Abstract
We evaluated West Nile virus (WNV) antibody persistence by using follow-up plasma samples from 35 blood donors who made viremic donations in 2005. At 26 to 34 days of follow-up, all of the donors (n = 33) were positive for WNV immunoglobulin M (IgM), IgA, and IgG. At 1-year of follow-up, 17% of the donors (n = 23) were positive for WNV IgM, 57% were positive for WNV IgA, and 100% were positive for WNV IgG.
West Nile virus (WNV) is now considered endemic to the United States because of the documentation of human WNV infections in all of the 48 contiguous states (2). Recent WNV infection is usually diagnosed on the basis of detection of WNV immunoglobulin M (IgM) in serum or plasma (5); however, the diagnostic utility of WNV IgM detection as an indicator of recent infection has come into question, largely because of a report documenting WNV IgM persistence for >500 days in 7 of 12 patients with WNV encephalitis (8). It has been suggested that WNV IgA detection may be better than WNV IgM detection as a marker of recent infection; in one study, WNV IgA was rarely detected in serum collected >50 days postinfection (4). To address these issues, we investigated WNV antibody persistence in blood donors who made a viremic donation in 2003; the results of that study (7) showed that IgM seroreversion occurred approximately 218 days after the viremic donation, contrasting with the findings reported for WNV encephalitis patients (8). Similarly, IgA seroreversion occurred approximately 232 days after the viremic donation (7), indicating that the diagnostic utility of WNV IgA detection as an indicator of recent infection is no better than that of WNV IgM detection. A limitation of the study was the small number of specimens (n = 11) collected >250 days after the viremic donation (7); therefore, we repeated the WNV antibody persistence study by using follow-up plasma samples from 35 viremic blood donors identified during the 2005 season.
WNV RNA-positive blood donors (n = 35) were identified by nucleic acid amplification test screening of donations made between June and November 2005, as previously described (1, 9). Plasma samples from donations confirmed as WNV RNA positive (hereafter referred to as the index donations), as well as plasma specimens collected during follow-up visits, were supplied by Blood Systems Research Institute, San Francisco, CA. Informed consent was obtained from all of the donors at the local blood donation site; protocols for nucleic acid amplification test screening and follow-up were approved by local institutional review boards and the Food and Drug Administration.
Plasma specimens were tested for WNV IgM and IgG by using Food and Drug Administration-cleared enzyme-linked immunosorbent assay kits manufactured by Focus Diagnostics (3, 7); in accordance with the kit inserts, an IgG sample-to-calibrator ratio (SCR) of >1.5 and an IgM SCR of >1.1 were considered positive. WNV IgA was measured in follow-up specimens with a laboratory-developed alpha-capture enzyme-linked immunosorbent assay as previously described (6, 7); an IgA SCR of >1.0 was considered positive.
Five follow-up (days postindex) time windows were selected for assessment of WNV antibody persistence; these time windows represent a targeted number of follow-up days ±15% of the targeted number of days. The five windows were thus 30 ± 4 days, 60 ± 9 days, 90 ± 14 days, 180 ± 27 days, and 365 ± 55 days. Results from one specimen per donor (if available) were included in each time window; if multiple results were available for a given donor within a given time window, the result for the sample collected closest to the time window target (i.e., 30, 60, 90, 180, or 365 days) was used.
Of the 35 viremic donors who participated in this study, 31 agreed to an interview, conducted 3 weeks after the index donation, regarding symptoms and medical care. Six (19%) of the 31 sought medical attention between the index donation date and the interview date, and 1 of these 6 donors was hospitalized with a diagnosis of West Nile fever. None of the interviewed donors were diagnosed with WNV neuroinvasive disease.
Of the 35 index donations, 27 were negative for both WNV IgM and IgG, 4 were positive for WNV IgM only, 3 were positive for both WNV IgM and IgG, and 1 was positive for WNV IgG only. Because of logistical limitations, plasma samples from most of the index donations were not available for WNV IgA testing.
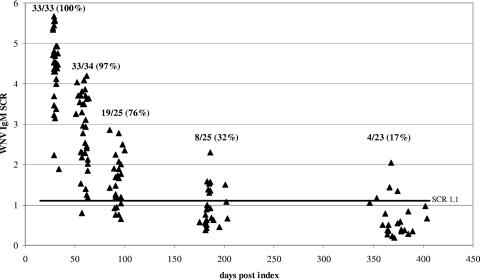
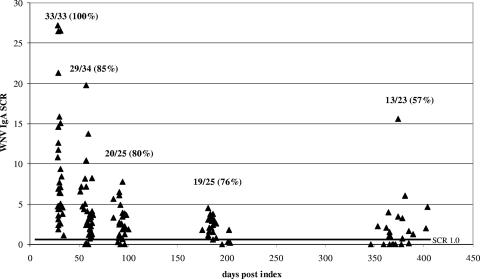
WNV IgM persistence results are shown in Fig. 1. All of the donors were positive for WNV IgM at the 30-day follow-up. The proportion of donors positive for WNV IgM at other follow-up time points decreased as a function of time, with only 17% of the donors still positive for WNV IgM at the 1-year follow-up. Similarly, all of the donors were positive for WNV IgA at the 30-day follow-up and 57% were still positive for WNV IgA at 1 year (Fig. 2). The proportion of donors positive for WNV IgA at the one-year follow-up was significantly higher than the proportion of donors positive for WNV IgM at the same follow-up time point (Fisher exact test, P = 0.013 [significance was defined as P < 0.05]). As expected, all of the donors were positive for WNV IgG at all of the follow-up time points (data not shown). All six of the donors who sought medical attention, including the donor hospitalized with West Nile fever, were negative for both WNV IgM and IgA at the 1-year follow-up time point.
FIG. 1.
Persistence of WNV IgM in blood donors who made a viremic donation. Within a cluster of points around the targeted days postindex (30, 60, 90, 180, and 365 days), each point represents the result for one donor. The values at the top of each cluster are the number of specimens that were positive for WNV IgM/the total number of specimens in the cluster, with the resulting percent positive in parentheses. The dark horizontal line indicates the SCR used to discriminate IgM-positive from IgM-negative results.
FIG. 2.
Persistence of WNV IgA in blood donors who made a viremic donation. Within a cluster of points around the targeted days postindex (30, 60, 90, 180, and 365 days), each point represents the result for one donor. The values at the top of each cluster are the number of specimens that were positive for WNV IgA/the total number of specimens in the cluster, with the resulting percent positive in parentheses. The dark horizontal line indicates the SCR used to discriminate IgA-positive from IgA-negative results.
These results confirm and extend our previous findings (7), demonstrating that WNV IgM persistence for a year or more in WNV-infected blood donors is not a common occurrence. The differences between our findings and those of Roehrig et al. (8), who found that WNV IgM persisted for more than a year in 7 of 12 patients with WNV encephalitis, most likely reflect differences in the study groups. Only 19% of participating donors in our study sought medical attention, and none were diagnosed with WNV neuroinvasive disease. Thus, assuming that blood donors are representative of the general population, the uncommon persistence of WNV IgM for a year or more in this group indicates that WNV IgM detection remains a useful diagnostic tool for identifying recent WNV infection.
Likewise, the findings presented here for WNV IgA persistence confirm and extend our previous findings (7) and clearly show that most infected blood donors have detectable WNV IgA well after 50 days postinfection; our results thus contrast with findings previously reported by another group of investigators (4). Indeed, the proportion of donors still positive for WNV IgA at 1 year of follow-up was significantly higher than the proportion still positive for WNV IgM, indicating that WNV IgA detection is inferior to WNV IgM detection as a marker of recent WNV infection. When these findings are taken together with published data demonstrating similar appearance kinetics for WNV IgA and IgM (7), there appears to be no role for WNV IgA measurements in either diagnosing or estimating the time since WNV infection.
Footnotes
Published ahead of print on 25 July 2007.
REFERENCES
- 1.Busch, M. P., S. Caglioti, E. F. Robertson, J. D. McAuley, L. H. Tobler, H. Kamel, J. M. Linnen, V. Shyamala, P. Tomasulo, and S. H. Kleinman. 2005. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N. Engl. J. Med. 353460-467. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Accessed 17 May 2004. 2004 West Nile virus activity in the United States. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/ncdod/cvbid/westnile/surv&control06Maps.htm.
- 3.Hogrefe, W. R., R. Moore, M. Lape-Nixon, M. Wagner, and H. E. Prince. 2004. The performance of West Nile virus recombinant (preM/E)-based immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assay for detection of West Nile virus- and other flavivirus-specific antibodies. J. Clin. Microbiol. 424641-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanciotti, R. S. Accessed 11 May 2004. Current laboratory protocols for West Nile virus. Centers for Disease Control and Prevention, Atlanta, GA. www.cdc.gov/ncidod/dvbid/westnile/conf/February_2004.htm.
- 5.Petersen, L. R., and A. A. Marfin. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137173-179. [DOI] [PubMed] [Google Scholar]
- 6.Prince, H. E., and M. Lape-Nixon. 2005. Evaluation of a West Nile virus immunoglobulin A (WNV IgA) capture enzyme-linked immunosorbent assay (ELISA). Clin. Diagn. Lab. Immunol. 12231-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince, H. E., L. H. Tobler, M. Lapé-Nixon, G. A. Foster, S. L. Stramer, and M. P. Busch. 2005. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J. Clin. Microbiol. 434316-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roehrig, J. T., D. Nash, B. Maldin, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 9376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stramer, S. L., C. T. Fang, G. A. Foster, A. G. Wagner, J. P. Brodsky, and R. Y. Dodd. 2005. West Nile virus among blood donors in the United States, 2003 and 2004. N. Engl. J. Med. 353451-459. [DOI] [PubMed] [Google Scholar]