Abstract
The MaxiK channel plays a critical role in the regulation of corporal smooth muscle tone and thereby erectile function. Given that ageing results in a decline in erectile function, we determined changes in the expression of MaxiK, which might impact erectile function. Quantitative-polymerase chain reaction demonstrated that although there is no significant change in transcription of the α- and β-subunits that comprise the MaxiK channel, there are significant changes in the expression of transcripts encoding different splice variants. One transcript, SV1, is 13-fold increased in expression in the ageing rat corpora. SV1 has previously been reported to trap other isoforms of the MaxiK channel in the cytoplasm. Correlating with increased expression of SV1, we observed in older rats there is approximately a 13-fold decrease in MaxiK protein in the corpora cell membrane and a greater proportion is retained in the cytoplasm (approximately threefold). These experiments demonstrate that ageing of the corpora is accompanied by changes in alternative splicing and cellular localization of the MaxiK channel.
Keywords: MaxiK channel, splicing, erectile dysfunction, smooth muscle, ageing
Introduction
Normal erectile function relies on the maintenance of the correct tone by corporal smooth muscle tissue.1 Alterations in the tone of smooth muscle tissue of the urogenital system can result in erectile dysfunction (ED) a problem that increases in men as they age.2 Of central importance to the regulation of smooth muscle tone is intracellular Ca2+, which regulates the contractile state of smooth muscle myosin through the action of the calcium–calmodulin complex that activates myosin light chain kinase.3 Intracellular Ca2+ stores (such as IP3 and ryanodine-sensitive sarcoplasmic reticulum calcium stores) and transmembrane calcium flux are critical sources of intracellular calcium during alterations in myocyte contractility.4 Sustained transmembrane calcium flux largely can be attributed to the presence of L-type, voltage-dependent calcium channels. The activity of the calcium channels is coupled to the membrane potential of corporal smooth muscle cells. Potassium channels are critically important in regulating membrane potential, as potassium flows out of the cell carrying positive charge the membrane becomes hyperpolarized, which has an inhibitory effect on the activity of voltage-dependent calcium channels. Although several types of potassium channels have been implicated in this process the large-conductance, voltage- and Ca2+-activated K+ (MaxiK (BK)) channels play a key role.5
The importance of the MaxiK channel to normal erectile function was recently demonstrated in MaxiK knockout mice, where the absence of MaxiK channels resulted in ED.6 In addition, in both aging and diabetic rat models of ED, gene transfer of hSlo (encoding the human α-subunit) was found to improve erectile function.7–9 These observations have led to the development and testing in clinical trials of gene transfer of the hSlo as a therapy for human ED.10–13 The MaxiK channels are assembled from at least two non-covalently associated subunits: the pore-forming α-subunit (encoded by the Slo gene) and a regulatory β1-subunit.14 Although the α-subunit is responsible for the basic ion flux function of MaxiK channels, the regulatory β1-subunit can dramatically affect channel conduction by changing channel kinetics, voltage/Ca2+ sensitivities and pharmacology.14–17 The Slo gene, through the process of alternative splicing, can generate several protein isoforms, each of which has unique biochemical properties.18 One of these splice variants, SV1, can act as a dominant-negative, trapping functional isoforms of the channel in the cytoplasm.19,20 Alternative splicing of the Slo gene therefore can have an impact on the smooth muscle physiology.
Although the reports cited above indicate the importance of the MaxiK channel in relaxation of smooth muscle tissue, there has been no systematic investigation describing how the MaxiK channel expression changes during ageing of the penis. Therefore, we have compared the transcription, splicing and intracellular localization of the MaxiK channel in young animals with good erectile function to older animals with impaired erectile function.
Materials and methods
Animals
Experiments were carried out using 4- to 5-month-old (young) rats (approximately 275 g) and 9- to 10-month-old (old) retired breeder male Sprague–Dawley rats (weighing more than 500 g). The study protocols were approved by the Animal Use Committee and Internal Review Board at Albert Einstein College of Medicine.
Measurement of intracorporal pressure/blood pressure in male rats
The methods for determining intracorporal pressure/blood pressure (ICP/BP) are based on methods previously described in papers from our laboratory.7–9 Five young and seven old animals were used in these experiments. Briefly, anaesthesia was induced in male animals by intraperitoneal injection (35 mg/kg) of sodium pentobarbital (Anpro Pharmaceuticals, Arcadia, CA, USA) and maintained during the course of the experimental protocol (2–3 h) by subsequent injection of pentobarbital (5–10 mg/kg) every 45–60 min, as described above. Animals were placed in the supine position, and the bladder and prostate were exposed through a mid-line abdominal incision. The inferior hypogastric plexus (i.e., the pelvic plexus or major pelvic ganglia), pelvic nerves and cavernous nerve (CN) were identified posterolateral to the prostate on both sides, and stainless steel bipolar wire electrodes were placed around these structures for electrical stimulation. The penis was denuded of skin; both crura (corpus cavernosa) were exposed by removing part of the overlying ischiocavernous muscles. To monitor ICP, a 23-gauge cannula was filled with 250 U/ml heparin solution, connected to polyethylene 50 tubing (Intramedic, Becton Dickinson, Franklin Lakes, NJ, USA), and was inserted into the right corpus cavernosum (crura). Another 23-gauge cannula preconnected to a 1-ml syringe was inserted into left corpus cavernosum for intracavernous drug injection (if necessary). Systemic arterial BP was monitored via a 25-gauge cannula placed into the carotid artery. A second cannula was placed in the external jugular vein for intravenous administration of the medication and test substance.
Both pressure lines then were connected to a pressure transducer, which was, in turn, connected via a Transducer amplifier (ETH 400, CB Sciences, Dover, NH, USA) to a data acquisition board (Mac Lab/8e; AD Instruments). Real-time display and recording of pressure measurements was performed on a Macintosh computer (Mac Lab software version 3.4; AD Instruments). The pressure transducers and analog-to-digital board were calibrated (in cmH2O) before each experiment. Direct electrostimulation of the CN was performed with a delicate stainless steel bipolar hook electrode attached to a multijointed clamp. Each probe was 0.2 mm in diameter; the two poles were separated by 1 mm. Monophasic rectangular pulses were delivered by a signal generator (custom made and with a built-in constant current amplifier). Stimulation parameters were as follows: frequency, 20 Hz; pulse width, 0.22 ms; duration, 1 min. The experimental protocol used current stimulation of 0.5 and 4 mA (Table 1).
Table 1.
Primers used in quantitative PCR
| Gene | Primer set | % Efficiency compared to GAPDH |
|---|---|---|
| GAPDH | Forward 5′-TCCTGCACCACCAACTGCTTAG-3′
Reverse 5′-GATGACCTTGCCCACAGCCTTG-3′ |
100 |
| α-subunit | Forward 5′-TACTTCAATGACAATATCCTCACCCT-3′
Reverse 5′-ACCATAACAACCACCATCCCCTAAG-3′ |
109 |
| β-subunit | Forward 5′-GTATCACACAGAAGACACTCGGGA-3′
Reverse 5′-AAGAAGGAGAAGAGGAGGATTTGGG-3′ |
90 |
| SVK | Forward 5′-GGGAGGAAACCAAGACTCC-3′
Reverse 5′-CTAATGTGGTTCCAGTTG-3′ |
105 |
| SV1 | Forward 5′-GCATTTGAAAGATCCTCATTG-3′
Reverse 5′-CATGAGGAGTCTAGGCATG-3′ |
99 |
| STREX | Forward 5′-CCAAGATGTCCATCTAC-3′
Reverse 5′-GGCACGGAAACTGGTGG-3′ |
101 |
At the end of the organ function experiments, animals were killed by placement within a CO2 gas chamber. The tissues of interest were to be divided as for immediate patch-clamping studies or stored at −70° until RNA extraction.
Generation of cDNA
RNA was prepared using the TRIzol method (In-Vitrogen, Carlsbad, CA, USA) according to the manufacturers instructions. RNA concentration was determined by optical density. The reverse transcriptase reaction used an avian-enhanced myeloblastosis virus (AMV) reverse transcriptase. RNA was first denatured in the presence of Oligo (dT) (a primer) at 70°C for 5 min. The sample then was cooled briefly on ice. The RNA was combined with the AMV reverse transcriptase, deoxynucleoside triphosphate (10 mM), and RT reaction buffer. The combination was heated for 60 min at 37°C.
Quantitative real-time polymerase chain reaction
Polymerase chain reaction (PCR) was performed in an Applied Biosystems 7300 real-time PCR system using 12.5 μl. SybrGreen (Stratagene, La Jolla, CA, USA) final, 0.5 μl cDNA product, 50–150 nM forward primer, 50–150 nM reverse primer (optimization of primer pair concentrations are performed before experiment), final volume made to 25 μl with water. The primers used in this study are shown in Table 1. A 10-min cycle at 95°C was followed by 40 cycles of 30 s, at 95°C, 60 s at 55°C and 1 min at 72°C. At completion of the PCR run, a dissociation curve was run to determine that a single product was generated. The crosshold threshold then was determined. The efficiency for each primer pair was determined by serial dilution; the efficiency is shown in Table 1. Products from the PCR reaction were separated by agarose gel electrophoresis and sequenced to confirm the identity of the products. In experiments, the comparative crossing threshold method was used to normalize the target gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Subcellular fractionation
The corpora from two young and two old animals were pulverized to powder in liquid nitrogen pre-chilled mortar and pestle followed by homogenization in buffer containing 10 mM N-2-hydroxyethyl-piperazine-N′-2-ethanesulphonic acid, pH 7.3, 250 mM sucrose, 2 mM ethylenediaminetetraacetic acid (EDTA), 5 mM dithiothreitol and Complet mini EDTA-free protease inhibitor (Roche Applied Science, Palo Alto, IN, USA) using Dounce homogenizer. The homogenized tissue was centrifuged at 600 g for 10 min at 4°C and resulting supernatant was used for subcellular fractionation. Subcellular fractionation was performed by centrifugation at 100 000 g for 60 min at 4°C. The resulting pellet was designated as membrane fraction and supernatant as cytosolic fraction. Protein concentration was determined by 2D quant kit (Amersham Bioscience, Piscataway, NJ, USA) with bovine serum albumin as standard.
Western blotting
Protein samples were loaded onto 10% sodium dodecyl sulphate-polyacrylamide gels. After electrophoresis, the resolved proteins were transferred to a nitrocellulose membrane. The membrane was blocked in 5% skim milk with 0.05% Tween-20 in phosphate-buffered saline (PBST) for 2 h at room temperature. The membrane was then incubated with anti-MaxiK antibody (1:250; BD Transduction Laboratories, Lexington, KY, USA) or anti-actin antibody (1:50 000; Sigma Aldrich Inc., St Louis, MO, USA) in 5% skim milk with PBST for 1 h at room temperature and washed five times for 5 min in PBST. The membrane was then incubated with mouse immunoglobulin G horseradish peroxidase-linked antibody (1:5000; Amersham Bioscience, NJ, USA) in PBST for 1 h at room temperature and subsequently the membrane was washed five times for 5 min with PBST. Proteins were visualized using enzymatic chemiluminescent Western blotting substrate (Pierce, Rockford, IL, USA) and images recorded on Kodak biomax light film (Eastman Kodak Company, Penver, CO, USA). The visualized proteins were quantified using model GS-700 imaging densitometer and molecular analyst (Bio-Rad, Hercules, CA, USA).
Results
Erectile function determined by ICP/BP response after electrostimulation in rats of two age groups
We determined the ICP/BP ratio in young and old rats as quantifiable measure of erectile function. As shown in Table 2, older rats had a significantly diminished erectile capacity indicated by a decrease in the ICP/BP ratio at both the 0.75-mA and 4-mA levels of electrostimulation. At both levels of stimulation the young animals had an ICP/BP ratio of >0.6 correlating to development of visible erections, whereas the older animals had ICP/BP ratios of <0.4, indicating impairment of erectile function. These observations are similar to previous reports in which ageing of rats is associated with the development of ED.7,21–23
Table 2.
Determination of erectile function by ICP/BP measurement in young and old rats
| ICP/BP ratio
|
|||
|---|---|---|---|
| Basal | 0.75 mA | 4 mA | |
| Young | 0.08 ± 0.006 | 0.67 ± 0.04 | 0.72 ± 0.02 |
| Old | 0.07 ± 0.007 | 0.18 ± 0.03* | 0.25 ± 0.03* |
Abbreviations: ICP/BP, intracavernosal pressure/ blood pressure. ICP/BP response to electrical stimulation of 0.75 and 4 mA to the cavernosal nerve in young and old rats.
Difference between ICP/BP value in young and old animals following electrostimulation is significantly different (P<0.01).
Expression of MaxiK channels transcripts and alternative splice forms of the Slo transcript
Previously, the expression of both the α- and β-subunits of the MaxiK channel have been reported to be downregulated in coronary arteries of aging rats.24.Therefore, using quantitative PCR, we looked at the expression of MaxiK α- and β-subunits in corporal tissue. There are several potential transcripts for the gene encoding the α-subunit of the MaxiK gene (Slo), which are generated by alternative splicing. Therefore, we also developed primers, which we predicted would specifically amplify these transcripts (STREX,25 SVK) primers based on human sequence as reported by Korovkina et al.26 and SV1.19 Isolation of RNA, generation of cDNA, and real-time amplification are described in Experimental Procedures. The PCR products were confirmed to be correct by sequencing.
We found that expression of the transcripts for the α- and β-subunits of MaxiK in young compared with older animals are not significantly changed in expression levels in the corporal tissue (Figure 1). However, although there was little change in the overall transcription of the α- or β-subunit in old compared with young animals, there were changes in the quantities of the splice variant. Of particular interest was that in the corpora, there was approximately a 13-fold increase in the expression of SV1 in the older animals. This splice variant, described by Zarei et al.19 acts as a dominant-negative inhibitor for MaxiK channel activity by trapping other isoforms in the cytosol.
Figure 1.
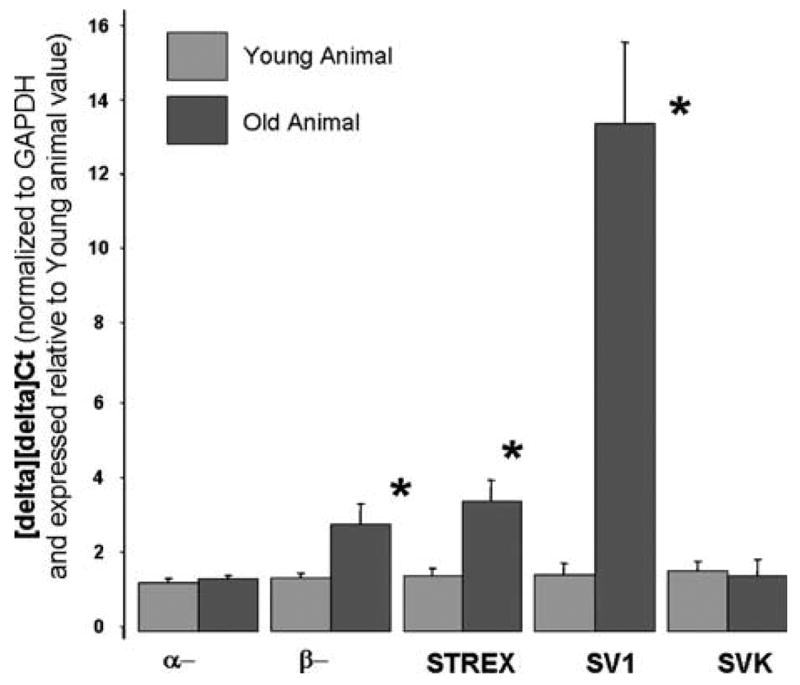
Quantitative real-time PCR of genes encoding MaxiK channel and splice variants in young and old male rats. Mean expression of the genes and transcript encoding the MaxiK channel in corporal smooth muscle tissues normalized to GAPDH using the 2−[delta][delta]Ct method expressed relative to expression in young animals (i.e. the calibrator, taken as 1). Primers the α- and β-subunits transcripts have been previously published,29 Primers for other splice variants were based on published sequences: STREX see Xie and McCobb,25 SVK see Korovkina et al.,26 SV1 see Zarei.19 The error bars represent the s.d. from the mean *significantly different expression of the transcript in older compared to younger animals (P<0.05).
Subcellular fractionation and Western blotting to localize expression of the MaxiK channel
The upregulation of the dominant-negative transcript, SV1, could potentially lead to trapping of functional isoforms of the MaxiK in the cytoplasm. Therefore, we investigated the subcellular localization of the MaxiK channel by sub-fractionating corporal cells from young and old animals by differential centrifugation into a membrane and a cytosolic fraction, and then probing for protein expression Western blot analysis. Blotting for actin was used to normalize protein loading. Results of a typical gel are shown in Figure 2a. The expression of protein (MaxiK and actin) in the sub-cellular fractions was determined by densitometry and expression of MaxiK was normalized by comparison with actin. These results are depicted graphically in Figure 2b. There is a significant decrease in the amount of MaxiK expressed in the membrane of the older animals of approximately 13-fold. Supporting the hypothesis that increased expression of SV1 would potentially lead to trapping of isoforms of the MaxiK in the cytoplasm, in older animals, there is a significant increase in the ratio of MaxiK in the cytosol compared to the membrane (approximately threefold).
Figure 2.
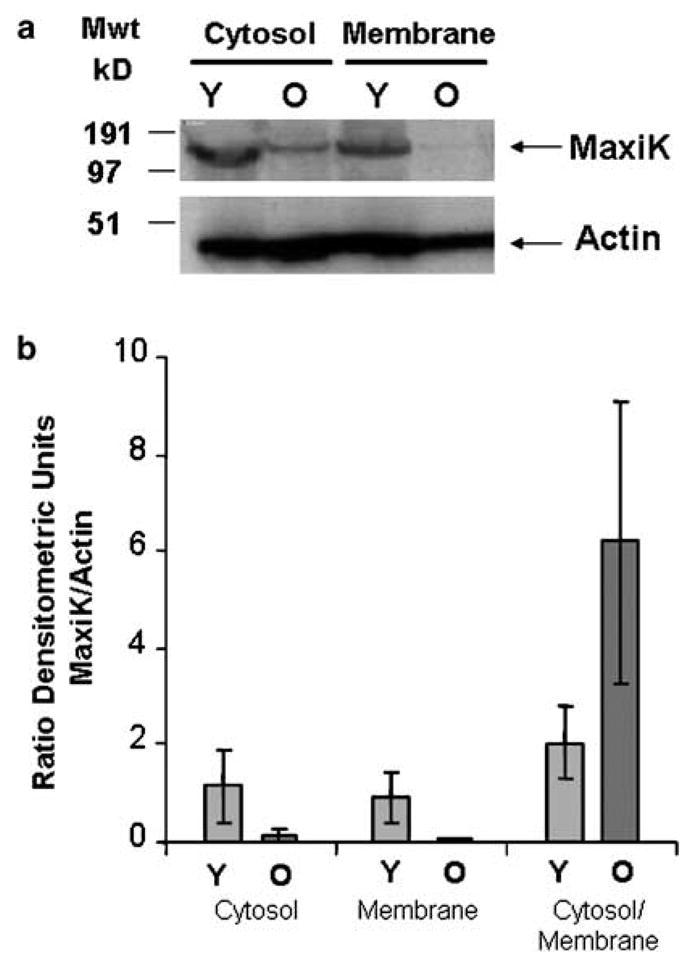
Subcellular fractionation and Western blotting to localize expression of the MaxiK channel. (a) A typical Western blot where expression of MaxiK in the cytosolic and membrane fractions of corpora is analysed in young (Y) and old (O) animals. Actin is used to normalize loadings. (b) Densitometric analysis of protein expression. Four animals, two young and two old were analysed by Western blot in duplicate. Expression of MaxiK and actin protein was determined in samples by densitometry. Expression of MaxiK was normalized by comparison with actin. The columns represent the mean of four analysis, and the error bars the average deviation from the mean. The expression of MaxiK is shown in the cytosol and membrane fractions, and also as a ratio of the amount in cytosol to membrane fractions in young (Y) and old (O) animals.
Discussion
As previously reported that as rats age, there is a decrease in erectile function.7,21–23 Because of the importance of the MaxiK channel in erectile function, we compared expression of the MaxiK channel in young to older rat corporal tissue. We observed that there is no change in the rate of transcription of the Slo transcript (encoding the MaxiK α-subunit), or changes in the level of expression of the transcript encoding the regulatory β-subunit. However, there are remarkable changes post-transcriptionally in the expression of MaxiK protein. In older animals, there is a significant decrease in the amount of MaxiK protein expressed in the membranes and an increase in the amount of MaxiK retained in the cytosol. The downregulated expression of the MaxiK channel correlates with a significant increase in the levels of a dominant-negative transcript of the Slo gene (SV1) in older animals.19 We propose that the increase in SV1 in older animals traps the MaxiK protein in the cytoplasm. It has been previously shown that dominant-negative mutants trapping MaxiK in the cytoplasm can lead to an overall decrease in the expression of the MaxiK protein probably through protein degradation pathways.27 This hypothesis is depicted graphically in Figure 3. Potentially, the physiological effect of downregulating the MaxiK channel activity could lead to heightened tone of the corporal smooth muscle tissue, although an increase in intracellular calcium, which results in heightened contractility and ED.
Figure 3.
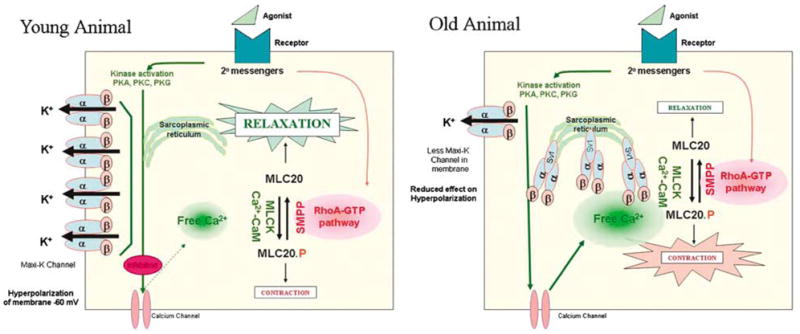
In young animals, the majority of the MaxiK channel is expressed on the surface of the corporal smooth muscle cells. Upon activation, they hyperpolarize the membrane, effect that inhibits the activity of the calcium channel. This results in a reduction in the intracellular free calcium levels and activity of the myosin light chain kinase (MLCK) through the action of the calcium-calmodulin complex. Less myosin light chain is in the phosphorylated (contracted) state, leaving the smooth muscle cell in a relaxed state. In the older animals, increased expression of the dominant negative MaxiK channel (SV1) reduces the amount of MaxiK expressed on the cell surface. The reduction in the ability of the MaxiK channels may cause hyperpolarization and thereby inhibit calcium channels, leads to an increase in intracellular calcium that ultimately results in the smooth muscle cell being in a more contracted state.
There is a strong correlation between the increase in the SV1 transcript (approximately 13-fold), and decrease in the amount of MaxiK protein (approximately 13-fold) expressed on the membrane surface of corporal smooth muscle cells from old compared to young animals. SV1 has been shown to trap isoforms of MaxiK in the cytoplasm. SV1 contains a 33-aa insert in S1 transmembrane domain and is retained in the endoplasmic SV1. The insert contains an endoplasmic reticulum (ER) retention/ retrieval motif, CVLF.20 The CVLF hydrophobic motif is sufficient to prevent surface expression of independently expressed membrane proteins by trapping them in the intracellular compartments, likely the ER. In addition, CVLF-containing channels are tetramerized and have similar solubility to hSlo variants expressed on the membrane. Therefore, retention by CVLF motif may be receptor mediated. In addition to trapping the α-subunits the SV1 isoform is also capable of trapping the β-subunits.
Comparing the results presented here with published papers, it seems that the effect of aging on expression of the MaxiK channels in is very organ, and even tissue specific. For example, in cerebral myocytes, expression and function of the MaxiK channel remains similar in both young (3- to 5-month-old) and old (25- to 30-month-old) rats.24 In contrast in rat coronary smooth muscle, there is downregulation of the transcripts for both the α- and the β-subunits correlating with a decrease in the activity of the channels seen with patch clamping.24,28–30 This suggests that therapies addressing age-related changes in the activity of MaxiK may need to be tailored to a particular organ.
In studies on ageing animals, gene transfer of plasmids expressing the hSlo gene has been used to treat age-related ED in rats.7 The use of gene transfer by the hSlo gene in restoring erectile function is being evaluated in a clinical phase trials.10–13 Our results suggest that if gene transfer of hSlo is successful in the treatment of ED in ageing human patients, the mechanism of action would likely involve overcoming post-transcriptional events that limit the expression of MaxiK channel activity.
Acknowledgments
This work was supported by grants P01-DK060037, R21- DK70229 (awarded to MR Chance) and K01-DK67270 (awarded to KP Davies) from the NIH, NIDDK.
References
- 1.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–S13. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 2.Shabsigh R, Perelman MA, Lockhart DC, Lue TF, Broderick GA. Health issues of men: prevalence and correlates of erectile dysfunction. J Urol. 2005;174:662–667. doi: 10.1097/01.ju.0000165389.73148.d1. [DOI] [PubMed] [Google Scholar]
- 3.Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2 + sensitivity in vascular smooth muscle. J Smooth Muscle Res. 2004;40:219–236. doi: 10.1540/jsmr.40.219. [DOI] [PubMed] [Google Scholar]
- 4.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, et al. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–556. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL. Potassium channels and erectile dysfunction. Vasc Pharmacol. 2002;38:61–71. doi: 10.1016/s1537-1891(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 6.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170:285–290. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- 8.Christ GJ, Rehman J, Day N, Salkoff L, Valcic M, Melman A, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998;275:H600–H608. doi: 10.1152/ajpheart.1998.275.2.H600. [DOI] [PubMed] [Google Scholar]
- 9.Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, et al. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–H1553. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 10.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur Urol. 2005;48:314–318. doi: 10.1016/j.eururo.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Melman A. Gene transfer for the therapy of erectile dysfunction: progress in the 21st century. Int J Impot Res. 2006;18:19–25. doi: 10.1038/sj.ijir.3901412. [DOI] [PubMed] [Google Scholar]
- 12.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. hMaxi-K gene transfer in males with erectile dysfunction: results of the first human trial. Hum Gene Ther. 2006;18:1165–1176. doi: 10.1089/hum.2006.17.1165. [DOI] [PubMed] [Google Scholar]
- 13.Schiff JD, Melman A. Ion channel gene therapy for smooth muscle disorders: relaxing smooth muscles to treat erectile dysfunction. Assay Drug Dev Technol. 2006;4:89–95. doi: 10.1089/adt.2006.4.89. [DOI] [PubMed] [Google Scholar]
- 14.Gribkoff VK, Starrett JE, Jr, Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Koike K, Alioua A, Shigenobu K, Stefani E, Toro L. Beta1-subunit of maxiK channel in smooth muscle: a key molecule which tunes muscle mechanical activity. J Pharmacol Sci. 2004;94:339–347. doi: 10.1254/jphs.94.339. [DOI] [PubMed] [Google Scholar]
- 16.Patterson AJ, Henrie-Olson J, Brenner R. Vasoregulation at the molecular level: a role for the beta1 subunit of the calcium-activated potassium (BK) channel. Trends Cardiovasc Med. 2002;12:78–82. doi: 10.1016/s1050-1738(01)00146-3. [DOI] [PubMed] [Google Scholar]
- 17.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fury M, Marx SO, Marks AR. Molecular bkology: the study of splicing and dicing. Sci STKE. 2002;2002:PE12. doi: 10.1126/stke.2002.123.pe12. [DOI] [PubMed] [Google Scholar]
- 19.Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel maxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–16239. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- 20.Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, et al. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2 +-activated K + channel splice variant. Proc Natl Acad Sci USA. 2004;101:10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabro A, Italiano G, Pescatori ES, Marin A, Gaetano O, Abatangelo G, et al. Physiological aging and penile erectile function: a study in the rat. Eur Urol. 1996;29:240–244. [PubMed] [Google Scholar]
- 22.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein a1 as a marker for erectile dysfunction. BJU Int. 2006;98:396–401. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajasekaran M, White S, Baquir A, Wilkes N. Rhokinase inhibition improves erectile function in aging male Brown–Norway rats. J Androl. 2005;26:182–188. doi: 10.1002/j.1939-4640.2005.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishimaru K, Eghbali M, Stefani E, Toro L. Function and clustered expression of maxiK channels in cerebral myocytes remain intact with aging. Exp Gerontol. 2004;39:831–839. doi: 10.1016/j.exger.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 26.Korovkina VP, Fergus DJ, Holdiman AJ, England SK. Characterization of a novel 132-bp exon of the human maxi-K channel. Am J Physiol Cell Physiol. 2001;281:C361–C367. doi: 10.1152/ajpcell.2001.281.1.C361. [DOI] [PubMed] [Google Scholar]
- 27.Kwon SH, Guggino WB. Multiple sequences in the c terminus of maxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci USA. 2004;101:15237–15242. doi: 10.1073/pnas.0404877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of maxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2 + )-activated K( + ) channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- 30.Toro L, Marijic J, Nishimaru K, Tanaka Y, Song M, Stefani E. Aging, ion channel expression, and vascular function. Vasc Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00128-3. [DOI] [PubMed] [Google Scholar]


