Abstract
The Entamoeba histolytica cell surface Gal/GalNAc-inhibitable lectin is a heterodimer between a heavy (170 kDa) subunit linked via a disulfide bond to a light (31 to 35 kDa) subunit. Five light subunit genes with high homology have been identified (Ehlgl1 to -5). We have previously shown that silencing of the expression of Ehlgl1, in the G3 trophozoites which had already been silenced in the amoebapore gene (Ehap-a), also suppressed the transcription of Ehlgl2 and -3 (strain RBV). The total absence of the lgl1 to -3 subunits in the RBV trophozoites affected their ability to cap the surface Gal-lectin molecules to the uroid region. We have now found that in the RBV trophozoites, the lgl4 and -5 subunits (31 kDa) are overexpressed and appear to compensate for the missing lgl1 to -3 in the heterodimer complex. Transcriptional silencing of Ehlgl5 was achieved by transfection of G3 trophozoites with a plasmid containing the open reading frame of Ehlgl5 ligated to the 5′ promoter region of the Ehap-a gene. The transfected trophozoites (strain L5) were silenced in Ehlgl5 and the closely related Ehlgl4, while the expression of the larger lgl1 to -3 subunits was upregulated. L5 trophozoites retained their ability to cap the Gal-lectin molecules. Attempts to simultaneously silence all of the Ehlgl genes have failed so far, possibly due to their crucial importance to the Gal-lectin functions. Our ability to silence part of the genes belonging to the same family can serve as a tool to study the relationships and functions of the members of other gene families.
The adherence of Entamoeba histolytica trophozoites to mucosal cells and red blood cells (RBC) as well as to some bacteria and colonic mucus is mostly mediated by the cell surface Gal/GalNAc-inhibitable lectin (19). Inhibition of lectin activity with millimolar concentrations of Gal or GalNAc prevents most of the contact-dependent cytotoxicity for which the organism is named (22). The cell surface Gal/GalNAc-lectin molecule is composed of a 260-kDa heterodimer of a disulfide-linked heavy (hgl) (170 kDa) subunit and a light (lgl) (35/31 kDa) subunit which is noncovalently associated with an intermediate subunit (igl) of 150 kDa (10). The structure, role, and functions of the 170-kDa subunit were largely investigated. It contains a carboxyl-terminal cytoplasmic tail and a transmembrane domain adjacent to a cysteine-rich extracellular domain. The carbohydrate recognition domain (CRD) is located within this cysteine-rich region (20). Five distinct E. histolytica genes (Ehhgl1 to Ehhgl5) encoding the lectin's heavy subunit have been identified and sequenced (16, 25). The group of the light subunit of the Gal/GalNAc-lectin molecule (Ehlgl) includes five genes with considerable homology and highly conserved regions (17, 22, 24) (Ehlgl1 to Ehlg5 [Fig. 1]). The light subunits appear to contain a glycosylphosphatidylinositol anchor at their C-terminal end. No function was originally ascribed to the light subunit, but this changed when we first reported that the avirulent E. histolytica strain Rahman has low transcriptional levels of the Ehlgl gene (3). Inhibition of expression of the 35-kDa Ehlgl1 light subunit by antisense RNA in the virulent strain HM-1:IMSS did not significantly affect adhesion activity to mammalian or bacterial cells but strongly inhibited cytopathic activity and cytotoxic activity as well as the ability to induce liver lesions in hamsters (3).
FIG. 1.
Nucleotide sequence comparison of the Ehlgl family genes. The different genes that encode the lgl proteins and their accession numbers are as follows: Ehlgl1, XM_651053.1; Ehlgl2, L20898; Ehlgl3, XM_649244.1; Ehlgl4, XM_646286.1 (previously annotated as Ehlgl6); and Ehlgl5, XM_652368.1.
The light subunit was shown to have a role in enabling the clustering of Gal/GalNAc-lectin complexes, which is required for virulence. This was shown following the dominant-negative expression of an N-truncated lgl1 subunit in the virulent strain (13). Furthermore, the transcriptional silencing of the three (Ehlgl1 to -3) genes in the RBV trophozoites also demonstrated the inability to cluster the Gal-lectin complexes (7). In the present report, we demonstrate that, in the RBV silenced trophozoites the two other lgl genes (Ehlgl4 and -5) are expressed. Moreover, Ehlgl4 and -5 appear to be up-regulated and continue to form heterodimers with the hgl subunits. It thus became interesting to investigate what would be the consequence of silencing the expression of Ehlgl4 and -5 and if it would be possible to simultaneously silence the expression of all five Ehlgl genes.
MATERIALS AND METHODS
E. histolytica culture conditions.
Trophozoites of E. histolytica strain HM-1:IMSS and of the plasmidless gene-silenced clone G3 (5) were grown at 37°C in TYI-S-33 medium (11). Transfection of G3 trophozoites was performed as previously described (5), and transfectants were selected by growing them in the presence of the neomycin derivative G418.
Plasmid constructs.
The pEhActNeo shuttle vector, which served as the basic construct, contains the Neo gene, which confers resistance to G418, flanked by the 5′ and 3′ regulatory sequences of the amoeba actin 1 gene Ehactin and an E. histolytica autonomous replication sequence, both cloned in pBluescript II SK (−) (2). For the construction of plasmid pL5, the 473-bp fragment of the 5′ upstream region of Ehap-a was amplified by PCR using primer TCCCCGCGGCTTGCTGCACCCTTTG as the sense primer with a SacII restriction site and CATGCCATGGTCATGATTGTTTGTAAGATATG as the antisense primer with a NcoI restriction site. Since we have previously shown that there was no need to include all of the open reading frame (ORF) of a second gene in order to silence it (7), we amplified a fragment of 311 bp from the 5′ end of the ORF of the Ehlgl5 gene. The Ehlgl5 gene fragment was amplified with sense primer CATGCCATGGTTACGTTGTTTTTATTG starting from the first ATG and with a NcoI restriction site and primer TCGAGCTCCATATCTAGTAGTTCCTTTTAC as the antisense primer from +311 bp of the ORF and with a SacI restriction site. The two fragments were digested with NcoI and ligated, and the cassette was then inserted into the above-mentioned pEhActNeo shuttle vector.
A plasmid (pRB9 [see Fig. 9]) was prepared for the transfection and simultaneous silencing of two genes at once (Ehlgl1 and EhCP-5) in the G3 clone which already has the Ehap-a gene silenced. Two DNA fragments were prepared by PCR amplification. The first, for silencing the Ehlgl1 gene, was done using a sense primer from the 5′ sequence upstream of the Ehap-a gene (TCCCCGCGGCTTGCTGCACCCTTTG) with a SacII restriction site and a specific antisense primer from a region within the ORF of the Ehlgl1 gene (bp 421; GCGGATCCGAAGTTCATTTCCTTGTTTCAATG) with a BamHI restriction site using pTL plasmid DNA (7) as the template. The second fragment was prepared by using the same 5′ sequence upstream of the Ehap-a gene primer, as described above, and an antisense primer from a region within the ORF of the EhCP-5 gene (bp 396; AGCGGATCCTTTGATCCAGCAACCAAC) with a BamHI restriction site, using the pAP-CP5 plasmid (7) as the DNA template. The two fragments were BamHI digested and ligated to each other, tail to tail, and the resulting cassette was then cloned into the pEhActNeo shuttle vector.
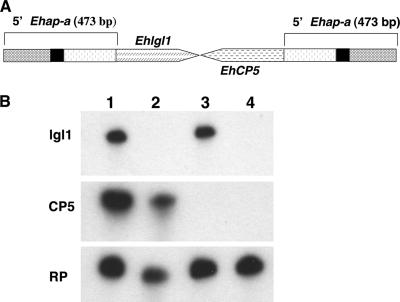
FIG. 9.
(A) Diagram of a plasmid construct pRB9 enabling the simultaneous silencing of two additional genes, Ehlgl1 and Ehcp5, in G3 trophozoites. (B) Northern blot analysis of amoebic RNA extracts. Lanes: 1, plasmid-less G3 clone; 2, RBV clone (7); 3, RB8 clone (7); 4, triple-gene-silenced RB9 clone. The probes used are as indicated. Note that the triple-silenced RB9 clone has no transcript for Ehlgl1 and EhCP5.
Northern blots and RT-PCR.
Total RNA was prepared using the RNA isolation kit TRI Reagent according to the manufacturer's protocol (Sigma, St. Louis, MO). RNA was size fractionated on a 4% polyacrylamide denaturing gel containing 8 M urea and subsequently blotted to a nylon membrane. Hybridization under stringent conditions was carried out with different probes randomly labeled using the Redi-Prime II kit (Amersham Life Science, Buckinghamshire, United Kingdom) and washed with a mixture of 0.1% sodium dodecyl sulfate (SDS) and 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). For reverse transcription-PCR (RT-PCR), RNA samples were treated with RNase-free DNase (Promega, Madison, WI) and after phenolation and sedimentation, 2 μg of RNA was reacted with avian myeloblastosis virus reverse transcriptase (Promega) according to the manufacturer's protocol using an oligo(dT) adaptor as a primer (Table 1). The cDNA product was diluted five times and used for the subsequent PCRs. In all of the RT-PCR experiments, a control in which the reverse transcriptase was omitted was performed. The results in all cases gave no PCR product, verifying that no DNA was present in the RT samples.
TABLE 1.
Sequences of the primers used in this study
| Primer | Sequence | Location | Orientation |
|---|---|---|---|
| 1 | GACTCGAGTCGACATCGATT (T16) | RT primer | |
| 2 | GACTCGAGTCGACATCGATT | Adaptor | |
| 3 | GATTACGTTGTTTTTATTGATAG | lgl5 | Sense |
| 4 | ATATTGTTCTTATTGATAAGCG | lgl4 | Sense |
| 5 | CTTTGATTATTTGTTCCTGG | lgl5 and lgl4 common primer | Antisense |
| 6 | GACCATTCAGAGGACATGG | rpL21 | |
| 7 (qPCR) | CGAAACACAAAAACAATTTACAAC | lgl5 | Sense |
| 8 (qPCR) | GTACTCTTCTTGGAATTGCAAATG | lgl5 | Antisense |
| 9 (qPCR) | GATCAGTTAGATTCCGTAATGGAGGG | lgl1 | Sense |
| 10 (qPCR) | GTTTGCTCCACCTCTAATATCATGAACTC | lgl1 | Antisense |
| 11 (qPCR) | CTTCCATTACCAGCTAAGAGGAC | rpL21 | Sense |
| 12 | GTGTGACATGCACTGAACAAAGGGC | igl1 | Sense |
| 13 | CATGCACTGACCAAAGTTCTG | igl2 | Sense |
| 14 | ATGGCTTACATTCACCATTTGAC | igl1 and igl2 common primer | Antisense |
| 15 | GTATGGACTACTTGTGAAACAACC | hgl | Sense |
| 16 | CATTATTCTTATGAACATCAATG | hgl | Antisense |
| 17 | GGTGATGATGCACCAAGAGC | Actin | Sense |
| 18 | GTCTGGTGTTAATACCTGG | Actin | Antisense |
qRT-PCR.
Quantitative real-time RT-PCR (qRT-PCR) was carried out in a 20-μl reaction mixture containing 1× Absolute SYBR green ROX master mix (ABgene, Epsom, United Kingdom), 200 nM primers (for each forward and reverse primer). The primers used are listed in Table 1. The PCR conditions were 95°C for 15 s and 60°C for 1 min for 40 cycles. The absence of primer-dimer formation was examined in the nontemplate controls. Two independent experiments were carried out. Each sample was examined in triplicate. Using relative quantification analysis, this method normalizes the expression of the specific gene versus the control reference. The threshold cycle value was defined as the PCR cycle number that crosses an arbitrarily placed threshold line. Quantitative analysis was performed using the GeneAmp7300 sequence detection system (PE Applied Biosystems [for further elaboration, see “Sequence detector user bulletin no. 2”]).
SDS-PAGE and Western blots.
For SDS-polyacrylamide gel electrophoresis (PAGE), soluble extracts from trophozoites prepared as previously described (5) were subjected to separation on a 12% polyacrylamide gel under reducing conditions. Gels were blotted on a nitrocellulose membrane and subjected to immunoreactions with the indicated antibodies. The blots were washed and incubated with horseradish peroxidase conjugated to donkey anti-rabbit immunoglobulin whole antibody (Amersham Pharmacia Biotech) and developed with an ECL enhanced chemiluminescence kit (Amersham, Little Chalfont, Buckinghamshire, United Kingdom).
Sucrose gradient flotation.
E. histolytica trophozoites were lysed in 1 ml of MES (morpholineethanesulfonic acid)-buffered saline (25 mM MES [pH 6.5], 150 mM NaCl, 1% cold Triton X-100, and the protease inhibitors leupeptin [0.2 mM], iodoacetamide [5 mM], 1,10-phenanthroline monohydrate [5 mM], phenylmethylsulfonyl fluoride [2 mM], sodium orthovanadate [1 mM], and sodium pyrophosphate [1 mM]), and incubated for 30 min on wet ice. Total cell lysates were adjusted to 40% sucrose by adding an equal volume of 80% sucrose and placed at the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was generated by overlaying this with 30% sucrose and 5% sucrose. Tubes were centrifuged at 4°C for 20 h at 39,000 rpm in an SW-41 rotor in a Beckman Ultracentrifuge. Following centrifugation, 12 fractions of 1 ml each were collected from the top of the gradient. Proteins were precipitated by the addition of trichloroacetic acid. Protein sediments were resuspended in sample buffer and subjected to SDS-PAGE and immunoblotted as described above.
Gal/GalNAc-lectin capping assay.
Capping or clustering of the Gal-lectin surface molecules was performed essentially as previously described (13). Freshly harvested trophozoites were washed in phosphate-buffered saline and divided into two tubes, each containing 2 × 106 cells per tube. To each tube, two monoclonal antibodies (3F4 and 7F4; a gift from Richard Vines, TechLab, Blacksburg, VA) against the heavy (170 kDa) subunit of the Gal-lectin (22) were added at 1:30 dilution. One of the tubes was kept at 4°C and the other at 37°C, to induce capping, for 20 min. Fixation of trophozoites was performed with the addition of paraformaldehyde to a final concentration of 3.7% for 15 min, followed by a wash with NH4Cl (50 mM) in order to block free aldehydes. Final blocking was done with 2% fetal calf serum. After washing, the fixed trophozoites were incubated with fluorescein isothiocyanate-labeled goat antimouse antibodies (Jackson Immuno Research, West Grove, PA) at a 1:200 dilution. Finally, samples were viewed with a confocal microscope (Fluoview FV500; Olympus, Tokyo, Japan).
Erythrophagocytosis.
Experiments were carried out in triplicate as previously described (18). Human erythrocytes (HRBC) and E. histolytica trophozoites were mixed in a ratio of 100:1 and incubated for 15 min at 37°C. The noningested erythrocytes were lysed with distilled water, the whole suspension was centrifuged, and the sedimented parasites were resuspended in formic acid. The hemoglobin that was released into the supernatant was determined in a spectrophotometer at 397 nm. The average number of HRBC per trophozoites was determined using a calibration curve.
RESULTS
Identification of the Ehlgl genes that are expressed in E. histolytica G3 and RBV trophozoites.
We have previously shown that silencing of the expression of the Ehlgl1 gene in the E. histolytica substrain G3, which already had the amoebapore genes Ehap-a, Ehap-b, and the saposin-like protein (Saplip1) silenced, caused a simultaneous down-regulation of the closely related Ehlgl2 and -3 (7). The plasmidless RBV trophozoites that were selected after the gene silencing were found to be devoid of the characteristic lgl protein bands which migrate at 35 kDa. RBV trophozoites continued, however, to express the lower-molecular-mass bands of 31 kDa which are seen in Western blots of the reduced PAGE that reacted with the polyclonal antibodies raised against lgl1 protein (7) (Fig. 2A).
FIG. 2.
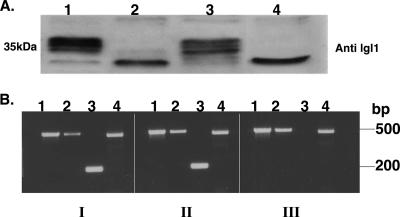
(A) Expression of the Ehlgl proteins in the various transfectants (7). The Western blot of reduced SDS-PAGE was reacted with polyclonal antibodies to lgl1. The different cell lysates are as follows: lane 1, G3; lane 2, pB33, the plasmid that induced the silencing of lgl1 (7); lane 3, pAY, a plasmid that did not silence lgl1; lane 4, RBV trophozoites, which were derived from the pB33-transfected trophozoites after the removal of the plasmid and in which lgl1 remained silenced (7). (B) RT-PCR of RNA samples derived from trophozoites of strain HM-1:IMSS (panel I), G3 (panel II), and RBV (panel III). Each sample underwent a PCR with primer sets for different regions (see Table 1) as follows: lane 1, Ehlgl5 (primers 3 and 5); lane 2, Ehlgl4 (primers 4 and 5); lane 3 Ehlgl1 (primers 9 and 10); and lane 4, ribosomal protein L21 (primers 6 and 2). Notice that Ehlgl1 does not appear in the RBV trophozoites, while Ehlgl5 and Ehlgl4 are transcribed.
In order to find out if the 31-kDa protein bands correspond to the other two light subunits of the Gal-lectin, lgl4 and -5, or are due to nonspecific signals, we searched for their transcripts in RNA extracts. RT followed by PCR of the RNA extracted from trophozoites of strains HM-1:IMSS, G3, and RBV revealed that both genes Ehlgl4 and -5 were transcribed in the RBV trophozoites that do not express Ehlgl1, -2, and -3 (Fig. 2B). The quantitative evaluation of the Ehlgl5 transcript level is shown in Fig. 5.
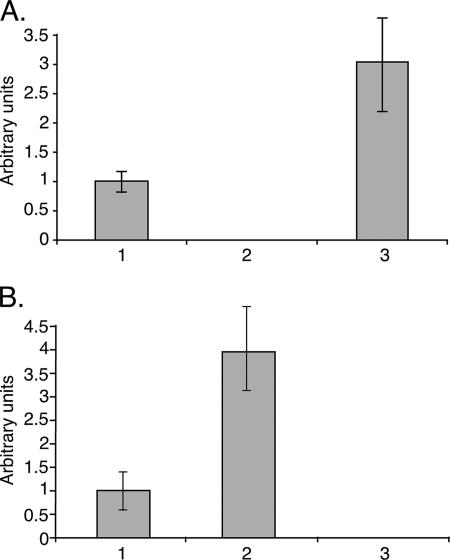
FIG. 5.
qRT-PCR to reverse-transcribed RNA from the G3 (bar 1), RBV (bar 2), and L5 (bar 3) trophozoites. The qPCR reactions were done with three sets of primers (see Table 1). The ribosomal protein L21 primers (EhRP-L21) (primers 11 and 2), which served as a control reference; the Ehlgl1-specific primers (primers 9 and 10); and Ehlgl5-specific primers (primers 7 and 8) were used. (A) Ratio between the transcripts of Ehlgl1 and those of EhRP-L21 in the above trophozoites. The value obtained for G3 was set to 1. (B) Transcript ratios between Ehlgl5 and EhRP-L21 (as described above). Two separate experiments, each done in triplicate, are summarized. The value of Ehlgl1 in the RBV trophozoites (A) and Ehlgl5 in the L5 trophozoites (B) was zero.
Distribution of Ehlgl4 and -5 in sucrose gradients of the parasite membranes.
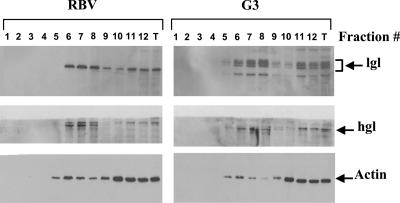
It was recently reported that the heavy, intermediate, and light subunits of the Gal/GalNAc-inhibitable lectin were present in raft-like fractions as well as in non-raft membrane fractions (14). A similar sucrose gradient separation was performed with the membranes of the two strains G3 and RBV. Western blots of the different fractions revealed that in membranes of RBV, only the lower (31 kDa) band was observed with antibodies against the lgl1 subunit in the different fractions, while in the parent G3 trophozoites, the typical profile of the various proteins (31 to 35 kDa) of the light subunit of the Gal-lectin appeared in the fractions (Fig. 3). The sucrose gradient separation profile of actin as well as that of the heavy subunit (hgl) of the Gal/GalNAc-inhibitable lectin appears to be the same in both preparations. These results further demonstrate that the lower band, which appears in RBV Western blots and reacted with antibodies against lgl1, represents the 31-kDa light subunits of lgl4 and -5 which prior to the reduction were covalently linked by a disulfide bond to the heavy hgl subunits.
FIG. 3.
Distribution of the Gal/GalNac-lectin subunits in sucrose gradients of trophozoite membranes. Total cell lysates prepared with cold Triton X-100 were subjected to sucrose gradient flotation (for details, see Materials and Methods). Twelve fractions were collected from the top of the gradient, and proteins were precipitated with trichloroacetic acid. Following SDS-PAGE and immunoblotting, the distribution of Gal-lectin heavy subunit hgl, Gal-lectin light subunit, lgl, and actin in each of the different fractions was analyzed by reacting Western blots with their respective antibodies. Fraction 1 is the top and fraction 12 the bottom of the gradient. T, total lysate.
Transcriptional silencing of Ehlgl4 and -5.
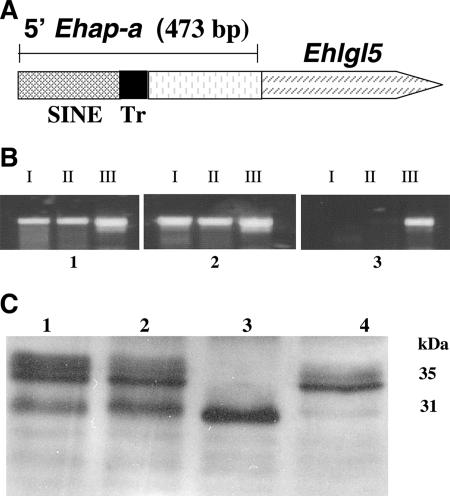
In order to silence the expression of Ehlgl5, a plasmid construct similar to the previous construct which silenced Ehlgl1 (7) was prepared (Fig. 4A). In brief, the 5′ upstream region of the Ehap-a gene (473 bp) was ligated to a truncated ORF (311 bp) of the Ehlgl5 gene, starting from the 5′ ATG, and cloned into the pEhActNeo vector as previously described (7). The plasmid pL5 was transfected into G3 trophozoites and following selection with G418, the growth of a culture (L5) was maintained. The transcription of the Ehlgl genes was examined in the transfectants and compared to those in the parent G3 as well as in RBV trophozoites. The results of RT-PCRs of L5 show that in addition to the silencing of the Ehlgl5 gene, also the Ehlgl4 gene showed no transcript (Fig. 4B). In contrast, genes Ehlgl1, -2, and -3 were transcribed as in strain G3. Western blots of the reduced SDS-PAGE that were reacted with antibodies raised against the lgl1 subunit clearly show that the band corresponding to the 31 kDa disappeared in the L5 trophozoites, which were silenced in lgl5 and -4 (Fig. 4C).
FIG. 4.
Transcriptional silencing of Ehlgl5 and Ehlgl4. (A) Diagram of plasmid pL5. The plasmid contains the 5′ Ehap-a promoter region (473 bp) ligated to a truncated ORF (311 bp) of the Ehlgl5 gene. (B) RT-PCR done on RNA extracted from the trophozoites G3 (panel 1), RBV (panel 2), and L5 (panel 3). Each panel contained three pairs of primers. The primers are listed in Table 1 and the legend to Fig. 2B. Lane I, Ehlgl5; lane II, Ehlgl4; and lane III, EhRP-L21, the ribosomal protein transcript. Note that in L5 the transcripts of both genes Ehlgl4 and Ehlgl5 are missing. (C) The Western blot of reduced SDS-PAGE was reacted with polyclonal antibodies to lgl1. The different cell lysates are as follows: lane 1, HM-1:IMSS; lane 2, G3; lane 3, RBV; and lane 4, L5. Notice that the 31-kDa bands are missing in L5.
Our results show that it is possible to differentiate between two groups of lgl lectin genes and that these two groups can be silenced separately. The first group includes genes Ehlgl1 to -3, which are cosuppressed when Ehlgl1 is silenced while the genes Ehlgl4 and -5 are expressed, whereas in the second group, silencing of Ehlgl5 also cosuppressed Ehlgl4 but not Ehlgl1 to -3.
Changes in the transcription levels of the expressed Ehlgl genes.
In order to obtain more accurate data for the levels of expression of Ehlgl5 in RBV trophozoites and the expression of Ehlgl1 in L5, a qRT-PCR was performed. Figure 5 shows the increase in the relative transcription level of Ehlgl5 in RBV trophozoites in the absence of transcription of Ehlgl1, and an inverted result was seen for the L5 trophozoites, namely a higher level of Ehlgl1 with no Ehlgl5 transcript. The overexpression of the lower 31-kDa protein bands, which corresponds to Ehlgl4 and -5, can also be clearly seen in the Western blots of the RBV trophozoites (see also Fig. 2A). These results indicate that overexpression happened among the members of one of the Ehlgl gene groups whenever the expression of the other group was suppressed, enabling the compensation for the missing lgl subunits which form the Gal/GalNAC-lectin heterodimer.
Silencing of the Ehlgl members does not influence the expression of the other gene members of the Gal/GalNAc-lectin complex.
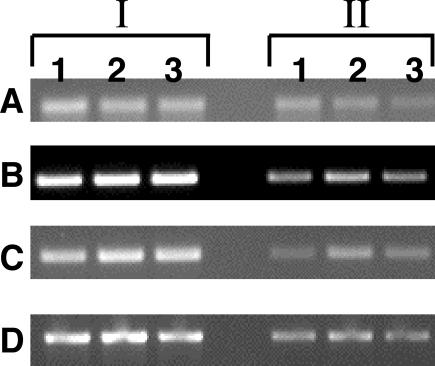
A semiquantitative RT-PCR (shown in two different concentrations in Fig. 6) indicated that silencing of either Ehlgl1 to -3 in RBV trophozoites or of Ehlgl4 and -5 in L5 trophozoites did not significantly alter the transcription of the heavy subunit Ehhgl or the Ehigl intermediate subunits, which are components of the Gal-lectin family (10).
FIG. 6.
Transcripts of the two other groups of the Gal/GalNAc-lectin subunits, Ehhgl and Ehigl, by semiquantitative RT-PCR in the different silent amoebae. The RNAs from G3 (lane 1), RBV (lane 2), and L5 (lane 3) trophozoites were extracted, reversed transcribed, and subjected to RT-PCR. Two concentrations (section I, 1; section II, 1/10) are shown with four sets of primers (see Table 1): A, Ehhgl (16.m00300) (primers 15 and 16); B, EhiglI (AF337950) (primers 12 and 14); C, EhiglII (AF337951) (primers 13 and 14); and D, Ehact (primers 17 and 18). The product of Ehact served as a loading control.
Comparison of phenotypes between G3, RBV, and L5 trophozoites.
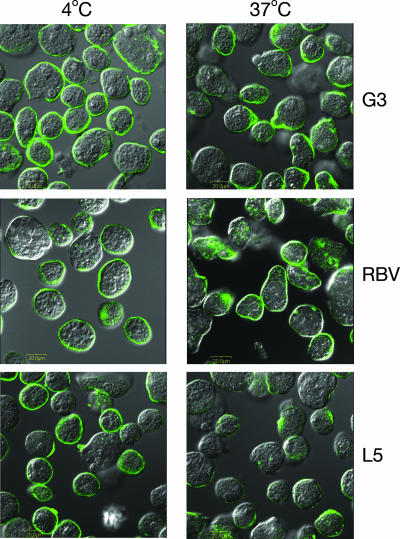
G3 trophozoites and all of the descendants of G3 trophozoites in which we have silenced additional genes are nonvirulent because of the lack of the toxic amoebapore proteins and cannot be tested for regular virulence properties (5) We have previously shown that RBV trophozoites that are devoid of lgl1, -2, and -3 were incapable of clustering or capping of the Gal/GalNAc-specific lectin molecules upon exposure to antibodies against the lectin (7). The inability of RBV trophozoites to induce the capping of the Gal-lectin occurred, although the light subunits lgl4 and -5 were expressed, but their presence did not enable the capping. As can be seen in Fig. 7, capping of the Gal-lectin complex in the L5 trophozoites which don't have the light subunits lgl4 and -5 (but do have lgl1, -2, and -3) was found to proceed at 37°C, as in the parent strain G3 but differed from RBV trophozoites. This indicates that the lgl4 and -5 subunits are not essential for the surface capping activity and the presence of lgl1 to -3 was sufficient for the capping formation.
FIG. 7.
Induction of capping of the Gal/GalNAc-lectin to the uroid region by the different trophozoites as shown by confocal microscopy. The left panels show trophozoites incubated at 4°C. In the right panels, trophozoites were incubated at 37°C for 20 min to observe the induction of capping using two monoclonal antibodies against the heavy subunit of the Gal-lectin (13). Each image shows the fluorescent anti-Gal-lectin antibodies superimposed on a Nomarski section. Samples as indicated on the figure are for trophozoites of strains G3, RBV, and L5.
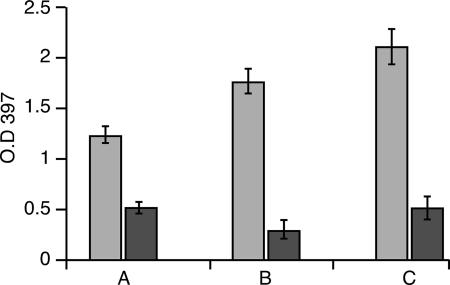
As previously shown, the erythrophagocytosis capability of the G3 amoeba was not impaired by the lack of amoebapore (9). This ability was also not affected in the different lgl-silenced amoeba, RBV or L5 (Fig. 8). A slight difference, however, was observed in the sensitivity of the erythrophagocytosis to inhibition by galactose (0.1 M). RBC ingestion by RBV trophozoites was most sensitive to galactose (89% inhibition); in L5, inhibition was 77%; while in the parent strain, G3, inhibition was only 63% (Fig. 8).
FIG. 8.
Erythrophagocytosis activity of the E. histolytica G3 (A), RBV (B), and L5 (C) trophozoites. The assay was performed in the absence (gray bars) or presence (black bars) of galactose (0.1 M). O.D. 397, optical density at 397 nm. The bars represent the mean and standard deviation of three independent experiments.
A somewhat similar result was seen when the adherence of the different trophozoites to fixed BHK cells was examined (not shown). This finding suggests that in the absence of lgl1 to -3, as in the RBV trophozoites, the CRD of the hgl (20) is more favorably exposed and the added galactose molecules are more effective at competing with the mammalian cell target for the carbohydrate binding domain.
Is it possible to silence all of the members of the light subunit of the Gal/GalNAc-lectin?
We have previously reported that we failed in our attempts to silence a third gene family following transfection of either RBV or RB8 trophozoites (7). We recently managed to simultaneously silence two additional genes in the G3 strain that is already silenced for the amoebapores (6). The two genes, Ehlgl1 and EhCP-5, were each ligated, as before, to the 473 bp of the upstream region of the Ehap-a gene. The two fragments were then ligated tail to tail to each other and cloned into the pEhActNeo vector described before (2). Transfection of the pRB9 plasmid (Fig. 9A) into G3 trophozoites caused the silencing of both the Ehlgl1and EhCP-5 genes in addition to the amoebapore genes that were already silenced (Fig. 9B). A plasmid built on the same principles described above, carrying the Ehlgl1 gene and the Ehlgl5 gene, was prepared and transfected into G3, but in the process of selection with G418, in all three cases that we tried, the transfected trophozoites died. It is possible that silencing of the entire family of Ehlgl genes is lethal for the trophozoites, but we cannot exclude, at this stage, experimental difficulties.
DISCUSSION
In recent years, a lot of unequivocal evidence has been published indicating that the Gal/GalNAc-inhibitable lectin plays an important role in the physiology of Entamoeba histolytica (3, 4, 13, 15, 20, 23). Among the three main protein subunits that compose the Gal/GalNAc-lectin complex, the heavy (hgl [170 kDa]) subunits were investigated the most, probably because they possess the CRD which mediates the trophozoite's ability to adhere to different targets and because antibodies to the hgl are usually found in human hosts infected with the parasites (1, 12). Much less is known about the function of the other two subunits that belong to the Gal/GalNAc-lectin family, the two genes of the intermediate subunits (Ehigl) (10), and the five genes found for the light subunits (Ehlgl) (3, 17, 24).
In the present investigation, we tried to learn more about the relationship between the different genes of the light subunit group and their function using our ability to silence the expression of only a part of these genes with a given plasmid construct. The members of the light subunit gene family bear high sequence homology in their nucleotide and protein sequences, and the polyclonal antibody raised against the lgl1 protein cross-reacts with all the light subunits (3, 13). The main sequence difference between the first group (lgl1 to -3) and the second group (lgl4 and -5) is a deletion of 17 amino acids, which also accounts for their smaller molecular mass. Moreover, from the distribution of the lgl and hgl proteins seen in the sucrose gradient fractions, we conclude that all of the lgl subunits are present on the amoeba surface as heterodimers. Differences were observed in the regulation of the transcription of the two groups of Ehlgl genes. As previously shown, silencing of the Ehlgl1 gene resulted in the down-regulation of the closely related Ehlgl2 and -3 genes (all of which are considered to belong to the 35-kDa proteins) in the RBV strain. In the present work, we show that Ehlgl4 and -5 were up-regulated when Ehlgl1, -2, and -3 were silenced in the RBV trophozoites. Moreover, when we prepared a plasmid construct containing the Ehlgl5 gene using the same principle as for the silencing of Ehlgl1 (7), the G3 transfected trophozoites that were obtained (strain L5) were found to be suppressed in the transcription of two genes—Ehlgl5, as expected, as well as Ehlgl4 (both produce 31-kDa proteins)—while the other genes, Ehlgl1 to -3 were transcribed. This indicates that it is possible to distinguish between two subgroups among the Ehlgl gene family members and that the expression of these two subgroups may be differently regulated.
It is interesting to mention in this context that when the down-regulation of the Ehlgl1 gene was performed by antisense transcripts (3), the major inhibitory effect was on all the 35-kDa proteins of the lgl family but no effect was seen on the 31-kDa proteins (see Fig. 3 in reference 3). Separate regulation of different members of gene families during a gene-silencing event was also observed in the case of the amoebapore genes, which were simultaneously silenced with the Ehap-a gene (Ehap-b and Saplip1). These genes have high sequence homology and are located on the same evolutionary branch (7, 8). Similarly, in the Ehlgl group there might be phylogenetic differences between the two groups of Ehlgl genes. Ehlgl4 and -5, which are the low-molecular-weight species, might have emerged later, whereas the other genes, Ehlgl1, -2, and -3, which have a distinct role in the clustering of the surface lectin molecules, probably appeared earlier.
The 260-kDa Gal/GalNAc-lectin complex that is present on the surface membrane of the trophozoite has been reported to consist of one molecule of the heavy (hgl) subunit (170 kDa) covalently ligated to one molecule of the light (lgl) subunit (35/31 kDa) (21, 22). The relative abundance of each of the light subunits in the lectin complex is, however, unknown. From the distribution of the lgl proteins in the RBV trophozoite membranes, we can conclude that lgl4 and -5 replace the missing lgl1, -2, and -3 subunits in the Gal/GalNAc-lectin heterodimer and their increased amount appears to compensate to some extent for the loss of lgl1 to -3. In the L5 trophozoites, the reverse occurred, namely lgl1, -2, and -3 compensate for the lack of lgl4 and -5. Our results also enable us to distinguish between the biological functions of the two subgroups of lgl subunits. lgl1, -2, and -3, as already shown (7), play a role in clustering of Gal/GalNAc-lectin molecules, a function that is lost when these lgls are down-regulated. In contrast, silencing of lgl4 and -5 did not have a significant effect on the usual clustering and capping of the lectin complex. We also noted that there was some slight difference between RBV and L5 trophozoites in their adherence to RBC or BHK cells. The adherence of RBV trophozoites which lack lgl1 to -3 as well as strain L5, which lacks lgl4 and -5 were more sensitive to inhibition by galactose molecules than the parent G3 strain. This suggests that the CRD of the hgl subunit maybe somewhat masked in the presence of lgl1 to -3 and somewhat less by lgl4 and -5, perhaps due to a slightly different conformation that the shorter lgl4 and -5 subunits confer to the 260-kDa lectin complex. It is of major interest to find out if the light subunit proteins of the Gal/GalNAc-inhibitable lectin are essential for parasite survival and adhesion functions. We attempted to silence all of the Ehlgl genes using a method that proved successful for the simultaneous silencing of Ehlgl1 to -3 and cysteine proteinase 5 (EhCP-5) in the same G3 trophozoites (6). Unfortunately, and despite our repeated efforts, the simultaneous silencing of all Ehlgl genes in the same G3 trophozoites was not successful in three separate transfections, and the transfectants died during the selection with G418. It is possible that the simultaneous silencing of expression of all the Ehlgl subunits could be lethal for the trophozoites, but more experiments are currently being conducted in order to exclude technical problems.
Acknowledgments
This investigation was supported by a grant from the Drake Family Foundation. The stipend of N.W. was funded by a donation from Mr. and Mrs. Henry H. Meyer, Jr.
We acknowledge the suggestion of Lesly A. Temesvari to investigate the Gal/GalNAc-lectin light subunits 4 and 5.
Footnotes
Published ahead of print on 31 August 2007.
REFERENCES
- 1.Abd-Alla, M. D., T. F. G. H. Jackson, G. C. Soong, M. Mazanec, and J. I. Ravdin. 2004. Identification of the Entamoeba histolytica galactose-inhibitable lectin epitopes recognized by human immunoglobulin A antibodies following cure of amebic liver abscess. Infect. Immun. 72:3974-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alon, R. N., R. Bracha, and D. Mirelman. 1997. Inhibition of expression of the lysine-rich 30 kDa surface antigen of Entamoeba dispar by the transcription of its antisense RNA. Mol. Biochem. Parasitol. 90:193-201. [DOI] [PubMed] [Google Scholar]
- 3.Ankri, S., F. Padilla-Vaca, T. Stolarsky, L. Koole, and D. Mirelman. 1999. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNAc lectin complex inhibits Entamoeba histolytica virulence. Mol. Microbiol. 33:2096-2102. [DOI] [PubMed] [Google Scholar]
- 4.Blazquez, S., M. C. Rigothier, M. Huerre, and N. Guillen. 2007. Initiation of inflammation and cell death during liver abscess formation by Entamoeba histolytica depends on activity of the galactose/N-acetyl-D-galactosamine lectin. Int. J. Parasitol. 37:425-433. [DOI] [PubMed] [Google Scholar]
- 5.Bracha, R., Y. Nuchamowitz, and D. Mirelman. 2003. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot. Cell 2:295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracha, R., Y. Nuchamowitz, M. Persky, N. Wender, and D. Mirelman. 2007. Epigenetic transcriptional silencing of multiple genes in Entamoeba histolytica, abstr. 024-6. Eur. J. Trop. Med. Int. Health 12(Suppl. 1):26. [Google Scholar]
- 7.Bracha, R., Y. Nuchamowitz, and D. Mirelman. 2006. Transcriptional silencing of multiple genes in trophozoites of Entamoeba histolytica. PLoS Pathogens 2:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruhn, H., and M. Leippe. 2001. Novel putative saposin-like proteins of Entamoeba histolytica different from amoebapores. Biochim. Biophys. Acta 1514:14-20. [DOI] [PubMed] [Google Scholar]
- 9.Bujanover, S., K. U. Bracha, R. Mirelman, and D. Links. 2003. A virulence attenuated amoebapore-less mutant of Entamoeba histolytica and its interaction with host cells. Int. J. Parasitol. 33:1655-1663. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, X.-J., M. A. Hughes, C. D. Huston, B. Loftus, C. A. Gilchrist, L. A. Lockhart, S. Ghosh, V. Miller-Sims, B. J. Mann, W. A. Petri, Jr., and H. Tachibana. 2001. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect. Immun. 69:5892-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 12.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 13.Katz, U., S. Ankri, T. Stolarsky, Y. Nuchamowitz, and D. Mirelman. 2002. Entamoeba histolytica expressing a dominant negative N-truncated light subunit of its gal-lectin are less virulent. Mol. Biol. Cell 13:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughlin, R. C., G. C. McGugan, R. R. Powell, B. H. Welter, and L. A. Temesvari. 2004. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect. Immun. 72:5349-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann, B. J., C. Y. Chung, J. M. Dodson, L. S. Ashley, L. L. Braga, and T. L. Snodgrass. 1993. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect. Immun. 61:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann, B. J., B. E. Torian, T. S. Vedvick, and W. A. J. Petri. 1991. Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:3248-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy, J. J., B. J. Mann, T. S. Vedvick, and J. W. A. Petri. 1993. Structural analysis of the light subunit of the Entamoeba histolytica galactose-specific adherence lectin. J. Biol. Chem. 268:24223-24231. [PubMed] [Google Scholar]
- 18.Mora-Galindo, J., F. Anaya-Velazquez, S. Ramirez-Romo, and A. Gonzalez-Robles. 2004. Entamoeba histolytica: correlation of assessment methods to measure erythrocyte digestion, and effect of cysteine proteinases inhibitors in HM-1:IMSS and HK-9:NIH strains. Exp. Parasitol. 108:89-100. [DOI] [PubMed] [Google Scholar]
- 19.Petri, W. A. 1996. Amebiasis and the Entamoeba histolytica Gal/GalNAc lectin: from lab bench to bedside. J. Investig. Med. 44:24-35. [PubMed] [Google Scholar]
- 20.Petri, W. A., Jr., B. J. Mann, and R. Haque. 2002. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 56:39-64. [DOI] [PubMed] [Google Scholar]
- 21.Petri, W. A., Jr., M. D. Chapman, T. Snodgrass, B. J. Mann, J. Broman, and J. I. Ravdin. 1989. Subunit structure of the galactose and GalNAc-inhibitable adherence lectin of Entamoeba histolytica. J. Biol. Chem. 264:3007-3012. [PubMed] [Google Scholar]
- 22.Petri, W. A., Jr., and R. L. Schnaar. 1995. Purification and characterization of galactose-and N-acetylgalactosamine-specific adhesin lectin of Entamoeba histolytica. Methods Enzymol. 253:98-104. [DOI] [PubMed] [Google Scholar]
- 23.Ravdin, J. I., and R. L. Guerrant. 1982. A review on the parasite cellular mechanism involved in the pathogenesis of amebiasis. Rev. Infect. Dis. 4:1185-1207. [DOI] [PubMed] [Google Scholar]
- 24.Tannich, E., F. Ebert, and R. D. Horstmann. 1992. Molecular cloning of cDNA and genomic sequences coding for the 35-kilodalton subunit of the galactose-inhibitable lectin of pathogenic Entamoeba histolytica. Mol. Biochem. Parasitol. 55:225-227. [DOI] [PubMed] [Google Scholar]
- 25.Tannich, E., F. Ebert, and R. D. Horstmann. 1991. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 88:1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]