaheri. 1989. Effect of measles, mumps, rubella vaccination on pattern of encephalitis in children. Lancet i31-34. [DOI] [PubMed] [Google Scholar]
- 21.Kudesia, G., S. Partridge, C. P. Farrington, and N. Soltanpoor. 2002. Changes in age related seroprevalence of antibody to varicella zoster virus: impact on vaccine strategy. J. Clin. Pathol. 55154-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupila, L., T. Vuorinen, R. Vainionpaa, V. Hukkanen, R. Marttila, and P. Kotilainen. 2006. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 6675-80. [DOI] [PubMed] [Google Scholar]
- 23.LaRussa, P., O. Lungu, I. Hardy, A. Gershon, S. P. Steinberg, and S. Silverstein. 1992. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 661016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaRussa, P., S. Steinberg, A. Arvin, D. Dwyer, M. Burgess, M. Menegus, K. Rekrut, K. Yamanishi, and A. Gershon. 1998. Polymerase chain reaction and restriction fragment length polymorphism analysis of varicella-zoster virus isolates from the United States and other parts of the world. J. Infect. Dis. 178(Suppl. 1)S64-S66. [DOI] [PubMed] [Google Scholar]
- 25.Law, B., N. MacDonald, S. Halperin, D. Scheifele, P. Dery, T. Jadavji, M. H. Lebel, E. Mills, R. Morris, W. Vaudry, R. Gold, V. Marchessault, and P. Duclos. 2000. The Immunization Monitoring Program Active (IMPACT) prospective five year study of Canadian children hospitalized for chickenpox or associated complications. Pediatr. Infect. Dis. J. 191053-1059. [DOI] [PubMed] [Google Scholar]
- 26.Lee, B. W. 1998. Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop. Med. Int. Health 3886-890. [DOI] [PubMed] [Google Scholar]
- 27.Lolekha, S., W. Tanthiphabha, P. Sornchai, P. Kosuwan, S. Sutra, B. Warachit, S. Chup-Upprakarn, Y. Hutagalung, J. Weil, and H. L. Bock. 2001. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am. J. Trop. Med. Hyg. 64131-136. [DOI] [PubMed] [Google Scholar]
- 28.Loparev, V. N., T. Argaw, P. R. Krause, M. Takayama, and D. S. Schmid. 2000. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strain using a VZV open reading frame 62-based PCR. J. Clin. Microbiol. 383156-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loparev, V. N., A. Gonzalez, M. Deleon-Carnes, G. Tipples, H. Fickenscher, E. G. Torfason, and D. S. Schmid. 2004. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J. Virol. 788349-8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loparev, V. N., K. McCaustland, B. P. Holloway, P. R. Krause, M. Takayama, and D. S. Schmid. 2000. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J. Clin. Microbiol. 384315-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir, W. B., R. Nichols, and J. Breuer. 2002. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J. Virol. 761971-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustonen, K., P. Mustakangas, M. Smeds, L. Mannonen, L. Uotila, A. Vaheri, and M. Koskiniemi. 1998. Antibodies to varicella zoster virus in the cerebrospinal fluid of neonates with seizures. Arch. Dis. Child. Fetal Neonatal Ed. 78F57-F61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltola, H., O. P. Heinonen, M. Valle, M. Paunio, M. Virtanen, V. Karanko, and K. Cantell. 1994. The elimination of indigenous measles, mumps, and rubella from Finland by a 12-year, two-dose vaccination program. N. Engl. J. Med. 3311397-1402. [DOI] [PubMed] [Google Scholar]
- 34.Preblud, S. R., W. A. Orenstein, and K. J. Bart. 1984. Varicella: clinical manifestations, epidemiology and health impact in children. Pediatr. Infect. Dis. 3505-509. [PubMed] [Google Scholar]
- 35.Quinlivan, M., K. Hawrami, W. Barrett-Muir, P. Aaby, A. Arvin, V. T. Chow, T. J. John, P. Matondo, M. Peiris, A. Poulsen, M. Siqueira, M. Takahashi, Y. Talukder, K. Yamanishi, M. Leedham-Green, F. T. Scott, S. L. Thomas, and J. Breuer. 2002. The molecular epidemiology of varicella-zoster virus: evidence for geographic segregation. J. Infect. Dis. 186888-894. [DOI] [PubMed] [Google Scholar]
- 36.Takayama, M., N. Takayama, N. Inoue, and Y. Kameoka. 1996. Application of long PCR method of identification of variations in nucleotide sequences among varicella-zoster virus isolates. J. Clin. Microbiol. 342869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenaar, T. R., C. Grose, V. N. Loparev, D. S. Schmid, and J. Breuer. 2003. Genomic analysis of varicella-zoster virus: primers for individual open reading frames. J. Clin. Virol. 28104-110. [DOI] [PubMed] [Google Scholar]