Abstract
Staphylococcal enterotoxin B (SEB) is a select agent because it is a potent mitogen that elicits life-threatening polyclonal T-cell proliferation and cytokine production at very low concentrations. Efforts are in progress to develop therapeutic reagents and vaccines that neutralize or prevent the devastating effects of this toxin. Because of its rapid binding to in vivo receptors, this toxin is difficult to detect in serum. This rapid binding also constitutes a major challenge for the development of effective therapeutic reagents that can neutralize the effects of the toxin in vivo. We have developed a highly sensitive capture enzyme-linked immunosorbent assay that detects SEB in body fluids at very low levels. With this assay, the peak levels of SEB in serum and renal clearance can be measured in mice. After either oral ingestion or nasal inhalation of SEB by mice, this assay documents the transcytosis of SEB across the mucosal membranes into serum within 2 h. Furthermore, this assay was used to compare the SEB levels in different murine models for SEB-induced lethal shock and demonstrated that the coadministration of toxin-enhancing chemicals, such as d-galactosamine and lipopolysaccharide, can alter the peak serum SEB levels. Hence, this assay is a potentially useful tool for the study of the pharmacokinetics of SEB and the effects of potential therapeutic reagents on serum SEB levels.
The staphylococcal enterotoxins compromise a family of distinct toxins (toxins A to E) that are excreted by various strains of Staphylococcus aureus (12). Staphylococcal enterotoxin B (SEB) is the primary cause of food poisoning, and ingestion of SEB induces emesis and diarrhea. At low serum concentrations, SEB can trigger toxic shock, profound hypotension, and multiorgan failure. SEB is the major enterotoxin associated with nonmenstrual toxic shock syndrome and accounts for the majority of intoxications that are not caused by toxic shock syndrome toxin 1 (TSST-1) (17).
SEB is a well-characterized 28-kDa protein that is most closely related to SEC and the streptococcal pyrogenic exotoxins A and C (12, 33). Like all of the aforementioned toxins, SEB is a superantigen and is one of the most potent mitogens described. SEB mediates its biological effects by binding to the major histocompatibility complex (MHC) class II complex at a distinct site and is different from other antigens in that it does not have to be preprocessed. The toxin is presented to a T-cell antigen receptor by an MHC class II molecule, forming ternary complexes that trigger cytokine production and T-cell proliferation (5, 13, 18, 33).
During the 1960s, when the United States had an offensive biological warfare program, SEB, then code named PG, was studied extensively as a biological incapacitant. The toxin was especially attractive as a biological agent because much lower quantities of SEB than of synthetic chemicals were needed to produce intoxicating effects. The dose of SEB that is incapacitating for 50% of the human population exposed to SEB was predicted to be 0.0004 μg/kg of body weight, and the 50% lethal dose was predicted to be 0.02 μg/kg by both the inhalational and the intravenous routes (36). SEB can easily be synthesized in large quantities and is considered a major agent of biological warfare. SEB is currently listed as a category B select agent (36).
Staphylococcus sepsis is very common and is associated with high rates of mortality and high costs in affected patients (29). The presence of genes encoding for SEB and several other bacterial superantigens in clinical S. aureus isolates has been described (10, 11). However, the contribution of SEB production to the outcome of staphylococcus-related diseases is not known. This is of particular importance, because the effects of toxins could potentially be neutralized with specific immune globulin therapy (2, 14, 15). Only a few clinical studies have actually demonstrated the presence of SEB in the serum of patients with staphylococcal infections (1), presumably because of the lack of highly sensitive detection methods. Most commercially available diagnostic tests detect SEB in the nanogram range (20, 22, 30-32, 34) Although mass spectrometry has created a niche for the analysis of proteinaceous toxins, its main drawbacks are its sophisticated instrumentation and its high costs (16). Hence, sensitive immunoassays continue to provide a more realistic alternative. We report on a highly sensitive capture enzyme-linked immunosorbent assay (ELISA) that can be used to further investigate the pathogenesis and treatment of SEB-induced disease.
MATERIALS AND METHODS
Toxins.
Purified toxins were obtained from Toxin Technologies (Sarasota, FL). SEB, SEA, SEC1, and TSST were obtained in lyophilized form. SEB stocks were prepared at 1 mg/ml in phosphate-buffered saline (PBS), and aliquots were stored at −20°C.
MAbs.
Monoclonal antibodies (MAbs) to SEB were generated in the Hybridoma Facility of the Cancer Center at the Albert Einstein College of Medicine. Three of the MAbs, 10F1 (immunoglobulin A [IgA]), 17C12 (IgG2a), and 20B1 (IgG1), were further used in these studies. A concentrated hybridoma supernatant containing MAb was used as a source of antibody. The antibody concentrations were determined by ELISA by the use of commercial isotype standards (MP Biomedicals) of known concentration.
Staphylococcus aureus strains.
S. aureus isolates from bacteremic patients were obtained from the microbiology laboratory at the Montefiore Medical Center (Bronx, NY). Isolates were maintained on blood agar plates and grown in brain heart infusion (BHI) medium (Bacto-BD) at 37°C with shaking. DNA was isolated with a commercially available kit (QIAGEN Inc.), following the manufacturer's instructions. The presence of the SEB gene in clinical strains was determined by PCR with previously published primers and by previously published protocols (9). Genetically engineered S. aureus strains (strains RN6734, PRN7114/RN9432, and PRN7116/RN9469) were kindly provided by R. P. Novick (Skirball Institute, New York University) and were used as control strains. These strains are described in detail elsewhere (37). RN6734 is an enterotoxin-negative strain, and PRN7114 contains an intact SEB gene on a plasmid, while PRN7116 was transformed with a plasmid containing a truncated SEB gene and, consequently, does not produce SEB. In addition, clinical S. aureus strains from our own collection were used; these included strains 2, 9, and 36, which are methicillin-resistant and SEB-positive strains, and strain 18, which is a methicillin-resistant SEB-negative strain.
Capture ELISAs.
The ELISAs were performed in 96-well polystyrene plates (Corning, Corning, NY). Capture ELISAs were configured in a double-sandwich format, similar to that used in previously published assays (4, 35), in which the “bottom” MAb was used to capture the antigen (SEB) and the “top” MAb was used to detect SEB. We developed two separate assays, both with the same “bottom” antibody but each with a different “top” or “detection” antibody of different isotypes. The protocols are as follows. First, the polystyrene plates were coated for 1 h at room temperature (RT) with 100 μl of 1 μg/ml goat anti-mouse IgA polyclonal antibody diluted in 0.02 M PBS (pH 7.2). Second, the plates were blocked with 5% milk in PBS overnight at 4°C. Third, the “bottom” or “capture” antibody, MAb 10F1 (IgA), was added at a volume of 100 μl per well at a concentration of 1 μg/ml diluted in 1% bovine serum albumin (BSA) in PBS (1% BSA-PBS). The plate was incubated for 30 min at RT and was then washed three times in an automated microtiter plate washer (Skanwasher 400; Molecular Devices). All washes were performed with PBS containing 0.1% Tween 20 and were interrupted by 1-min soaks. Fourth, the samples were added to each well and serially diluted across 12 wells. Purified SEB (1 μg/ml) diluted in 1% BSA-PBS was used as the concentration standard. The samples and the SEB standard were incubated for 1 h at RT. Fifth, the “top” or “detection” MAb, MAb 20B1 (IgG1) or MAb 17C12 (IgG2a), was added at 1 μg/ml in 1% BSA-PBS, and the plates were incubated for 30 min at RT. Sixth, 1 μg/ml of alkaline phosphatase-conjugated goat anti-mouse IgG1 or IgG2a in 1% BSA-PBS for MAb 20B1 or 17C12, respectively, was added for 30 min at RT. Lastly, for colorimetric detection, 50 μl per well of phosphatase substrate (1 mg/ml p-nitrophenylphosphate in 0.001 M MgCl2 and 0.05 M Na2CO3, pH 9.8) was added for 15 to 30 min at RT. The plates were read at 405 nm with a microtiter plate reader (Emax micro plate reader; Molecular Devices).
ELISA specificity for SEB.
To determine the specificity of the ELISA for SEB compared with its specificity for other staphylococcal enterotoxins, we diluted purified SEB, SEC1, SEA, and TSST-1 to a concentration of 2 μg/ml in assay diluent (BSA-PBS) and measured these samples in the ELISA described above.
Staphylococcal protein A interference.
Purified SEB was diluted to 1 μg/ml into a solution of recombinant staphylococcal protein A (Pierce) at concentrations ranging from 1,000 to 15.6 ng/ml in assay diluent (BSA-PBS). The samples were assayed by the capture ELISA with the anti-SEB specific antibodies described above, and the values were compared to those for SEB diluted in assay diluent alone to determine possible interference from protein A. Recombinant protein A was used to avoid possible enterotoxin contamination.
SEB detection in culture supernatants and whole-cell lysates.
Single colonies from the S. aureus isolates were grown in BHI medium overnight. For SEB quantification, these cultures were diluted into fresh medium to an A600 of 0.1 and were cultured for an additional 24 h. Over the first 7 h, aliquots of the supernatant were collected hourly and the absorbance at 600 nm was measured. The time period of supernatant sampling corresponded to the logarithmic growth phase of the cultures. All supernatant samples were frozen, and the SEB concentration was determined by the capture ELISA. For the detection of SEB in whole-cell lysates, approximately 10 μl of cells was scraped directly from a blood agar plate. The cells were boiled in 100 μl of PBS for 30 min and then cooled to 4°C. The cellular debris was pelleted by centrifugation, and the SEB concentration in the resulting supernatant was measured by ELISA.
Analysis of biological specimens.
The ability to accurately measure SEB in biological fluids was determined by diluting a known amount (1 μg/ml) of SEB in solutions of 50% human serum, 50% mouse serum, or 15 mg/ml mouse feces. The levels in these samples, as well as those in a standard sample of SEB diluted in assay diluent, were measured by the ELISA described above. Prior to these experiments, the biological fluids used were analyzed for the presence of preexisting anti-SEB antibodies by a direct-binding ELISA and were determined to have nondetectable levels (data not shown).
Mouse experiments.
BALB/c mice (NCI) (n = 5 to 10 mice per group) were challenged by intraperitoneal (i.p.) injection, oral gavage feeding, or intranasal (i.n.) administration of SEB. Mice were injected i.p. with either 25 mg of d-galactosamine or 70 μg of lipopolysaccharide (LPS; Sigma) in PBS, followed by 50 μg of purified SEB dissolved in 100 μl of PBS. HLA-DR3 transgenic mice (27) (a generous gift of C. S. David at the Mayo Clinic) were also injected i.p. with 50 μg of purified SEB dissolved in 100 μl of PBS. This concentration was suggested by other investigators to be the 100% lethal dose within 48 h for mice (25). Sera were collected by retro-orbital bleeding at 8 h postinjection and frozen. Urine release was induced by rubbing the mouse's lower abdomen, and the urine was collected in a microcentrifuge tube. The mice were also fed 200 μg of SEB in PBS by oral gavage. The mice developed mild symptoms of intoxication (decreased mobility) within 24 h but survived. For i.n. administration, the mice were briefly anesthetized and 20 μg of SEB in 20 μl of PBS was placed on their nostrils, which allowed inhalation of the toxin. These mice developed no symptoms. SEB levels were also measured in the serum of mice 2, 8, and 24 h after infection with 109 CFU of clinical S. aureus strains that were SEB positive (strain 36) and SEB negative (strain 18).
Statistical analysis.
Student t tests, standard curves, concentrations, and trend lines were generated and calculated by using Excel X software (Microsoft Corporation).
RESULTS
Configuration of capture ELISA.
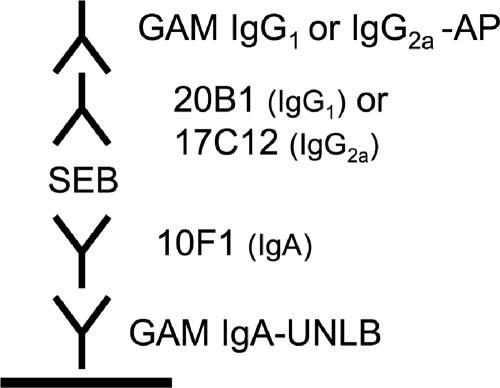
Five different SEB-specific MAbs (two IgG1-specific, one IgG2a-specifc, and two IgA-specific MAbs) were evaluated in 16 different combinations to identify the combination that produced the most sensitive ELISAs (Table 1). Four MAb combinations yielded good standard curves that allowed the fitting of trend lines used for the quantification of SEB. The other combinations either did not bind to the toxin, bound to the toxin only at high concentrations of SEB, or did not yield values with a good linear fit. On the basis of antibody availability and detection cutoff levels, we focused on further testing of two capture ELISA configurations that used the IgA MAb for the capture of SEB and either the IgG1 or IgG2a MAb for the detection of SEB. The configurations of the ELISAs used in the experiments described below are outlined in Fig. 1. ELISA-based binding assays with both the detection and the capture MAbs (MAbs 10F1 and 17C12, respectively, as well as MAbs 10F1 and 20B1, respectively) simultaneously revealed no competition, from which we conclude that the two MAbs do not recognize the same epitope on SEB.
TABLE 1.
Sensitivities of different capture ELISAs tested
| Capture MAb (isotype) | Detection MAb | Isotype | Sensitivitya |
|---|---|---|---|
| 6D3 (IgG1) | 7H3 | IgA | ND |
| 10F1 | IgA | 50 pg/ml | |
| 17C12 | IgG2a | ND | |
| 20B1 (IgG1) | 7H3 | IgA | ND |
| 10F1 | IgA | 4 ng/ml | |
| 17C12 | IgG2a | 16.9 pg/ml | |
| 7H3 (IgA) | 6D3 | IgG1 | ND |
| 20B1 | IgG1 | ND | |
| 17C12 | IgG2a | ND | |
| 10F1 (IgA) | 6D3 | IgG1 | 50 pg/ml |
| 20B1 | IgG1 | 50 pg/ml | |
| 17C12 | IgG2a | 4 ng/ml | |
| 17C12 (IgG2a) | 6D3 | IgA | ND |
| 20B1 | IgG1 | ND | |
| 7H3 | IgA | 40 ng/ml | |
| 10F1 | IgA | ND |
Sensitivity is defined by the lowest concentration of SEB from which the starting concentration (1 μg/ml) could be calculated by using the linear-fit standard curve generated in each ELISA. Antibody combinations without a sensitivity value listed did not successfully bind SEB in this assay; hence, calculations were not done (ND).
FIG. 1.
Configuration of capture ELISA for SEB detection. GAM, goat anti-mouse; AP, alkaline phosphatase; UNLB, unlabeled.
Sensitivity and specificity of capture ELISA.
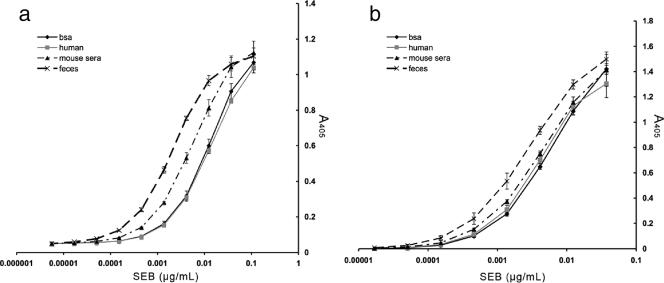
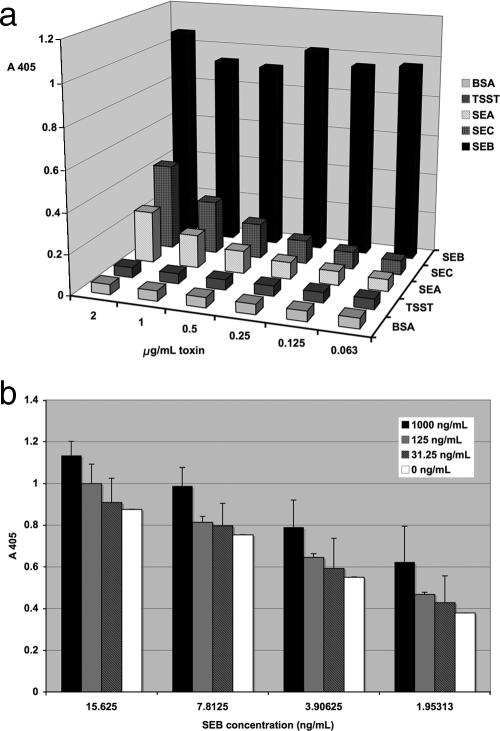
A standard curve was constructed for both ELISA combinations by plotting the absorbance (y axis) against the concentration (x axis) by using the results for purified toxin diluted to 1 μg/ml and serially diluted in either 1% BSA, 50% mouse or human serum, or feces (Fig. 2). Linear regression trend lines were generated for the lower part of the standard curves to calculate the concentrations in unknown SEB-containing samples. The samples were analyzed in triplicate, and the calculated concentrations from different diluents (serum, feces, etc.) were compared to determine the sensitivity and the accuracy of the assay. The lower limit was defined as the lowest dilution from which the original concentration of toxin (1 μg/ml) could be calculated from the equation for linear fit. The sensitivity of the test was comparable in mouse serum as well as human serum and BSA (Table 2). The accuracy was mildly reduced (twofold) with feces. Most importantly, the two assays showed that the quantification of SEB was accurate and yielded calculated values that were very close to the actual amount of toxin added (Table 2). The ELISA with the combination of MAbs 10F1 and 20B1 was slightly more sensitive, with toxin detected at a level of 20 pg/ml. We analyzed the specificities of these capture ELISAs for SEB by assaying other staphylococcal enterotoxins. Our data demonstrate that the ELISAs also detect SEC1 and SEA, although the sensitivities were much lower than that for SEB (Fig. 3a). Staphylococcal protein A, a staphylococcal cell wall protein, is known to interfere with ELISAs due to the ability of the N terminus of the protein to bind to IgG (7). To address this possible interference, we examined the sensitivities of these ELISAs in the presence of increasing concentrations of protein A. These tests determined that in the presence of high concentrations of protein A (1,000 ng/ml) the levels of SEB measured were slightly increased. This effect was not seen at lower protein A concentrations (Fig. 3b) and was not inhibitory of detection. In addition, the supernatants from non-SEB-producing strains (RN6734 and PRN7116/RN9469) resulted in absorbance levels comparable to the background levels, as measured by the ELISA (Fig. 4). Thus, these findings demonstrate that staphylococcal protein A, which may be shed during in vitro growth (and which may also be shed by non-SEB-producing strains) does not interfere with SEB measurements by ELISA. In summary, two highly sensitive capture ELISAs were developed. These ELISAs reproducibly detected SEB in serum and feces. Cross-reactivity with other enterotoxins was present but limited, and interference by protein A was observed only at high concentrations and increased the SEB level measured, reducing the accuracy but not limiting detection.
FIG. 2.
SEB detection by two different MAb sandwich ELISAs. (a) MAbs 10F1 and 20B1; (b) MAbs 10F1 and 17C12. A comparison of SEB detection in 50% murine serum, 50% human serum, feces, and BSA is shown. The test was performed in triplicate, and error bars represent standard deviations.
TABLE 2.
Results of ELISAs with MAbs 10F1 and 20B1 and MAbs 10F1 and 17C12
| MAbs and quantity of SEB added (ng/ml) | Quantity of SEB (ng/ml) determined in the following diluents:
|
|||
|---|---|---|---|---|
| 1% BSA | 50% human serum | 50% mouse serum | 15 mg/ml mouse feces | |
| MAbs 10F1 and 20B1 | ||||
| 37.04 | 37.24 ± 0.015 | 26.26 ± 0.008 | 34.56 ± 0.003 | 440.1 ± 0.005 |
| 12.35 | 13.71 ± 0.001 | 15.60 ± 0.003 | 16.59 ± 0.0003 | 24.89 ± 0.002 |
| 4.12 | 3.94 ± 0.0002 | 4.61 ± 0.001 | 5.28 ± 0.0003 | 8.89 ± 0.0007 |
| 1.37 | 1.36 | 1.53 | 1.85 | 2.64 ± 0.0003 |
| 0.46 | 0.50 | 0.56 | 0.75 | 1.17 ± 0.0002 |
| 0.15 | 0.15 | 0.17 | 0.23 | 0.42 |
| 0.05 | 0.04 | 0.05 | 0.08 | 0.14 |
| 0.02 | 0.02 | 0.01 | 0.02 | 0.04 |
| MAbs 10F1 and 17C12 | ||||
| 37.04 | 37.99 ± 0.006 | 30.75 ± 0.0011 | 32.13 ± 0.0141 | 67.15 ± 0.01 |
| 12.35 | 12.15 ± 0.002 | 10.90 ± 0.0007 | 13.45 ± 0.0046 | 47.25 ± 0.005 |
| 4.12 | 4.03 ± 0.0005 | 3.91 ± 0.0002 | 4.68 ± 0.0011 | 21.46 ± 0.001 |
| 1.37 | 1.61 ± 0.0002 | 1.50 ± 0.0001 | 1.74 ± 0.0001 | 7.27 ± 0.0002 |
| 0.46 | 0.50 ± 0.0001 | 0.46 ± 0.0001 | 0.65 ± 0.0001 | 2.85 ± 0.0003 |
| 0.15 | 0.1 | 0.1 | 0.19 | 1.03 ± 0.0002 |
| 0.05 | 0.04 | 0.31 ± 0.0001 | ||
| 0.02 | 0.05 | |||
FIG. 3.
(a) Measurement of equal amounts of SEA, SEC, TSST-1, and SEB by ELISA (with MAbs 10F1 and 20B1). As expected, some cross-reactivity is observed at the highest dose of SEC, which is closely related to SEB. (b) Measurement of SEB by ELISA in the presence of increasing staphylococcal protein A concentrations. The test was performed in triplicate, and error bars represent standard deviations. Note that the measurements were affected only by the presence of high levels of protein A (1,000 ng/ml), as the capture ELISA measures slightly increased SEB levels compared to the levels measured for the control.
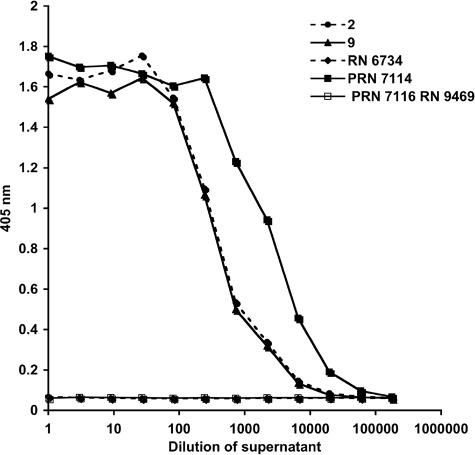
FIG. 4.
SEB was measured by capture ELISA in diluted supernatants of three SEB-positive S. aureus strains (strains 2, 9, and PRN7114) after overnight growth in BHI medium at 37°C. The supernatants from S. aureus strains that contained either no SEB gene or a defective gene were used as controls (PRN7116/RN9469, RN6734). Beginning with undiluted samples, the supernatant was serially diluted 1:3 across 12 wells. SEB-negative strains demonstrated background detection (A405, approximately 0.06) for this ELISA. Note that more SEB is produced by PRN7114.
Applications of SEB capture ELISA. (i) Detection of SEB in culture supernatants and whole-cell preparations.
Next we tested the usefulness of the ELISA for the detection of SEB production by clinical and laboratory S. aureus strains grown in BHI medium. SEB levels varied between samples and were dependent on the time of growth (Fig. 4). Two clinical strains (strains 2 and 9) produced an average of 660 ng/ml SEB after 6 h of logarithmic growth, while genetically engineered strain PRN7114/RN9432 produced 5.7 μg/ml of SEB under the same growth conditions. This S. aureus strain was transformed with a plasmid containing the gene for SEB toxin. As expected, no SEB was detected in the supernatants of cultures that contained either no SEB gene (RN6734) or a truncated SEB gene (PRN7116/RN9469). The accumulation of toxin in supernatants was noted to be maximal after overnight growth (approximately 16 to 18 h), which corresponded to late stationary phase. The supernatant cultures of clinical strains accumulated up to 5 μg/ml, whereas strain PRN7114/RN9432 produced up to 27 μg/ml of SEB. SEB was also detected in the whole-cell lysates of S. aureus colonies. The clinical strains produced approximately 4 ng/ml, whereas PRN7114 produced 1.2 μg/ml, and no SEB was detected in the control strains. In summary, SEB was detected in samples in which toxin was either cell associated or shed into the supernatant.
(ii) SEB detection in body fluids of SEB-exposed and SEB-positive S. aureus-infected mice.
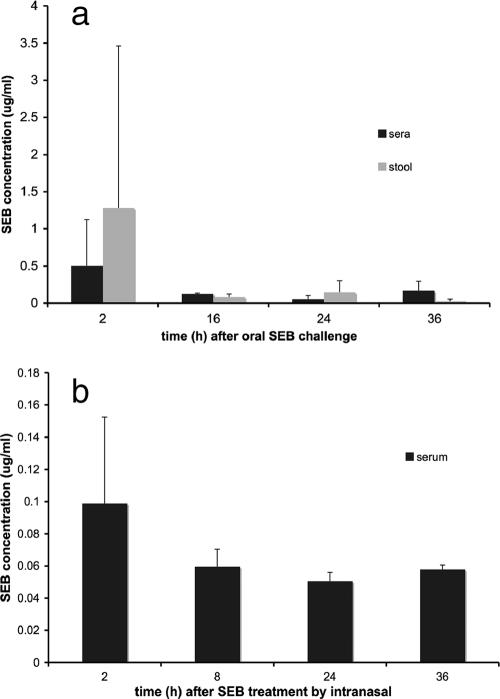
The capture ELISA was used for the detection of toxin in body fluids. First, mice were injected i.p. with a lethal dose of 20 μg purified SEB after sensitization with d-galactosamine. Serum and urine were collected 8 h after injection. Serum SEB levels were variable and ranged from 28 to 150 ng/ml (median, 74 ng/ml). The SEB levels in the urine 8 h after i.p. injection ranged from 10 to 47 ng/ml (median, 40 ng/ml). Presumed transcytosis of SEB across mucosal membranes was measured in models of airborne and food-borne SEB exposure (Fig. 5a). SEB (200 μg) was fed to the mice via a gavage needle. In a different experiment, 20 μg of purified SEB was given i.n. (Fig. 5b). SEB levels were measured by ELISA in the stool and blood at 2, 16, 24, and 36 h and in the blood after i.n. exposure at 2, 8, 24, and 36 h. SEB was consistently detected in the stools of mice fed SEB. Transcytosis of SEB from the gut, as well as from the alveolar space, into the bloodstream was rapid and could be documented as early as 2 h postexposure. As expected, the serum SEB levels after i.n. challenge were lower than those measured in serum after oral challenge with a 10-fold higher SEB dose. Next, we infected mice intravenously with 109 CFU from SEB-producing and non-SEB-producing (no SEB gene) S. aureus clinical strains. SEB was detected in three of five mice that were infected with an SEB-positive strain and in none of the mice that were infected with the SEB-negative strain. The serum SEB levels were higher at 2 h postinfection (10.3 ± 7.4 μg/ml) than at 24 h postinfection (2.0 ± 1.3 μg/ml), consistent with the higher average numbers of CFU (log 4.5 at 2 h versus log 3.9 at 24 h) early in the course of the infection.
FIG. 5.
Measurement of SEB by ELISA in body fluids of challenged mice. The average SEB levels that were measured by capture ELISA in the sera and stools of BALB/c mice after oral and i.n. challenges with purified SEB are shown. Error bars reflect standard deviations (n = 5 to 10 mice per group). (a) SEB levels detected in the sera and stools after oral challenge with 200 μg SEB at the given times; (b) SEB can also be detected in the serum of mice that were challenged with a 10-fold lower dose (20 μg) of SEB i.n. Prior to intoxication, SEB was not detectable in the sera or the stools of the mice (data not shown).
(iii) Measurement of SEB levels in different mouse models for SEBILS.
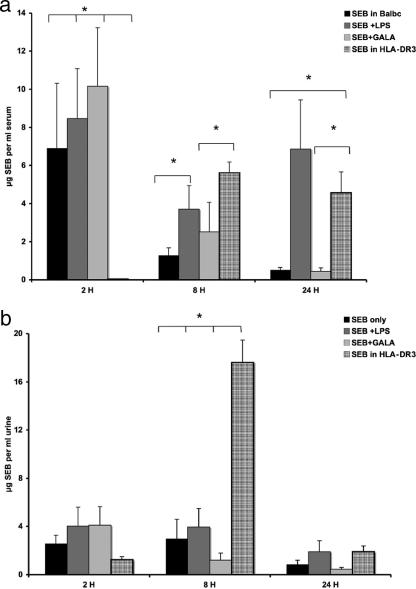
Several murine models for SEB-induced lethal shock (SEBILS) are available. These models can be used to determine the potential efficacies of therapeutic reagents. Traditionally, murine animal models for SEBILS required the coadministration of d-galactosamine or LPS to achieve mortality because mice are more resistant to SEB intoxication than humans. The precise mechanism by which these substances enhance the toxicity of SEB is not known. Mice that are transgenic for human HLA-DR3 are more sensitive, and mortality can be achieved without coadministration of d-galactosamine or LPS (26-28). The measurement of SEB levels by the capture ELISA demonstrated that BALB/c mice treated with SEB only or in combination with d-galactosamine or LPS initially exhibited higher serum SEB levels than HLA-DR3 transgenic mice that received equivalent doses of SEB (Fig. 6a). SEB-injected BALB/c mice cleared the toxin from serum rapidly, whereas both LPS (with or without d-galactosamine)-treated BALB/c mice and HLA-DR3 mice maintained higher levels of toxin in the serum at 8 h after toxin exposure. d-Galactosamine- and LPS-treated mice died faster than HLA-DR3 mice. They were all dead by 48 h, and 40 to 50% died within 24 h, whereas only five of nine HLA-DR3 mice had died by 72 h. All SEB-treated BALB/c mice survived. SEB was detected in the urine of all the mice, but the levels were noted to be much higher in HLA-DR3 mice 8 h after exposure than in BALB/c mice (Fig. 6b).
FIG. 6.
Comparison of serum SEB levels in different mouse models for SEBILS. Shown are the average SEB levels in the sera (a) and urine (b) of BALB/c mice, as measured by capture ELISA, after i.p. injection of (i) 50 μg SEB only, (ii) SEB + 25 mg d-galactosamine, or (iii) SEB plus 75 μg LPS and (iv) average levels in serum of transgenic HLA-DR3 mice after i.p. challenge with 50 μg SEB. Error bars denotes standard deviations within a group of mice (n = 5 to 10 mice). *, significant differences in SEB levels by Student t test (P < 0.05). Note that the peak SEB levels in serum differ significantly between HLA-DR3 and BALB/c mice, as SEB appears later in the serum of HLA-DR3 mice (a). At 8 h SEB levels in urine are also significantly higher in HLA-DR3 mice than in BALB/c mice (b).
DISCUSSION
The sensitive and specific detection of toxins is of critical importance, especially for those toxins associated with biological weapon warfare. The generation of several SEB-specific murine MAbs allowed us to identify combinations that could be successfully used to generate two highly sensitive sandwich ELISAs. These ELISAs can be used to detect SEB both in vitro and in vivo. The use of two different IgG subtypes as detection MAbs permits the quantification of SEB in the serum of mice that are treated with SEB-specific MAbs, as long as the epitopes do not overlap and the “treatment” and “detection” MAbs are of different isotypes. These ELISAs are more sensitive than many published detection methods (20, 22, 30-32, 34), maintain their sensitivities with serum, and are easy to use in standard laboratories. This ELISA constitutes a useful tool for the diagnosis of SEB intoxication, as well as infection with SEB-producing S. aureus strains. These ELISAs could be useful for testing of the efficacies of vaccines or therapeutic reagents, as well as investigation of important aspects of in vivo regulation and the clearance of SEB.
In general, SEB toxin levels are expected to be very low, in the range of pg/ml, in the serum of exposed humans who either survived or are symptomatic from SEB intoxication (36). The capture ELISA described here is specific and highly sensitive for the detection of toxin in the pg/ml range. Neither culture supernatants nor serum, urine, or stool products interfered with the performance of this assay. The extent to which preexisting antibodies in the serum of already exposed patients could interfere with the sensitivity of the ELISA is unknown at this point. Our data indicate that SEB-injected mice develop significant levels of SEB in their serum after 8 h and excrete the toxin renally within that time frame. In addition, our results demonstrate that transcytosis of ingested or inhaled SEB occurs across mucosal barriers within a short time. In contrast to other studies, we were able to detect the presumed transcytosis of SEB into serum by ELISA, whereas others demonstrated transcytosis only by biological assays, which would not be useful in the clinical setting (8). Given that we challenged mice with the same dose used in other published studies, we believe that our ability to detect SEB was most likely a result of the enhanced sensitivity of our test.
Up to 30% of the human population is colonized with S. aureus, and SEB is produced by 5 to 10% of clinical S. aureus strains (6, 11). Humans colonized with toxin-producing S. aureus often exhibit neutralizing antibodies (11). The protective efficacy of human antibodies in the situation of a massive exposure following an attack with SEB toxin is not known. Although neutralizing IgM antibodies may be available in preimmune subjects, intoxication with a superantigen in the setting of a high level of SEB exposure could potentially interfere with the mobilization of memory B cells. This ELISA will be useful in studying these aspects as well as studying the effects of reagents that protect transcytosis across mucosal barriers.
SEB is thought to rapidly bind to the MHC and T-cell antigen receptor in the host, and hence, the design of an effective therapeutic intervention remains a challenge. SEB has a much higher affinity for human MHC than mouse MHC, and consequently, murine animal models have their limitations in answering these important questions. To enhance the toxicity of SEB in murine models, d-galactosamine, an agent toxic for the liver, is usually used (18). The exact mechanism by which this chemical affects SEB levels is not known, but it has been suggested that it may augment liver toxicity and cytokine levels (19). More recent studies have proposed the use of mice that are transgenic for HLA-DR3, which renders them more sensitive to SEB intoxication (27, 28). Our data indicate that the serum SEB levels in these murine models vary over a considerable range and that the clearance of SEB from the serum of d-galactosamine- and LPS-cotreated mice may be significantly different from that from the serum of HLA-DR3 transgenic mice. More extensive studies will have to be done to determine which model best mimics the clearance of the toxin in humans. This is of great importance in settings in which drugs that prevent the rapid binding of this toxin to MHC and T-cell receptors are to be developed (24, 25, 27, 28).
S. aureus is the second most common pathogen recovered in nosocomial bloodstream infections in the United States (38). The regulation of staphylococcal enterotoxins has been studied predominately in in vitro experiments, in which toxin levels are determined by protein precipitation of the supernatant (37). Recent studies reveal that the in vivo regulation may not correlate with the in vitro regulation of the toxin (21, 23), possibly because the pathogen burden and/or the in vivo growth conditions potentially affect toxin production (39-41). The contribution of SEB to the outcome of staphylococcal infection is still unclear, mainly because tools for the measurement of low SEB toxin levels in serum and tissue are lacking (1, 6). Even if only a subset of S. aureus infections are caused by SEB toxin-producing strains, these may be more likely to cause lethal disease and may be more likely to infect patients who do not have protective antibodies. Accordingly, although several studies have shown that vaccination can prevent toxin-mediated disease, no systemic clinical investigations with patients have been undertaken (3). Our ELISA is more sensitive than other published SEB detection assays and may be helpful in future clinical investigations.
Acknowledgments
We thank C. S. David for providing us with the HLA-DR3 transgenic mice. We thank R. P. Novick for providing us with S. aureus strains. We thank A. Casadevall for critical review of the manuscript.
This work was funded by Northeast Biodefense Center (grant U54-AI057158 and grant 5U54AI057158-05 to P. I. Lipkin). Part of this work was done in collaboration with the Northeast Biodefense Center Monoclonal Antibody Core (P. I. Scharff).
Footnotes
Published ahead of print on 18 July 2007.
REFERENCES
- 1.Azuma, K., K. Koike, T. Kobayashi, T. Mochizuki, K. Mashiko, and Y. Yamamoto. 2004. Detection of circulating superantigens in an intensive care unit population. Int. J. Infect. Dis. 8292-298. [DOI] [PubMed] [Google Scholar]
- 2.Bavari, S., R. G. Ulrich, and R. D. LeClaire. 1999. Cross-reactive antibodies prevent the lethal effects of Staphylococcus aureus superantigens. J. Infect. Dis. 1801365-1369. [DOI] [PubMed] [Google Scholar]
- 3.Boles, J. W., M. L. Pitt, R. D. LeClaire, P. H. Gibbs, E. Torres, B. Dyas, R. G. Ulrich, and S. Bavari. 2003. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin. Immunol. 10851-59. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 15427-35. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner, L., A. Cooper, C. Fantino, D. M. Altmann, and S. Sriskandan. 2005. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J. Immunol. 1756870-6877. [DOI] [PubMed] [Google Scholar]
- 6.Ferry, T., D. Thomas, A. L. Genestier, M. Bes, G. Lina, F. Vandenesch, and J. Etienne. 2005. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin. Infect. Dis. 41771-777. [DOI] [PubMed] [Google Scholar]
- 7.Freed, R. C., M. L. Evenson, R. F. Reiser, and M. S. Bergdoll. 1982. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl. Environ. Microbiol. 441349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamad, A. R., P. Marrack, and J. W. Kappler. 1997. Transcytosis of staphylococcal superantigen toxins. J. Exp. Med. 1851447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lovenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Broker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 724061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtfreter, S., and B. M. Broker. 2005. Staphylococcal superantigens: do they play a role in sepsis? Arch. Immunol. Ther. Exp. (Warsaw) 5313-27. [PubMed] [Google Scholar]
- 11.Holtfreter, S., K. Roschack, P. Eichler, K. Eske, B. Holtfreter, C. Kohler, S. Engelmann, M. Hecker, A. Greinacher, and B. M. Broker. 2006. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 1931275-1278. [DOI] [PubMed] [Google Scholar]
- 12.Iandolo, J. J., and W. M. Shafer. 1977. Regulation of staphylococcal enterotoxin B. Infect. Immun. 16610-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappler, J. W., A. Herman, J. Clements, and P. Marrack. 1992. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J. Exp. Med. 175387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeClaire, R. D., and S. Bavari. 2001. Human antibodies to bacterial superantigens and their ability to inhibit T-cell activation and lethality. Antimicrob. Agents Chemother. 45460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeClaire, R. D., R. E. Hunt, and S. Bavari. 2002. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect. Immun. 702278-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ler, S. G., F. K. Lee, and P. Gopalakrishnakone. 2006. Trends in detection of warfare agents. Detection methods for ricin, staphylococcal enterotoxin B and T-2 toxin. J. Chromatogr. A 11331-12. [DOI] [PubMed] [Google Scholar]
- 17.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 5577-104. [DOI] [PubMed] [Google Scholar]
- 18.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 17591-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaki, M., Y. Muto, H. Ohnishi, S. Yasuda, K. Sano, T. Naito, T. Maeda, T. Yamada, and H. Moriwaki. 1994. Hepatic injury and lethal shock in galactosamine-sensitized mice induced by the superantigen staphylococcal enterotoxin B. Gastroenterology 106450-458. [DOI] [PubMed] [Google Scholar]
- 20.Nedelkov, D., and R. W. Nelson. 2003. Detection of Staphylococcal enterotoxin B via biomolecular interaction analysis mass spectrometry. Appl. Environ. Microbiol. 695212-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novick, R. P. 2003. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid 4993-105. [DOI] [PubMed] [Google Scholar]
- 22.Peruski, A. H., L. H. Johnson III, and L. F. Peruski, Jr. 2002. Rapid and sensitive detection of biological warfare agents using time-resolved fluorescence assays. J. Immunol. Methods 26335-41. [DOI] [PubMed] [Google Scholar]
- 23.Pragman, A. A., and P. M. Schlievert. 2004. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol. Med. Microbiol. 42147-154. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan, G., K. Iijima, M. Singh, H. Kita, R. Patel, and C. S. David. 2006. Intranasal exposure to bacterial superantigens induces airway inflammation in HLA class II transgenic mice. Infect. Immun. 741284-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopalan, G., M. M. Sen, and C. S. David. 2004. In vitro and in vivo evaluation of staphylococcal superantigen peptide antagonists. Infect. Immun. 726733-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajagopalan, G., M. M. Sen, M. Singh, N. S. Murali, K. A. Nath, K. Iijima, H. Kita, A. A. Leontovich, U. Gopinathan, R. Patel, and C. S. David. 2006. Intranasal exposure to staphylococcal enterotoxin B elicits an acute systemic inflammatory response. Shock 25647-656. [DOI] [PubMed] [Google Scholar]
- 27.Rajagopalan, G., M. K. Smart, S. Cheng, C. J. Krco, K. L. Johnson, and C. S. David. 2003. Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M. Tissue Antigens 62149-161. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan, G., M. K. Smart, C. J. Krco, and C. S. David. 2002. Expression and function of transgenic HLA-DQ molecules and lymphocyte development in mice lacking invariant chain. J. Immunol. 1691774-1783. [DOI] [PubMed] [Google Scholar]
- 29.Reed, S. D., J. Y. Friedman, J. J. Engemann, R. I. Griffiths, K. J. Anstrom, K. S. Kaye, M. E. Stryjewski, L. A. Szczech, L. B. Reller, G. R. Corey, K. A. Schulman, and V. G. Fowler, Jr. 2005. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control Hosp. Epidemiol. 26175-183. [DOI] [PubMed] [Google Scholar]
- 30.Ruan, C., K. Zeng, O. K. Varghese, and C. A. Grimes. 2004. A staphylococcal enterotoxin B magnetoelastic immunosensor. Biosens. Bioelectron. 20585-591. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki, T., Y. Terano, T. Shibata, H. Kawamoto, T. Kuzuguchi, E. Kohyama, T. Watanabe, T. Ohyama, and M. Gemba. 2005. Establishment of highly specific and quantitative immunoassay systems for staphylococcal enterotoxin A, B, and C using newly-developed monoclonal antibodies. Microbiol. Immunol. 49589-597. [DOI] [PubMed] [Google Scholar]
- 32.Schotte, U., N. Langfeldt, A. H. Peruski, and H. Meyer. 2002. Detection of staphylococcal enterotoxin B (SEB) by enzyme-linked immunosorbent assay and by a rapid hand-held assay. Clin. Lab. 48395-400. [PubMed] [Google Scholar]
- 33.Swaminathan, S., W. Furey, J. Pletcher, and M. Sax. 1992. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature 359801-806. [DOI] [PubMed] [Google Scholar]
- 34.Tempelman, L. A., K. D. King, G. P. Anderson, and F. S. Ligler. 1996. Quantitating staphylococcal enterotoxin B in diverse media using a portable fiber-optic biosensor. Anal. Biochem. 23350-57. [DOI] [PubMed] [Google Scholar]
- 35.Todaro-Luck, F., E. Reiss, R. Cherniak, and L. Kaufman. 1989. Characterization of Cryptococcus neoformans capsular glucuronoxylomannan polysaccharide with monoclonal antibodies. Infect. Immun. 573882-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulrich, R. G., S. Sidell, T. J. Taylor, C. L. Wilhelmsen, and D. R. Franz. 2001. Textbook of military medicine: medical aspects of chemical and biological warfare. www.bordeninstitute.army.mil/cwbw/default.htm. Borden Institute, Washington, DC.
- 37.Vojtov, N., H. F. Ross, and R. P. Novick. 2002. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc. Natl. Acad. Sci. USA 9910102-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39309-317. [DOI] [PubMed] [Google Scholar]
- 39.Yarwood, J. M., J. K. McCormick, and P. M. Schlievert. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 1831113-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 381797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarwood, J. M., and P. M. Schlievert. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 1121620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]