Abstract
Background
Screening of high-risk groups for peripheral arterial disease has been advocated because the condition underdiagnosed and secondary prevention can reduce cardiovascular event rates.
Aim
To establish the feasibility of screening for peripheral arterial disease in people aged 60 years or over with hypertension, and to estimate the potential to improve secondary preventive treatment.
Design of study
Pilot study and cross-sectional survey.
Setting
Large general practice in north-east Scotland.
Method
People aged 60 years or over with hypertension but no cardiovascular disease or diabetes were identified from computer records and invited to a screening clinic. Data were collected on ankle brachial pressure index (ABPI), preventive treatment, and risk factors.
Results
Of 705 potentially eligible patients, 443 (63%) agreed to participate. Sixty-four were excluded and 364 of 379 patients (96%) attended screening. Thirty patients had peripheral arterial disease (ABPI of 0.9 or less), of whom 24 (7%; 95% confidence interval [CI] = 4 to 10%) were previously undiagnosed. Fifteen (50%) patients took antiplatelets, 13 (45%) had cholesterol <5mmol/l, and 16 (53%) had blood pressure below 140/85 mmHg. Twenty-two (73%) patients were non-smokers, 14 (47%) had low-fat diets, two (7%) were physically active, and three (10%) ate recommended amounts of fruit and vegetables.
Conclusions
It is feasible to screen for peripheral arterial disease in primary care, but its prevalence is lower than anticipated. There is room for improvement in secondary preventive treatment and lifestyle, so a structured programme could still have important benefits for survival.
Keywords: general practice, peripheral vascular diseases, pilot study, prevention and control, screening
INTRODUCTION
People with peripheral arterial disease are at high risk of cardiovascular events and deaths. As this condition often goes undiagnosed, opportunities for secondary prevention are missed in primary care.1 Peripheral arterial disease is common, with prevalence estimated at 16% in those aged over 55 years and 29% in high-risk groups.1,2Screening of high-risk groups has been advocated because most patients are asymptomatic; peripheral arterial disease can be detected by a simple, non-invasive, and accurate test (ankle brachial pressure index [ABPI]); and secondary preventive treatment can prevent vascular events and death.1,3 Patients with diabetes and cardiovascular disease are at a high risk for peripheral arterial disease, but are already targeted for secondary prevention, so have little to gain from screening. However, three other high-risk groups may, benefit from more aggressive (secondary) prevention if diagnosed with peripheral arterial disease: smokers; people with hypertension; and people with dyslipidaemia. Those with dyslipidaemia are difficult to identify because population-wide lipid measurement is not routine in UK general practice. On the other hand, recording of blood pressure and smoking status is routine and encouraged by contractual incentives.4 Of patients with peripheral arterial disease, 80% have hypertension, which is a higher proportion than ex- or current smokers, therefore the researchers judged that people with hypertension were the most promising target group.2 The aim of this study was to establish the feasibility of screening for peripheral arterial disease in this group and to estimate the potential to improve secondary preventive treatment.
How this fits in
Peripheral arterial disease is an indication for treatment to prevent cardiovascular events, but it remains largely undiagnosed. Screening of high-risk groups has been advocated, with epidemiological studies suggesting yields as high as 29%. Screening of patients aged 60 years or over with hypertension was feasible in primary care, but the yield (8%) was lower than anticipated because patients already targeted for secondary prevention were excluded. Despite the lower yield, there was room for improvement in secondary preventive treatment, which could still improve survival.
METHOD
The project was set in one large general practice in north-east Scotland. Patients with hypertension, aged 60 years or over and without diabetes, coronary heart disease, or cerebrovascular disease were identified using searches of pre-existing hypertension registers and computerised records. The resultant list was screened by GPs to exclude patients with terminal illness or dementia, and the remaining patients were invited to take part in the study. After obtaining patient consent, case notes were screened by the researchers to ensure eligibility. Finally, eligible patients were invited to attend a peripheral arterial disease screening clinic.
Research nurses reviewed general practice case notes to collect data on prescribed medications and comorbidities. Participants were asked to complete the following with the help of the research nurse: Edinburgh Claudication Questionnaire;5 Rose Angina Questionnaire;6 a list of prescribed and over the counter medications (including aspirin); Health Practices Index questions on smoking status and alcohol intake;7 Godin leisure time exercise questionnaire;8 and HEA2 Diet Questionnaire.9 To calculate participants' ABPI, systolic blood pressure was measured in both arms and in the dorsalis pedis and posterior tibial arteries of both legs using a Doppler ultrasound probe and standard sphygmomanometer. For each leg, the ABPI was calculated by dividing the higher ankle pressure in that leg by the higher brachial systolic pressure. The lower ABPI for each patient was used in subsequent analysis. A venous blood sample was collected for estimation of random lipids and glucose. Exhaled carbon monoxide concentration was measured (Bedfont Micro-Smokerlyzer, Bedfont Scientific Ltd, UK).10
The main outcome was peripheral arterial disease defined by an ABPI of 0.90 or less. Coronary and cardiovascular risk were calculated by applying Framingham equations to age (capped at 74 years), sex, blood pressure and lipid measurements from the clinic, and self-reported smoking status. This procedure was intended to replicate normal clinical practice used to support treatment decisions as closely as possible. Preventive lifestyle and treatment were defined as:3 non-smoking (6 ppm carbon monoxide); moderate exercise (Godin leisure time exercise questionnaire score ≥24); healthy diet (<33% energy from fat; ≥5 fruit and vegetables per day); antiplatelet agents (aspirin and/or clopidogrel); optimisation of management of hypertension (blood pressure <140/85 mmHg); and optimisation of lipids (total cholesterol <5mmol/l). Data were analysed using SPSS for Windows (version 11.0) and CIA (version 2). Descriptive statistics were used to summarise characteristics of patients with and without low ABPI. χ2 tests were used to compare differences in categorical variables and Student's t-test or the non-parametric Mann–Whitney test were used for continuous data. Finally, preventive treatments for participants with and without peripheral arterial disease were compared. Statistical significance was set as P ≤0.05.
RESULTS
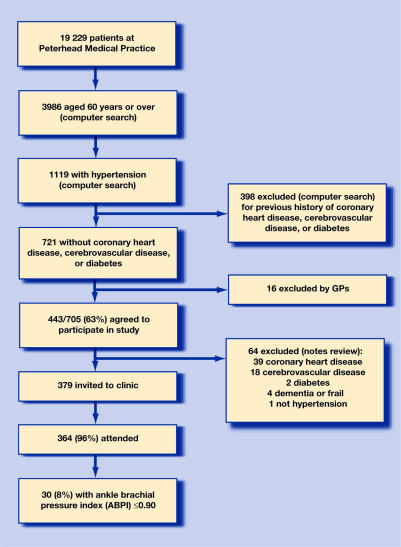
From a general practice of 19 229 patients, 705 were identified as potentially eligible, of whom 443 (63%) agreed to participate (Figure 1). Screening and data collection took place during 1 day per week for 40 weeks and involved two nurses. Of 379 patients confirmed eligible, 364 (96%) attended. Thirteen (4%) had peripheral arterial disease recorded in their medical history: eight with affected legs (three with investigative evidence, five with clinical diagnoses), four with abdominal aortic aneurysms, and one with acute ischaemic colitis. ABPIs for 363 participants were obtained, of whom 30 (8%; 95% confidence interval [CI] = 6 to 12%) had peripheral arterial disease (ABPI ≤ 0.9). Of these, 24 were previously undiagnosed (7%; 95% CI = 4 to 10%) and 10 had claudication (Table 1). The number needed to screen to detect one patient with peripheral arterial disease was n = 12, and to make one new diagnosis was n = 15.
Figure 1.
Study profile.
Table 1.
Characteristics of patients with and without ABPI ≤ 0.90, and comparison of their preventive treatment.
| ABPI | |||
|---|---|---|---|
| ≤ 0.90 (n = 30) | > 0.90 (n = 333) | P-valuea | |
| Sex: male | 21 (70) | 152 (46) | 0.011 |
| Mean (SD) age, years | 70.1 (6.3) | 70.0 (5.9) | 0.922 |
| Edinburgh questionnaireb | |||
| Definite claudication | 10 (34) | 9 (3) | |
| Atypical claudication | 1 (4) | 11 (3) | <0.001 |
| No claudication | 17 (61) | 296 (94) | |
| Rose anginab | |||
| Yes | 2 (7) | 26 (8) | 0.802 |
| No | 28 (93) | 301 (92) | |
| Random glucose, mmol/lb | |||
| ≤ 7.0 | 27 (90) | 292 (88) | |
| 7.1–11.0 | 2 (7) | 37 (11) | |
| 10-year CVD riskb,c | |||
| >20% | 19 (66) | 162 (49) | 0.087 |
| 10 year CHD risk, %b,c | |||
| ≥30 | 2 (7) | 5 (2) | |
| 20 to <30 | 9 (31) | 39 (12) | |
| 15 to <20 | 4 (14) | 60 (18) | |
| <15 | 14 (48) | 227 (69) | - |
| Blood pressure, mmHg | |||
| <140/85 | 16 (53) | 157 (47) | 0.516 |
| Mean (SD) systolic | 139(19) | 139 (17) | 0.801 |
| Mean (SD) diastolic | 78 (14) | 78 (11) | 0.943 |
| Number of blood pressure drugs | |||
| 0 or 1 | 9 (30) | 141 (42) | |
| 2 | 14 (47) | 121 (36) | 0.395 |
| 3–5 | 7 (23) | 71 (21) | |
| Cholesterol, mmol/l | |||
| <5.0 | 13/29 (45) | 110/331 (33) | 0.207 |
| Mean (SD) cholesterol | 5.2 (1.1) | 5.4 (1.0) | 0.538 |
| Statins prescribed | 13 (43) | 75 (23) | 0.011 |
| On aspirin or clopidogrel | 15 (50) | 96 (29) | 0.016 |
| Smoking status | |||
| Never smoked (self report) | 7 (23) | 186 (56) | |
| Ex-smoker (self report) | 16 (53) | 113 (34) | |
| Current smoker (self report) | 7 (23) | 34 (10) | 0.001 |
| Exhaled CO >6.0 ppmd | 8 (27) | 37 (11) | 0.013 |
| Exercise (Godin score) | |||
| ≥24 | 2 (7) | 70 (21) | 0.058 |
| Median (IQR) | 9 (0–21) | 18 (2–21) | 0.020 |
| Diet | |||
| <33% energy from fat | 14 (47) | 174 (52) | 0.558 |
| Mean (SD) % energy from fat | 33.6 (5.2) | 32.8 (5.9) | 0.474 |
| 5 or more daily portions fruit and vegetables | 3 (10) | 38 (11) | 0.815 |
| Mean (SD) daily portions fruit and vegetables | 2.7 (1.4) | 2.8 (1.7) | 0.717 |
Values are numbers (percentages within columns) unless otherwise stated. CHD = coronary heart disease; CO = carbon monoxide; CVD = cardiovascular disease; IQR = interquartile range; SD = standard deviation.
P-values from the χ2 (proportions), independent samples t-test (means), or Mann–Whitney U test (Godin score).
Missing values were: Edinburgh questionnaire n = 19; Rose angina n = 4; glucose n = 2; Framingham scores n = 3.
Calculated using Framingham equations.
Bedfont Micro-Smokerlyzer, Bedfont Scientific Ltd, UK.
More selective screening was then considered. The yield from current smokers was higher (17%), but most cases (n = 23) would have been missed. Screening current and ex-smokers would have detected n = 23 (yield 14%), missing n = 7. Framingham equations were less helpful than smoking status for selecting patients, whatever cut-off point was used.
About half of the cardiovascular prevention opportunities were being taken among patients with peripheral arterial disease: compared with those without peripheral arterial disease, they were more likely to be treated with statins and antiplatelets, less likely to be physically active, and more likely to smoke (Table 1).
DISCUSSION
Summary of main findings
Screening for peripheral arterial disease in a high-risk group is feasible in primary care, and there is potential to improve secondary prevention among those identified, but the yield is lower than expected from previous population and primary care research.1,2
Strengths and limitations of the study
This study was limited to one general practice, but it was large and well represented in terms of socioeconomic characteristics and travelling distances from the surgery, both of which are predictors of screening uptake.11,12 This study's prevalence rates for coronary heart disease and hypertension and quality indicators for secondary prevention in coronary heart disease are similar to, or between, average figures for Scotland and England, suggesting that these findings may be widely relevant, at least in the UK.13,14
Comparison with existing literature
Uptake among those invited to participate (63%) was reasonable given the age group of the sample and the presentation of screening as a research project rather than for health benefit. There was a lower uptake than for breast (75%) and cervical screening (79%),12 but higher than for colorectal screening (57%).15 However, the prevalence of peripheral arterial disease was found to be lower than expected from previous population studies. In epidemiological surveys of general populations over 55 years, symptomatic peripheral arterial disease has been reported to affect 4 to 7%, and asymptomatic disease 9 to 12%.16–19 Recent studies in primary care from Europe and North America (all from outside the UK) have reported total prevalence of 15 to 29%.20–23 Two factors may explain the difference. First, the above estimates do not exclude those with diabetes and coronary or cerebrovascular disease, groups with particularly high rates of peripheral arterial disease. These were excluded because they are already targeted for secondary prevention. Secondly, advantage was taken of universal patient registration to attempt to recruit all eligible patients: most previous primary care studies have recruited patients attending for appointments, in whom levels of morbidity are likely to be higher. One recent report from Italy, which also took advantage of universal registration, found the prevalence of symptomatic peripheral arterial disease among 40–80 year olds to be only 1.6%.24
This study's findings on uptake of secondary prevention in peripheral arterial disease are similar to those from other countries. Reported antiplatelet use is from 33 to 79%, with higher levels in those with known peripheral arterial disease than those identified through screening.21,23,25,26 Reported statin prescribing varies from 31 to 56%, with between 43 and 71% having total cholesterol levels above 5.2 mmol/l.20,21,23,27 In one study, 34% had systolic blood pressure levels above 140 mmHg.27 A study in the US found current smoking rates (31%) and findings on physical activity similar to the present study.20
Implications for future research and clinical practice
Screening will only benefit patients if it detects disease substantially earlier than it would otherwise be detected and if it provides opportunities to improve outcome. Data from longitudinal studies suggest that few patients detected by screening would present with symptoms, but that they are already at high risk of morbidity and mortality. Fewer than 10% developed claudication during 5 years of follow-up in the Edinburgh Artery Study, during which time 7% suffered non-fatal myocardial infarctions and 10% cardiovascular death.17 Secondary preventive treatment can reduce cardiovascular events and death, and 97 opportunities were found to improve secondary prevention (Table 2). However, not all of these opportunities can be achieved as past experience of secondary prevention in coronary heart disease demonstrates. Taking 24 opportunities would achieve targets equivalent to maximum points for secondary prevention in coronary heart disease in the revised Quality and Outcomes Framework of the general medical services contract.4 Translation of previous findings on structured nurse-led clinics suggests 16 improvements would be made.28 Without structured care or targets, there may be fewer improvements. On the other hand, accumulated incremental benefits can translate into meaningful reductions in cardiovascular events and deaths.29
Table 2.
Opportunities for improved management and possible responses.
| Opportunities for improvement (n = 30) | |||
|---|---|---|---|
| Total | To achieve targets similar to CHD QOFa | Predicted from nurse-led clinics in CHDb | |
| Antiplatelet drugs | 15 | 12 | 3 |
| Cholesterol <5.0 mmol/l (n = 29) | 16 | 7 | 6 |
| Blood pressure <140/85 mmHg | 14 | 5 | 3 |
| Non-smoking | 8 | - | 0 |
| Moderate exercise | 28 | - | 2 |
| Low-fat diet | 16 | - | 2 |
| Total | |||
| Per 30 patients | 97 | 24 | 16 |
| Per patient | 3.2 | 0.8 | 0.5 |
Maximum targets for secondary prevention of coronary heart disease (CHD) in the Quality Outcomes Framework (QOF) of the UK general medical services contract: 90% on antiplatelet drugs, 70% achieving cholesterol and blood pressure targets.4
Based on outcomes achieved in a previous randomised controlled trial: 9% more on antiplatelet drugs, 21% more achieving cholesterol targets, 10% more achieving blood pressure targets, no change in smoking, 6% more exercising, and 8% more on a low-fat diet.28
Two further factors need consideration and research. First, this study presents yields from first-pass screening; yields are likely to be lower with subsequent rounds. Secondly, screening requires resources, which include nurse time, a Doppler ultrasound probe, accommodation, and administrative help. In this study, each diagnosis took approximately 3 days of nurse time, but most of this was associated with research activities. In clinical practice, time requirements may be nearer 1 day per diagnosis.
In conclusion, screening in primary care for peripheral arterial disease to improve secondary prevention is likely to have a lower yield than might have been expected previously, but may still be worthwhile. A large randomised trial would be needed to confirm or refute these estimates of benefits, although this will be a considerable undertaking given the lower than expected yield from screening.
Acknowledgments
Grateful thanks to the staff and patients at Peterhead Medical Practice for all their help with this study.
Funding body
The study was funded by a research grant (CZG/2/136) from the Chief Scientist Office of the Scottish Executive. Neil Campbell is funded by a Career Scientist Award (CZP/4/3) from the Chief Scientist Office of the Scottish Executive. The funding source had no involvement in the research or its interpretation
Ethics committee
The study was granted ethical approval by Grampian Research Ethics Committee (1) (04/S0801/42)
Competing interests
Julie Brittenden has done consultancy work for Sanoffi and Astra-Zeneca. All the other authors declare that they have no competing interests
REFERENCES
- 1.Belch JJF, Topol EH, Agnelli C, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163(8):884–892. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ. 2003;326(7389):584–588. doi: 10.1136/bmj.326.7389.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Medical Association. Quality and Outcomes Framework guidance. www.bma.org/ap.nsf/Content/qof06 (accessed 1 Mar 2007)
- 5.Leng GC, Fowkes FGR. The Edinburgh claudication questionnaire: an improved version of the WHO/Rose questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45(10):1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 6.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 7.Berkman LF, Breslow L. Health and ways of living. The Alameda County study. Oxford University Press: Oxford; 1983. [Google Scholar]
- 8.Godin G, Shepherd R. A simple method to assess exercise behaviour in the community. Can J Appl Sport Science. 1985;19:141–146. [PubMed] [Google Scholar]
- 9.Little P, Barnett J, Kinmonth AL, et al. Can dietary assessment in general practice target patients with unhealthy diets? Br J Gen Pract. 2000;50(450):43–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 11.Bentham G, Hinton J, Haynes R, et al. Factors affecting non-response to cervical cytology screening in Norfolk, England. Soc Sci Med. 1995;40(1):131–135. doi: 10.1016/0277-9536(94)e0048-w. [DOI] [PubMed] [Google Scholar]
- 12.ISD Scotland. Scottish Health Statistics. Cancer (updated 7 June 2006). www.isdscotland.org/cancer (accessed 1 Mar 2007)
- 13.ISD Scotland. Scottish Health Statistics. Quality and Outcomes Framework (updated 29 September 2005). www.isdscotland.org/isd/qof (accessed 1 Mar 2007)
- 14.NHS. The Information Centre. Quality and Outcomes Framework 2004/05 (updated 28 September 2005) www.ic.nhs.uk/services/qof/data (accessed 1 Mar 2007)
- 15.UK Colorectal Cancer Screening Pilot Group. Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ. 2004;329:133–135. doi: 10.1136/bmj.38153.491887.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooi JD, Kester AD, Stoffen HE. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153(7):666–672. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 17.Leng GC, Lee AJ, Fowkes FGR, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25(6):1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 18.Lowe GD, Fowkes FG, Davies J, et al. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Siscovick DS, Manolio TA, et al. Ankle arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) collaborative research group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 20.Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 21.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163:1469–1474. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 22.Hayoz D, Bounameaux H, Canova CR. Swiss Atherothrombosis Survey: a field report on the occurrence of symptomatic and asymptomatic peripheral arterial disease. J Int Med. 2005;258:238–243. doi: 10.1111/j.1365-2796.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 24.Brevetti G, Oliva G, Silvestro A, et al. Prevalence, risk factors and cardiovascular comorbidity of symptomatic peripheral arterial disease in Italy. Atherosclerosis. 2004;175:131–138. doi: 10.1016/j.atherosclerosis.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Lange S, Diehm C, Darius H, et al. High prevalence of peripheral arterial disease but low antiplatelet treatment rates in elderly primary care patients with diabetes. Diabetes Care. 2003;26(12):3357–3358. doi: 10.2337/diacare.26.12.3357. [DOI] [PubMed] [Google Scholar]
- 26.Bongard V, Cambou JP, Leizorovicz A, et al. Comparison of cardiovascular risk factors and drug use in 14 544 French patients with a history of myocardial infarction, ischaemic stroke and/or peripheral arterial disease. Eur J Cardiovasc Prev Rehabil. 2004;11(5):394–402. doi: 10.1097/00149831-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rehring TF, Sandhoff BG, Stolcpart RS, et al. Atherosclerotic risk factor control in patients with peripheral arterial disease. J Vasc Surg. 2005;41(5):816–822. doi: 10.1016/j.jvs.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Campbell NC, Ritchie LD, Thain J, et al. Secondary prevention in coronary heart disease: a randomised trial of nurse-led clinics in primary care. Heart. 1998;80(5):447–452. doi: 10.1136/hrt.80.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murchie P, Campbell NC, Ritchie LD, et al. Secondary prevention clinics for coronary heart disease: four year follow up of a randomised trial in primary care. BMJ. 2003;326(7380):84–87. doi: 10.1136/bmj.326.7380.84. [DOI] [PMC free article] [PubMed] [Google Scholar]