Abstract
The developing fetus is uniquely sensitive to perturbation by chemicals with hormone-like activity. The adverse effects of prenatal diethylstilbestrol (DES) exposure are a classic example. Since concern has been mounting regarding the human health and environmental effects of bisphenol A (BPA), a high-production-volume chemical with estrogenic activity used in the synthesis of plastics, we investigated its long-term effects in an experimental animal model that was previously shown useful in studying the adverse effects of developmental exposure to DES. Outbred female CD-1 mice were treated on days 1-5 with subcutaneous injections of BPA (10, 100 or 1000 μg/kg/day) dissolved in corn oil or corn oil alone (Control). At 18 months, ovaries and reproductive tract tissues were examined. There was a statistically significant increase in cystic ovaries and cystic endometrial hyperplasia (CEH) in the BPA-100 group as compared to Controls. Progressive proliferative lesion (PPL) of the oviduct and cystic mesonephric (Wolffian) duct remnants were also seen in all of the BPA groups. More severe pathologies of the uterus following neonatal BPA treatment included adenomyosis, leiomyomas, atypical hyperplasia, and stromal polyps. These data suggest that BPA causes long-term adverse effects if exposure occurs during critical periods of differentiation.
Keywords: Endocrine Disruptors, Ovary, Uterus, Reproduction, Developmental
Introduction
It is well known that perinatal exposure to environmental chemicals with estrogenic activity can have long lasting consequences if exposure occurs during critical periods of development [1]. Recent studies have further proposed that hormonal perturbations during fetal or neonatal development may predispose individuals to disease and/or dysfunction later in life such as hypertension and coronary disease [2], obesity [3-5], and reproductive problems including infertility/subfertility, and increased tumors such as uterine fibroids (leiomyomas) [6] and breast cancer [7]; also see Keri et al. review in this issue. The benign and carcinogenic effects of prenatal exposure to diethylstilbestrol (DES), a potent synthetic estrogen used to prevent miscarriage in the late 1940s-1970s, is an unfortunate reminder of the harmful effects that estrogenic chemicals can cause during development, some of which were not apparent until much later in adulthood or in subsequent generations [8, 9]. Although the use of DES during pregnancy was discontinued over 30 years ago, infants and children continue to be inadvertently exposed to a wide number of environmental chemicals, many with hormone–like activity.
Bisphenol A (BPA), used in the manufacture of polycarbonate plastics and epoxy resins, is one such hormonally active chemical that is receiving increased attention due to its potential for human exposure. BPA has been shown to leach from food cans [10], beverage containers [11], and dental sealants and composites [12], suggesting that humans are routinely exposed to this compound. Experimental animal studies have reported that BPA given during development can act at very low doses in the range of human and wildlife exposures [13]. Further, an increasing number of studies have implicated perinatal BPA exposure in a variety of abnormalities in the female reproductive tract including earlier onset of vaginal opening [14], early puberty [15], altered estrus cyclicity [16], altered plasma LH levels [17], altered ovarian morphology [16], and altered body weight [17]. Also, reports of perinatal BPA exposure have been linked to alterations in the mammary gland [18] and uterus [13] but no studies have looked at BPA effects in mature animals exposed as neonates. This study was designed to determine if exposure to BPA at environmentally relevant doses can cause long-term adverse effects in the female reproductive tract in 18 month old animals.
Materials and Methods
Animals
Adult outbred female CD-1 [Crl:CD-1 (ICR) BR] mice were obtained from Charles River Breeding Laboratories (Raleigh, NC) and bred to male mice of the same strain in the breeding facility at the National Institute of Environmental Health Sciences (NIEHS; Research Triangle Park, NC). Vaginal plug detection was considered day 0 of pregnancy. Pregnant mice were individually housed in plastic cages with hardwood chip bedding under controlled lighting (12 h light and 12 h dark) and temperature (21-22C) conditions. Mice were fed NIH 31 mouse chow, which contained a moderately low amount of genistein (46 μg/g) (14) and fresh water ad libitum. All animal procedures complied with NIEHS/NIH animal care guidelines. At delivery, pups from all litters were pooled, then separated by sex, and randomly standardized to 8 female pups per dam. (Male pups were used in another experiment.) Female pups were treated by daily subcutaneous injections on days 1-5 with BPA (10, 100, or 1000 μg/kg) dissolved in corn oil or corn oil alone (as Control). These groups are referred to subsequently as BPA-10, BPA-100, BPA-1000 or Control (n=24 female pups per treatment group). These doses were chosen since they were in the range previously reported to cause effects in experimental animals [13, 19]; also see Richter et al review in this issue. Also, the route of administration, namely subcutaneous injections, is considered relevant for assessing the potential for developmental effects of BPA in humans since newborn mice do not demonstrate the rapid first pass metabolism of BPA as orally dosed adults do (see Richter et al review this issue). Mice were weaned on day 21, housed 4 per cage, and held without further treatment. Mice were sacrificed by CO2 asphyxiation at 18 months of age. Reproductive tract tissues plus ovaries were removed, fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 6 microns. Tissue sections were stained with hematoxylin and eosin (H & E) and evaluated by light microscopy. If a microscopic lesion was observed, additional serial sections were made to include the entire area of pathological change. Lesions diagnosed as uterine leiomyomas were stained with Masson's trichrome special stain to confirm their smooth muscle cell origin.
Statistical Analysis
Cochran-Armitage trend tests were used to test for dose-related changes in lesion incidence. Lesion incidences in each dose group were separately compared to the Control group incidence using one-sided Fisher's exact tests. P-values ≤ 0.05 were considered statistically significant.
Results
At 18 months of age, body weights were not statistically different between Controls and mice treated neonatally with BPA (54.23 ± 2.10 Controls; 59.99 ± 1.45 BPA-10; 51.62 ± 2.68 BPA-100; and 55.13 ± 2.03 BPA-1000).
A comparison of ovarian and oviductal abnormalities in Control and BPA treated mice is shown in Table 1. At 18 months, cystic ovaries were common in all treatment groups (39% in Controls, 35% in BPA-10, 70% in BPA-100 and 38% in BPA-1000) but only the BPA-100 group was statistically different (p≤0.05) from Controls. Corpora lutea (CL) were seen in all 100% (18/18) of the Controls and 96% (22/23) of BPA-10, 90% (18/20) of BPA-100, and 88% (14/16) of BPA-1000 mice suggesting a decreasing trend in CLs as the BPA dose increased. Para-ovarian cysts of mesonephric origin were not observed in any Controls but were seen in all groups of the BPA-treated mice [4% (1/23) in BPA-10, 10% (2/20) in BPA-100, and 6% (1/16) in BPA-1000].
Table 1.
Incidence of Benign and Malignant Abnormalities in 18-month old Mice Treated Neonatally with Bisphenol A
| Treatment | Ovary/Oviduct | Uterus | ||
|---|---|---|---|---|
| Control | 18/18 | CL (100)a | 1/18 | CEH (6) c |
| 7/18 | Ovarian Cysts (39) | 1/18 | Adenomyosis (6) | |
| 0/18 | Paraovarian Cysts (0) | 0/18 | WD Remnants in Uterine Wall (0) d | |
| 0/18 | PPL (0) b | 0/18 | Leiomyoma (0) | |
| 0/18 | Atypical Hyperplasia (0) | |||
| 1/18 | Stromal Polyp (6) | |||
|
| ||||
| BPA-10 | 22/23 | CL (96) | 5/23 | CEH (22) |
| 8/23 | Ovarian Cysts (35) | 2/23 | Adenomyosis (9) | |
| 1/23 | Paraovarian Cysts (4) | 3/23 | WD Remnants in Uterine Wall (13) d | |
| 3/23 | PPL (13) | 1/23 | Leiomyoma (4) | |
| 1/23 | Atypical Hyperplasia (4) | |||
| 1/23 | Stromal Polyp (4) | |||
|
| ||||
| BPA-100 | 18/20 | CL (90) | 9/20 | CEH (45) ** |
| 14/20 | Ovarian Cysts (70) * | 4/20 | Adenomyosis (20) | |
| 2/20 | Paraovarian Cysts (10) | 2/20 | WD Remnants in Uterine Wall (10) d | |
| 3/20 | PPL (15) | 2/20 | Leiomyoma (10) | |
| 1/20 | Atypical Hyperplasia (5) | |||
| 5/20 | Stromal Polyps (25) | |||
|
| ||||
| BPA-1000 | 14/16 | CL (88) | 4/16 | CEH (25) |
| 6/16 | Ovarian Cysts (38) | 3/16 | Adenomyosis (19) | |
| 1/16 | Paraovarian Cysts (6) | 3/16 | WD Remnants in Uterine Wall (19) d | |
| 1/16 | PPL (6) | 1/16 | Leiomyoma (6) | |
| 0/16 | Atypical Hyperplasia (0) | |||
| 1/16 | Stromal Polyp (6) | |||
Outbred female CD-1 mice were treated by subcutaneous injections on Days 1-5 of neonatal life with bisphenol A and sacrificed at 18 months. The numbers in parenthesis represent percent incidence. The number in the denominator represents the number of mice that survived to 18 months and that had sufficient tissue sections for evaluation.
CL = corpora lutea.
PPL = progressive proliferative lesion of the oviduct.
CEH = cystic endometrial hyperplasia.
WD = Wolffian remnants
Statistical significance determined by Fisher's exact test.
p ≤ 0.05
p< 0.01
In the oviduct, progressive proliferative lesion (PPL) (Figure 1) was not observed in Controls but was seen in all groups of the BPA treated mice [13% (3/23) in BPA-10, 15% (3/20) in BPA-100, and 6% (1/16) in BPA-1000]. This oviductal abnormality was previously reported in perinatally DES-treated mice [20] and, it histologically resembled the DES lesions; the pattern of tubal plications of oviductal mucosa was often distorted and characterized by irregularities in the size and shape of the mucosal folds relative to the Control animals. The mucosal folds had an adenomatous (gland-like) appearance but maintained connection with the oviductal lumen. This abnormality in proliferation was termed progressive proliferative lesion (PPL) since it did not spread along the serosal surface or metastasize to surrounding tissues [20].
Figure 1.
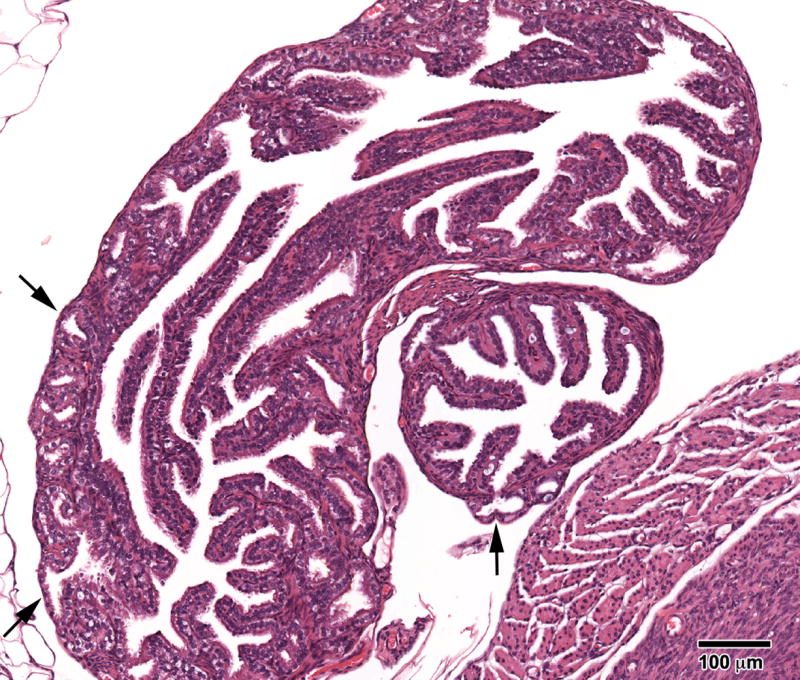
Progressive proliferative lesion (PPL) of the oviduct in an 18- month mouse treated neonatally with BPA (100 mg/kg/day) on days 1-5 of life. The epithelial folds of the oviduct are of normal height, but note areas where the epithelium resembles “gland-like structures” which extend into and through the muscularis (arrows). (H & E; bar = .5mm)
The range of uterine abnormalities is also shown in Table 1. The incidence of cystic endometrial hyperplasia (CEH) was increased in all BPA groups [6% (1/18) Controls, 22% (5/23) BPA-10, 45% (9/20) BPA-100, and 25% (4/16) BPA-1000] indicating excessive estrogen stimulation; the BPA-100 group was statistically significant from Controls (p<0.01). Adenomyosis characterized by benign invasion of endometrial glands into the myometrium occurred in all groups with an increasing trend in the two highest BPA groups [6% (1/18) Controls, 9% (2/23) BPA-10, 20% (4/20) BPA-100, and 19% (3/16) BPA-1000] (Figure 2); adenomyosis was previously reported to be prevalent in DES treated mice [21, 22] but unlike DES, the BPA treated mice appeared to have a well developed uterine muscle wall. Atypical hyperplasia of the uterus, a precursor lesion to estrogen–associated uterine adenocarcinoma, occurred in 4% (1/23) of the BPA-10 and 5 % (1/20) BPA-100 but not in Controls; Figure 3A shows a stromal polyp and Figure 3B shows atypical hyperplasia in the polyp. Uterine leiomyomas which were well-demarcated and discrete lesions were also seen in BPA treated mice [none (0/18) in Controls, 4% (1/23) in BPA-10; 10% (2/20) in BPA-100; 6% (1/16) in BPA-1000] (Figure 4) and they were primarily located within the uterine horn. One mouse in the BPA-100 group had multiple lesions. Histologically, the leiomyoma cells were spindle shaped with elongated blunted nuclei typical of smooth muscle cells. These leiomyoma tumor cells stained red with Masson's trichrome stain confirming their smooth muscle origin.
Figure 2.
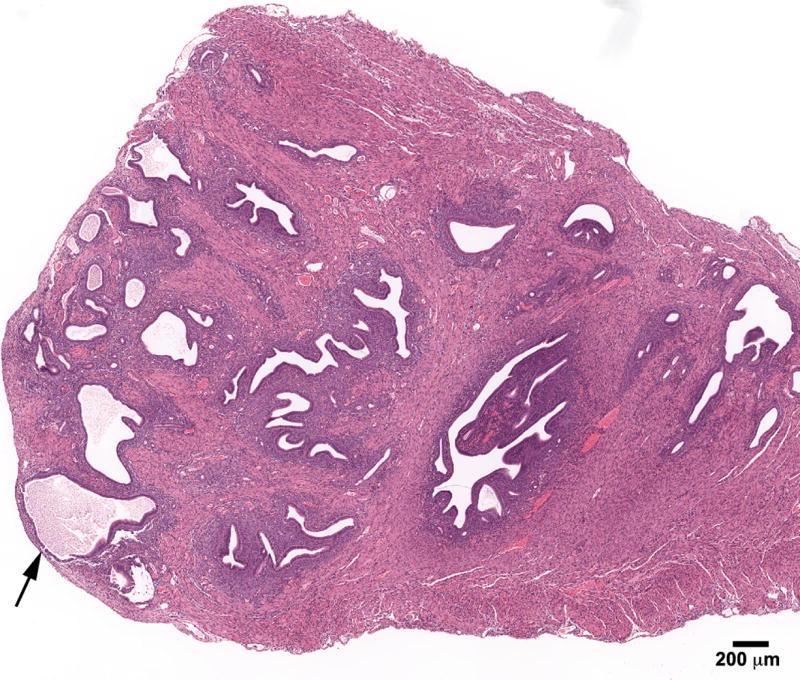
Adenomyosis of the uterus in an 18- month mouse treated neonatally with BPA (100 mg/kg/day) on days 1-5 of life. Note the benign invasion of endometrial glands that are growing into the myometrium (arrow). (H & E; bar = .5mm)
Figure 3.
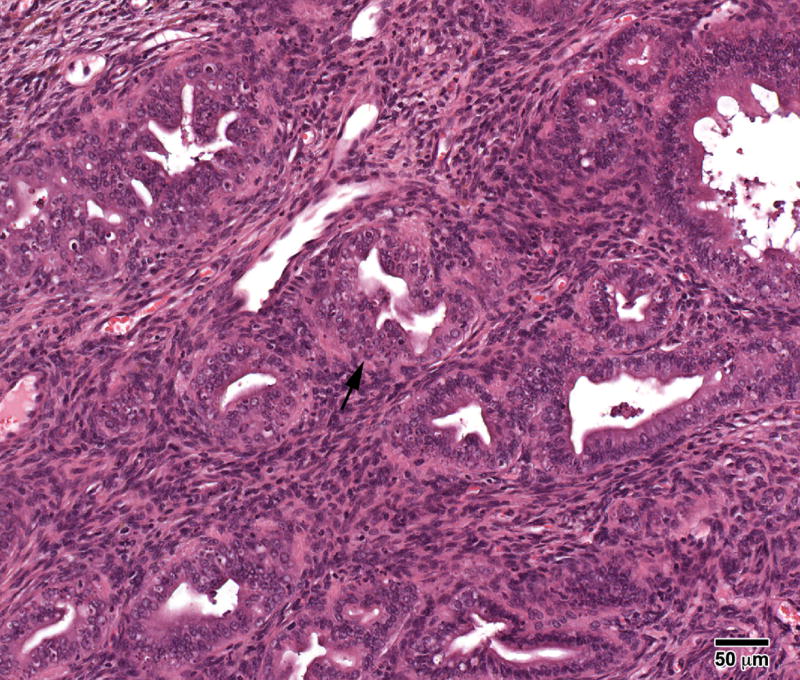
A) Atypical hyperplasia in a large uterine stromal polyp in an 18- month mouse treated neonatally with BPA (100 mg/kg/day) on days 1-5 of life. Note the irregularly shaped glands with little intervening stroma. B) High power shows “piling up” of cells (arrow) with hyperchromatic nuclei and many mitotic figures. (H & E; bar = .5mm)
Figure 4.
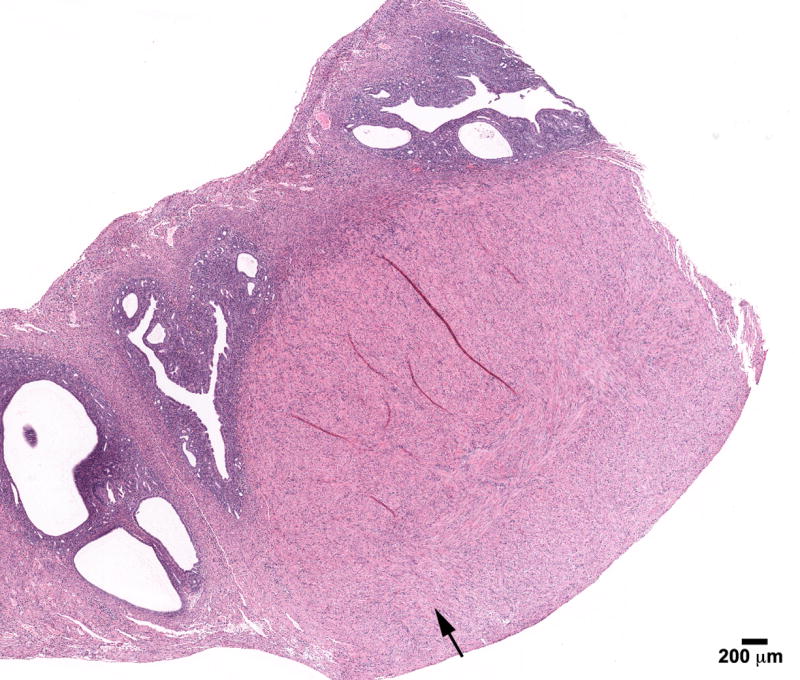
Leiomyoma in the uterus in an 18- month mouse treated neonatally with BPA (100 mg/kg/day) on days 1-5 of life. Note well-circumscribed tumor (arrow). CEH characterized by enlarged dilated spaces can be seen in the remainder of the uterine horn (H & E; bar = .5mm)
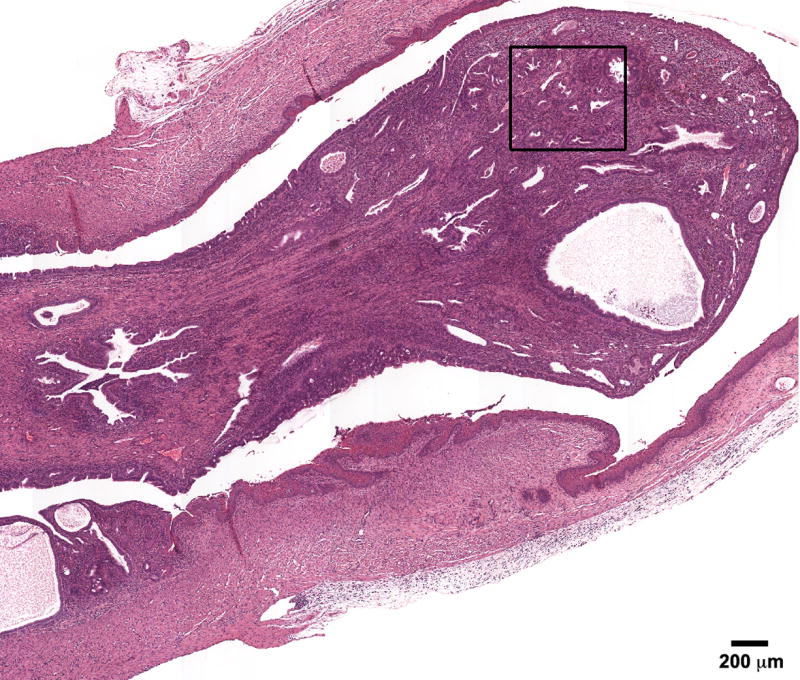
In addition, stromal polyps were seen in all groups but there was a high 25% (5/20) incidence of this neoplastic lesion observed in the BPA-100 group (Figure 3A). These lesions have been reported to be associated with the development of stromal cell sarcomas. Similar to DES, enlarged mesonephric (Wolffian) duct remnants (Figure 5) were also found in BPA treated mice [one (1/18) in the Controls, 13% (3/23) BPA-10, 10% (2/20) BPA-100, and 19% (3/16) in BPA-1000].
Figure 5.
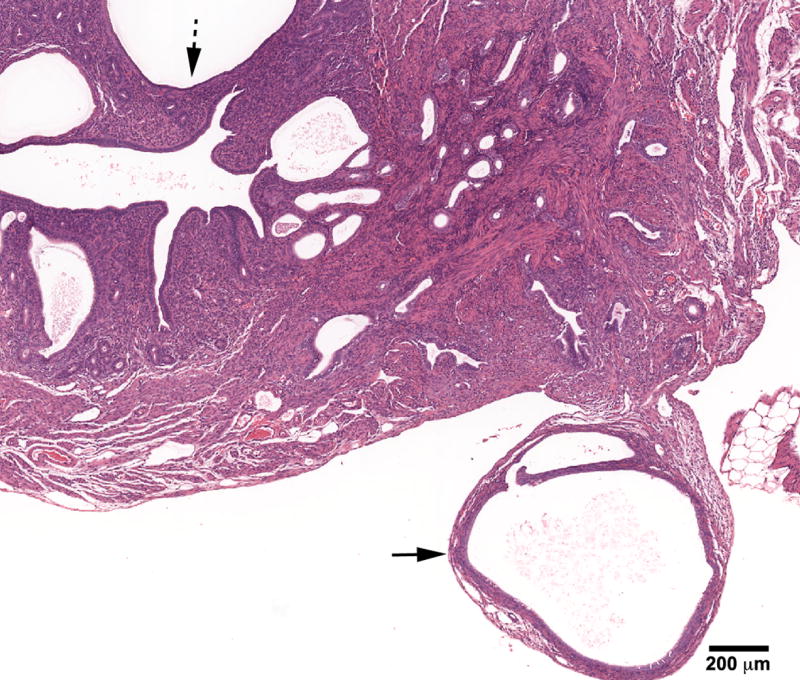
Cystic mesonephric (Wolffian) remnant (solid arrow) in an 18- month mouse treated neonatally with BPA (100 mg/kg/day) on days 1-5 of life. Remnant is located in close proximity to the uterus and ovary. CEH (dotted arrow) with changes which vary from enlarged spaces lined by columnar or flattened epithelium. (H & E; bar = .5mm)
Discussion
This report describes the induction of numerous abnormalities in the ovary and reproductive tract of aged female mice treated neonatally with BPA. The doses of BPA chosen were low and within the range of human exposures [13]; further, these doses have been reported to cause changes in male [23]; also see Richter et al. review in this issue and female mice [13]; also see A. Soto review in this issue. Skeptics of low dose endocrine disrupting effects criticized early BPA studies because they only described changes in tissues weights and morphology but did not document specific adverse effects. However, more recent studies report adverse effects including premalignant changes in mammary gland tissues [18] and abnormal gene imprinting in the prostate [24, 25] following developmental exposure to BPA. In addition, higher doses of BPA have been shown to cause alterations in estrus cyclicity, ovulation, and ovarian morphology [26]. Our current study adds to the growing body of literature that reports adverse effects following developmental exposure to low doses of BPA. Our study is the first to show BPA-induced developmental abnormalities in the mature female reproductive tract which include benign, premalignant and neoplastic changes.
Among the significant benign abnormalities was an elevated incidence of ovarian cysts (70% in the BPA-100 group). Although these ovarian cysts are histologically similar to those seen in our aged controls, the incidence is significantly higher (39%). In fact, the incidence is higher than we have seen following developmental exposure to DES .001 mg/kg (58%) [22], genistein 50 mg/kg (41%) [27], or tamoxifen (60%) [28] suggesting that the ovary may be a particularly sensitive target for the effects of BPA. Other studies describing the disruption of early oogenesis in the mouse by BPA have also shown the sensitivity of the ovary [29]. Ongoing studies in our laboratory are investigating this possibility as well as mechanisms involved in the formation of ovarian cysts.
Paraovarian cysts of mesonephric (Wolffian) duct origin were also seen in neonatally BPA treated mice in this study. This increased incidence combined with the finding of cystic Wolffian duct remnants in the uterine wall of BPA mice also suggests that the mesonephric duct system (Wolffian duct) may be a target of BPA. Interestingly, mesonephric-derived tissues were noted to be sensitive to the effects of perinatal DES exposure in both male [30] and female mice [31].
Another BPA-induced abnormality that has been described in perinatal DES exposed mice is PPL of the oviduct. DES was previously shown to interfere with the normal differentiation of the Mullerian duct (the precursor of the oviduct) resulting in structural (prenatal exposure) [32] and cellular (neonatal exposure) alterations [20, 33]. A molecular mechanism is likely involved since studies reported that HOX genes were involved in the differentiation of the reproductive tract [34] and prenatal DES was shown to delay the expression of these genes [35]. Subsequent studies suggest DES works through multiple genes pathways [36, 37]. Thus, molecular “misprogramming” is responsible for DES-induced oviductal alterations and is most likely associated with the similar BPA-induced oviductal changes.
The benign lesions of CEH and adenomyosis also occurred in the uterus following neonatal BPA treatment; CEH was statistically increased in the BPA-100 group. These lesions were histologically similar to those seen in aged Controls; however, their severity and the extensive involvement of the uterine horns were increased in BPA treated groups as compared to Controls.
Of particular significance in this study is the occurrence of more severe uterine lesions. Leiomyomas were seen in all BPA treated groups similar to those seen following DES treatment [38]. Previously, many experimental animal studies examining the tumorigenic potential of environmental estrogens have focused on epithelial lesions of the reproductive tract such as uterine adenocarcinoma [39]; however, alterations in smooth muscle cells following exogenous estrogen administration have also been shown [21, 40]. Further, we have recently shown an association of prenatal DES exposure in women with the development of uterine leiomyomas [41]. Since smooth muscle lesions are seen in our mice following BPA treatment, it gives validity to the concerns that endocrine disrupting chemicals in the environmental, in particular those with estrogen-like activity, are contributing to the increased incidences of various human diseases; leiomyomas have been proposed to be one such lesion [42]. Although uterine adenocarcinoma was not seen in this study, its premalignant lesion of atypical hyperplasia was observed. Finally, there was an increased incidence of stromal polyps following BPA-100 treatment. These lesions are considered neoplastic in experimental rodent models because they are often the site for the development of endometrial stromal sarcoma [43]. Historically, we have rarely seen these lesions in CD-1 mice although a stromal polyp was seen in one Control mouse in this study.
Although adverse effects in the reproductive tract were identified in all BPA treated groups, it is interesting that the BPA-100 dose was the most affected with more leiomyomas, adenomyosis, mesonephric (Wolffian) duct remnants, ovarian cysts, and stromal polyps than the other doses. The significance of this is uncertain but non-linear dose response curves have been commonly reported in endocrinology studies[19].
In this study, body weights were not different between BPA treated and Control mice. This lack of difference is most likely due to the advanced age of the mice in the study. It has been previously reported by our lab and others that developmental exposure to BPA, DES, and other environmental chemicals with endocrine disrupting effects was associated with obesity in mice after they reach puberty and throughout maturity [3, 4, 44, 45]; however, these animals were not examined as they aged to 18 months. Since we have also shown that significant differences observed in body weight in DES mice as compared to Controls at 6-8 months get more difficult to detect as the animals age because of increased individual variability among all mice [4], this variability probably accounts for lack of detection of body weight differences in this study. Most importantly, in this study, there was no apparent correlation of body weight and tumor occurrence either in individual animals or groups.
The findings of the present developmental study raise concerns over BPA contained in products used by infants and children. Further, other studies have shown that BPA transforms Syrian hamster embryo cells (SHE) [46] and induces aneuploidy suggesting that BPA in genotoxic [47]. There is also some evidence of BPA carcinogenicity following adult exposure [48-50] although the focus of current concern is developmental exposure and long term consequences. Additional studies are needed to determine the potential adverse effects to humans exposed to BPA during critical stages of neonatal or early development.
Acknowledgments
The authors wish to thank Dr. Grace Kissling, NIEHS, for the statistical analysis of the data described in this report and Norris Flagler, NIEHS, for the photomicrographs. We would also like to thank Dr. Bill Bullock, Department of Pathology, Wake Forest University School of Medicine, Wake Forest University, Winston Salem, NC for consultation in pathology. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bern H. The fragile fetus. In: Colborn T, Clement C, editors. Chemically-Induced Alterations in Sexual and Functioal Development: The Wildlife/Human Connection. Princeton: Princeton Scientific Publishing Co; 1992. [Google Scholar]
- 2.Sallout B, Walker M. The fetal origin of adult disease. J Obstet Gynaecol. 2003;23:555–560. doi: 10.1080/0144361031000156483. [DOI] [PubMed] [Google Scholar]
- 3.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol. 2005;73:478–480. doi: 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- 4.Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23:290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 6.Newbold RR, DiAugustine RP, Risinger JI, Everitt JI, Walmer DK, Parrott EC, Dixon D. Advances in uterine leiomyoma research: conference overview, summary, and future research recommendations. Environ Health Perspect. 2000;108 5:769–773. doi: 10.1289/ehp.00108s5769. [DOI] [PubMed] [Google Scholar]
- 7.Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environmental Health Perspectives. 1993;101:372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newbold R. Lessons Learned from Perinatal Exposure to Diethylstilbestrol (DES) Toxicology and Applied Pharmacology. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Blatt J, Van Le L, Weiner T, Sailer S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J Pediatr Hematol Oncol. 2003;25:635–636. doi: 10.1097/00043426-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Brotons JA, Olea-Serrano MF, Villalobos M, Olea N. Xenoestrogens released from lacquer coating in food cans. Environmental Health Perspectives. 1994;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biles JE, McNeal TP, Begley TH, Hollifield HC. Determination of bisphenol-A in reusable polycarbonate food-contact plastics and migration to food simulating liquids. Journal Agric Food Chem. 1997;45:3541–3544. [Google Scholar]
- 12.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 14.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 15.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 16.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 17.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 19.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc National Acad Sciences USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newbold RR, Bullock BC, McLachlan JA. Progressive proliferative changes in the oviduct of mice following developmental exposure to diethylstilbestrol. Teratogenesis Carcinogenesis and Mutagenesis. 1985;5:473–480. doi: 10.1002/tcm.1770050610. [DOI] [PubMed] [Google Scholar]
- 21.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Research. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 22.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Research. 1990;50:7677–7681. [PubMed] [Google Scholar]
- 23.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16:107–116. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 27.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–4328. [PubMed] [Google Scholar]
- 28.Newbold RR, Jefferson WN, Padilla-Burgos E, Bullock BC. Uterine carcinoma in mice treated neonatally with tamoxifen. Carcinogenesis. 1997;18:2293–2298. doi: 10.1093/carcin/18.12.2293. [DOI] [PubMed] [Google Scholar]
- 29.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newbold RR, Bullock BC, McLachlan JA. Lesions of the rete testis in mice exposed prenatally to diethylstilbestrol. Cancer Research. 1985;45:5145–5150. [PubMed] [Google Scholar]
- 31.Haney AF, Newbold RR, Fetter BF, McLachlan JA. Paraovarian cysts associated with prenatal diethylstilbestrol exposure: comparison of the human with a mouse model. American Journal of Pathology. 1986;124:405–411. [PMC free article] [PubMed] [Google Scholar]
- 32.Newbold RR, Tyrey S, Haney AF, McLachlan JA. Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethylstilbestrol. Teratology. 1983;27:417–426. doi: 10.1002/tera.1420270316. [DOI] [PubMed] [Google Scholar]
- 33.Newbold RR, Bullock BC, McLachlan JA. Animal model of human disease: diverticulosis and salpingitis isthmica nodosa (SIN) of the fallopian tube: estrogen-induced diverticulosis and SIN of the mouse oviduct. American Journal of Pathology. 1984;117:333–335. [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 35.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 36.Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat Genet. 1998;20:228–230. doi: 10.1038/3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development. 1994;120:335–345. doi: 10.1242/dev.120.2.335. [DOI] [PubMed] [Google Scholar]
- 38.Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30:611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- 39.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 40.Hodges LC, Hunter DS, Bergerson JS, Fuchs-Young R, Walker CL. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:100–111. doi: 10.1111/j.1749-6632.2001.tb03991.x. [DOI] [PubMed] [Google Scholar]
- 41.Baird DD, Newbold RR. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol. 2005;20:81–84. doi: 10.1016/j.reprotox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Olden K, Newbold RR. Women's health and the environment in the 21st century. Environ Health Perspect. 2000;108 5:767–768. doi: 10.1289/ehp.00108s5767. [DOI] [PubMed] [Google Scholar]
- 43.Davis B, Dixon D, Herbert R. Ovary Oviduct Uterus and Vagina. Vienna, IL: Cache River Press; 1999. [Google Scholar]
- 44.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 45.Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20:2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsui T, Tamura Y, Suzuki A, Hirose Y, Kobayashi M, Nishimura H, Metzler M, Barrett JC. Mammalian cell transformation and aneuploidy induced by five bisphenols. Int J Cancer. 2000;86:151–154. doi: 10.1002/(sici)1097-0215(20000415)86:2<151::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, Yamaguchi F, Barrett JC. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer. 1998;75:290–294. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Huff J. Carcinogenicity of bisphenol-A in Fischer rats and B6C3F1 mice. Odontology. 2001;89:12–20. doi: 10.1007/s10266-001-8179-y. [DOI] [PubMed] [Google Scholar]
- 49.Huff J. Carcinogenicity bioassays of bisphenol A, 4-vinylcyclohexene diepoxide, and 4-vinycyclohexene. Toxicol Sci. 2001;64:282–283. doi: 10.1093/toxsci/64.2.282. author reply 284. [DOI] [PubMed] [Google Scholar]
- 50.NTP. Technical report TR-215. National Toxicology Program; Research Triangle Park, NC: 1982. Carcinogenesis bioassay of Bisphenol A [CAS No. 80-05-7] in F344 rats and B6C3F1 mice [feed study] [PubMed] [Google Scholar]


