Abstract
Bloodstream form Trypanosoma theileri degrades glucose to acetate (47%) and succinate (45%) and, therefore, does not solely rely on glycolysis for ATP production. This trypanosomatid does not use amino acids for energy metabolism. These results refute the prevailing hypothesis that substrate availability determines the type of energy metabolism of trypanosomatids.
The energy metabolism of Trypanosomatidae varies considerably, not only between species but also between distinct life cycle stages of the same species (7, 17). Several Trypanosomatidae rely on glycolysis of carbohydrates for their ATP production, whereas the energy metabolism of other Trypanosomatidae comprises substantial mitochondrial metabolism in which amino acids are often used as substrates for ATP production. Bloodstream form Trypanosoma brucei, which lives in a glucose-rich environment, is entirely dependent on degradation of glucose into pyruvate by glycolysis, whereas procyclic T. brucei living in the gut of tsetse flies degrades carbohydrates and amino acids by a more-complex metabolism involving substantial mitochondrial metabolism (2, 5, 15). Next to carbohydrate abundance, the type of available nutrients seems to correlate with the differences in energy metabolism in trypanosomatids as well. Insect stages of African trypanosomes and of Trypanosoma cruzi and Leishmania spp. that inhabit arthropod intestines, as well as trypanosomatids that live in intracellular compartments of the endocytotic pathway of mammalian cells, such as amastigote stages of T. cruzi and Leishmania spp., use the abundantly available amino acids in their environment as substrates for energy metabolism involving mitochondrial pathways (4, 7, 17). Therefore, multiple observations argue for the suggestion that nutrient supply in the natural environment correlates with the energy metabolism present in the distinct trypanosomatid species and life cycle stages.
To investigate this possible correlation, we studied the energy metabolism of bloodstream form trypanosomes of the species Trypanosoma theileri, which are extracellular, blood-dwelling parasites of mammals, living in a habitat identical to that of bloodstream form T. brucei (3). Although these two bloodstream form trypanosomes inhabit the same environment, these species are not closely related. T. theileri belongs to the subgenus Megatrypanum and, therefore, belongs to the stercorarian section of trypanosomatids, whereas African trypanosomes belong to the section of salivarian trypanosomes (11). T. theileri is a ubiquitous parasite that infects cattle with high incidence all around the world, but these infections are generally considered not to be pathogenic and parasitemia is very low (12, 18).
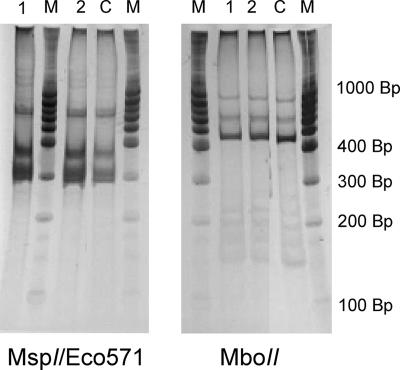
Bloodstream form T. theileri isolates were obtained from blood samples derived from Holstein-Frisian cows in The Netherlands. These isolated bloodstream form trypanosomes were expected to be T. theileri, since T. theileri is the only trypanosome species endemic in cows in northwestern Europe (11, 18, 20). The length of the isolated trypanosomal cells was between 30 and 80 μm, which corresponds to that of T. theileri trypanosomes and is longer than those of other trypanosomes (9, 11, 20). Furthermore, a species-specific diagnostic PCR test (6) using genomic DNA derived from the isolated trypanosomes demonstrated that the isolated cells were T. theileri trypanosomes (Fig. 1). The isolated bloodstream form trypanosomes were, therefore, confirmed to be T. theileri by analyses of both genetic and morphological features.
FIG. 1.
Identification of bloodstream form T. theileri. Restriction fragment length polymorphism analysis using MspI/Eco571 (left) and MboII (right) digestion on PCR amplicons obtained by 18S rDNA amplification. Lane M contains the 100-bp DNA marker (MBI Fermentas, Lithuania), lane C shows the result of the restriction enzyme analysis derived from control DNA of T. theileri, whereas lanes 1 and 2 show the results derived from genomic DNA of isolated bloodstream form trypanosomes used in this study, in a 1/1 and 1/10 dilution, respectively.
It appeared to be impossible to sustain long-term growth of bloodstream form T. theileri trypanosomes for multiple passages in semidefined media, such as HMI-9 containing 10% fetal calf serum, which is used to culture bloodstream form T. brucei cells. Therefore, isolated bloodstream form T. theileri trypanosomes were cultured at 37°C in the presence of 5% CO2 in a humidified incubator in RPMI 1640 medium containing 50 IU/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine (all from Invitrogen-Gibco), 10% (vol/vol) fetal calf serum (Bodinco, The Netherlands), 5 × 10−5 M β-mercaptoethanol (Flow Laboratories, Ivume, United Kingdom), and 2 × 106 (irradiated) peripheral blood mononuclear cells (PBMCs) per ml. The necessity of blood cells or cell products in the culture medium for T. theileri species is in agreement with earlier reports (8, 18, 19).
Metabolic experiments with T. theileri cells were started with 2 × 106 T. theileri cells per ml and maximally 3 × 105 irradiated PBMC per ml in sealed 25-ml Erlenmeyer flasks containing 5 ml standard incubation medium (see above). In parallel, control incubations were prepared with the equivalent number of isolated irradiated PBMCs or with RPMI incubation medium only. All metabolic incubations were started after addition of either 5 μCi d-[6-14C]glucose (2.07 Gbq/mmol), 5 μCi l-[U-14C]proline (9.47 Gbq/mmol) (both from Amersham Biosciences), or 5 μCi l-[U-14C]glutamine (8.8 GBq/mmol) (DuPont-NEN Research Products). After the incubations, carbon dioxide was trapped in NaOH, and radioactivity counted as described previously (13). Analysis of the excreted, labeled end products occurred by anion-exchange chromatography as described previously (14).
Analysis of radioactively labeled products in the medium demonstrated acetate and succinate as the main end products of the carbohydrate metabolism in the T. theileri culture, whereas PBMCs produced mainly lactate (Table 1). The observed variation in the rate of end product formation is large. However, when calculated within each incubation as a percentage of the glucose broken down to the various end products, it turned out that the ratio of end products produced by T. theileri is remarkably constant. Glucose was degraded by T. theileri for 47 ± 3% to acetate and for 45 ± 4% to succinate (calculated from the data in Table 1). Only a very limited amount of the degraded [6-14C]glucose (less than 1%) was broken down by T. theileri to 14C-labeled CO2, the end product resulting from full Krebs cycle activity.
TABLE 1.
Radioactively labeled end products of [6-14C]glucose breakdown by bloodstream form T. theileri cells and PBMCs
| End producta | Production (nmol · h−1 · mg protein−1) with:
|
|
|---|---|---|
| T. theileri cells | PBMCs | |
| Acetate | 594 ± 320 | —b |
| Succinate | 542 ± 224 | 19 ± 27 |
| Lactate | — | 191 ± 195 |
| Pyruvate | 9 ± 2 | 5 ± 5 |
| CO2 | 31 ± 20 | — |
All values represent the means ± standard deviations of four (T. theileri) or three (PBMC) incubations, in two completely independent experiments for each cell type. End product formation per milligram of protein was calculated using the amount of trypanosomal or PBMC protein at the end of the incubation. All values of PBMC incubations were corrected for blank incubations, and T. theileri incubations were corrected for the contribution by the PBMCs.
—, not detectable.
As these experiments were performed in the presence of a small number of irradiated PBMCs, 10 times less in number than the bloodstream form T. theileri, the carbohydrate metabolism of PBMCs was also investigated in order to exclude the possibility that the detected end products acetate and succinate were produced by PBMCs. Metabolic incubations with isolated irradiated PBMCs, equal to the amount present in the T. theileri incubations, demonstrated that these cells produced largely lactate plus traces of succinate and pyruvate (Table 1). These observations of the glucose metabolism of bovine PBMCs are in agreement with previous studies (21). These results demonstrate that the main end products of glucose metabolism by bloodstream form T. theileri are acetate and succinate.
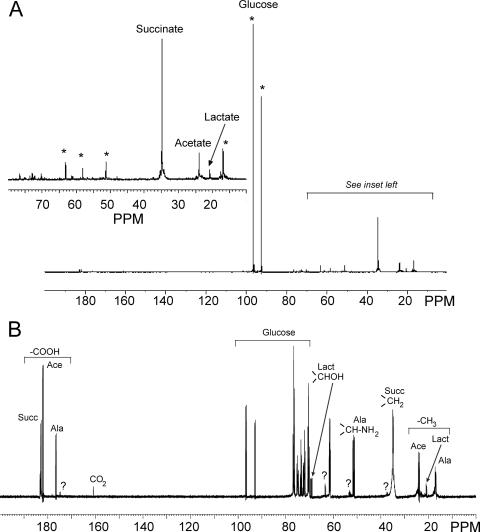
The identities of the major end products produced in the [14C]glucose incubations was confirmed by separate experiments using 13C-nuclear magnetic resonance (NMR) spectroscopy. For that purpose, 4 × 107 bloodstream form T. theileri cells were incubated for 17 h in 5 ml of the same medium described above, except that all the glucose (10 mM) normally present in RPMI was replaced by 10 mM d-[U-13C]glucose or d-[1-13C]glucose (both from Sigma). 13C-spectra of the incubation media were measured using a one-dimensional pulse sequence with WALTZ-16 power-gated broadband 1H-decoupling. A total of 30,000 transients were acquired with a recycle delay of 2 s and a spectral width of 50,506 Hz. During each cycle, 4,096 data points were collected during 40.6103 ms after a 90° pulse of 4 μs. The data were zero-filled to 16,384 data points before processing with a 5-Hz exponential multiplication followed by a Fourier transformation. Chemical shifts were measured with respect to C-1 of β-glucose at 96.6 ppm relative to tetramethylsilane at 0 ppm (13). Next to the characteristic resonance peaks of acetate and succinate, peaks of alanine and lactate could also be detected in both [1-13C]glucose and [U-13C]glucose incubations (Fig. 2). It should be noted that in the [14C]glucose incubations, labeled alanine cannot be measured because it coelutes with glucose in the anion-exchange chromatography methods. The detected 13C-labeled lactate is produced by the PBMCs present in the incubation (see above), whereas the detected 13C-labeled alanine is probably produced by the T. theileri cells, because alanine formation is common in trypanosomatids and not in PBMCs (17, 21).
FIG. 2.
13C-NMR spectra of excreted end products of [1-13C]glucose or [U-13C]glucose metabolism by bloodstream form T. theileri. Incubations were performed in RPMI medium containing either 10 mM d-[1-13C]glucose (panel A) or 10 mM d-[U-13C]glucose (panel B). Resonance peaks of carboxyl-groups (COOH) and methyl-groups (CH3) were detected between 170 and 190 ppm and 16 and 30 ppm, respectively. Peaks marked by a question mark are minor unidentified end products. The asterisks indicate peaks that are also present in the blank incubations. Abbreviations: Ace, acetate; Ala, alanine; Lact, lactate; Succ, succinate.
The amino acid metabolism of bloodstream form T. theileri was investigated by metabolic incubations in the presence of [U-14C]-labeled proline or [U-14C]glutamine, because many trypanosomatids are known to catabolize significant amounts of amino acids for ATP production. However, no end products of amino acid breakdown, such as carbon dioxide, acetate, or succinate, could be detected in the medium after incubation with bloodstream T. theileri, which demonstrated that these amino acids are not used in energy metabolism in these cells.
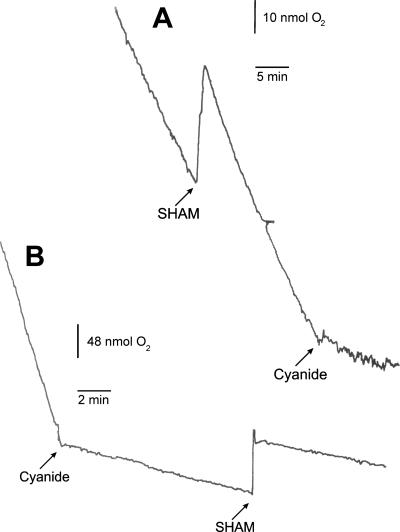
We investigated the presence and activity of the terminal oxidases of the respiratory chain, because the presence and activity of these distinct respiratory chain complexes differ drastically between distinct trypanosomes and even between life cycle stages of the same species. Oxygen consumption was measured using a Clark-type electrode at 37°C. Bloodstream form T. theileri cells were resuspended in fresh RPMI medium at a density of approximately 2 × 106 trypanosomes and 2 × 105 irradiated PBMCs per ml. Oxygen consumption was recorded for 15 min in a total volume of 2 ml. In control experiments, performed with the equivalent number of irradiated PBMCs, no oxygen consumption could be detected, which implies that these cells did not interfere with the measurements. Bloodstream form T. theileri cells consumed 22 ± 11 nmol oxygen per minute per mg protein (Fig. 3), which is threefold lower than the rate for bloodstream form T. brucei cells (1). Addition of SHAM (salicylhydroxamic acid) did not result in a decrease in oxygen consumption, whereas subsequent addition of cyanide resulted in a 90% reduction in oxygen consumption (Fig. 3A). Furthermore, when cyanide was added first, oxygen consumption could not be further inhibited by subsequent addition of SHAM (Fig. 3B). These results demonstrate that a plant-like alternative oxidase is not active in bloodstream form T. theileri, if present at all.
FIG. 3.
Oxygen consumption by intact bloodstream form T. theileri cells. Oxygen consumption was measured by a Clark-type electrode. Two representative experiments (out of five) are shown, containing 4 × 106 (trace A) or 8 × 106 (trace B) bloodstream form T. theileri cells, to which cyanide (final concentration, 1 mM) or SHAM (final concentration, 0.5 mM) were added in a distinct order.
T. theileri inhabits the same natural environment as bloodstream form T. brucei: bovine blood vessels. Therefore, these trypanosomes were expected to rely solely on glycolysis for their energy metabolism, just like bloodstream form T. brucei. However, analysis of their carbohydrate metabolism using 13C- and 14C-labeled glucose demonstrated that bloodstream form T. theileri did not excrete the glycolytic end product pyruvate, but instead acetate and succinate were excreted as the main end products. Hence, the energy metabolism of bloodstream form T. theileri is more complex and involves mitochondrial metabolism, as is indicated by a cyanide-sensitive electron transport chain and because acetate is produced inside the mitochondrion in trypanosomatids (10, 16). Although mitochondrial metabolism is involved in the energy metabolism of bloodstream form T. theileri, amino acids did not seem to be a substrate for ATP production, as no end products could be detected of radioactively labeled proline and glutamine, which are amino acids commonly used for ATP production in other trypanosomes. Our experiments further demonstrated that a respiratory chain with cytochrome-containing complexes is present in T. theileri cells, whereas no alternative oxidase activity could be detected.
Compared to the energy metabolism of bloodstream form T. brucei, that of T. theileri is more efficient, since glycolytic end products are further catabolized to acetate and succinate and because reoxidation of the produced cofactors occurs via electron-transport complexes that translocate protons, which strongly suggests that additional ATP is produced by oxidative phosphorylation. Therefore, apparently the presence of high concentrations of carbohydrates in the natural environment of trypanosomatids does not correlate with the presence of an inefficient energy metabolism that depends entirely on glycolysis and ATP production by substrate level phosphorylation.
Acknowledgments
Part of this study was supported by a grant from the Technology Foundation (STW) of the Dutch Research Council (NWO), grant number STW-UDG5589.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Bakker, B. M., P. A. M. Michels, F. R. Opperdoes, and H. V. Westerhoff. 1997. Glycolysis in bloodstream form Trypanosoma brucei can be understood in terms of the kinetics of the glycolytic enzymes. J. Biol. Chem. 272:3207-3215. [DOI] [PubMed] [Google Scholar]
- 2.Besteiro, S., M. P. Barrett, L. Rivière, and F. Bringaud. 2005. Energy generation in insect stages of Trypanosoma brucei: metabolism in flux. Trends Parasitol. 21:185-191. [DOI] [PubMed] [Google Scholar]
- 3.Böse, R., K. T. Friedhoff, S. Olbrich, G. Buscher, and I. Domeyer. 1987. Transmission of Trypanosoma theileri to cattle by Tabanidae. Parasitol. Res. 73:421-424. [DOI] [PubMed] [Google Scholar]
- 4.Cazzulo, J. J. 1992. Aerobic fermentation of glucose by trypanosomatids. FASEB J. 6:3153-3161. [DOI] [PubMed] [Google Scholar]
- 5.Clayton, C. E., and P. A. M. Michels. 1996. Metabolic compartmentation in African trypanosomes. Parasitol. Today 12:465-471. [DOI] [PubMed] [Google Scholar]
- 6.Geysen, D., V. Delespaux, and S. Geerts. 2003. PCR-RFLP using Ssu-rDNA amplification as an easy method for species-specific diagnosis of Trypanosoma species in cattle. Vet. Parasitol. 110:171-180. [DOI] [PubMed] [Google Scholar]
- 7.Hannaert, V., F. Bringaud, F. R. Opperdoes, and P. A. M. Michels. 2003. Evolution of energy metabolism and its compartmentation in Kinetoplastida. Kinetoplastid Biol. Dis. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHolland-Raymond, L. E., N. Kingston, and M. S. Trueblood. 1978. Continuous cultivation of Trypanosoma theileri at 37 C in bovine cell culture. J. Protozool. 25:388-394. [DOI] [PubMed] [Google Scholar]
- 9.Mehlhorn, H. 2001. Encyclopedic reference of parasitology. Springer-Verlag, Berlin, Germany.
- 10.Rivière, L., S. W. H. Van Weelden, P. Glass, P. Vegh, V. Coustou, M. Biran, J. J. Van Hellemond, F. Bringaud, A. G. M. Tielens, and M. Boshart. 2004. Acetate:succinate CoA-transferase in procyclic Trypanosoma brucei: gene identification and role in carbohydrate metabolism. J. Biol. Chem. 279:45337-45346. [DOI] [PubMed] [Google Scholar]
- 11.Roberts, L. S., and J. Janovy. 1996. Foundations of parasitology. William C. Brown Publishers, Dubuque, IA.
- 12.Rodrigues, A. C., M. Campaner, C. S. Takata, A. Dell'Porto, R. V. Milder, G. F. Takeda, and M. M. Teixeira. 2003. Brazilian isolates of Trypanosoma (Megatrypanum) theileri: diagnosis and differentiation of isolates from cattle and water buffalo based on biological characteristics and randomly amplified DNA sequences. Vet. Parasitol. 116:185-207. [DOI] [PubMed] [Google Scholar]
- 13.Tielens, A. G. M., A. M. Horemans, R. Dunnewijk, P. van der Meer, and S. G. van den Bergh. 1992. The facultative anaerobic energy metabolism of Schistosoma mansoni sporocysts. Mol. Biochem. Parasitol. 56:49-57. [DOI] [PubMed] [Google Scholar]
- 14.Tielens, A. G. M., P. van der Meer, and S. G. van den Bergh. 1981. The aerobic energy metabolism of the juvenile Fasciola hepatica. Mol. Biochem. Parasitol. 3:205-214. [DOI] [PubMed] [Google Scholar]
- 15.Van Hellemond, J. J., B. M. Bakker, and A. G. M. Tielens. 2005. Energy metabolism and its compartmentation in Trypanosoma brucei. Adv. Microb. Physiol. 50:199-226. [DOI] [PubMed] [Google Scholar]
- 16.Van Hellemond, J. J., F. R. Opperdoes, and A. G. M. Tielens. 1998. Trypanosomatidae produce acetate via a mitochondrial acetate:succinate CoA transferase. Proc. Natl. Acad. Sci. USA 95:3036-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hellemond, J. J., and A. G. M. Tielens. 1998. Differences in energy metabolism between Trypanosomatidae. Parasitol. Today 14:265-271. [DOI] [PubMed] [Google Scholar]
- 18.Verloo, D., J. Brandt, M. N. Van, and P. Buscher. 2000. Comparative in vitro isolation of Trypanosoma theileri from cattle in Belgium. Vet. Parasitol. 89:129-132. [DOI] [PubMed] [Google Scholar]
- 19.Wells, E. A. 1971. Studies on Trypanosoma theileri-like trypanosomes of cattle. I. Culture and storage of isolations. Br. Vet. J. 127:466-475. [DOI] [PubMed] [Google Scholar]
- 20.Wells, E. A. 1971. Studies on Trypanosoma theileri-like trypanosomes of cattle. II. The characteristics of infection in a single Ayrshire cow. Br. Vet. J. 127:476-484. [DOI] [PubMed] [Google Scholar]
- 21.Wu, G., and L. W. Greene. 1992. Glutamine and glucose metabolism in bovine blood lymphocytes. Comp. Biochem. Physiol. B 103:821-825. [DOI] [PubMed] [Google Scholar]