Abstract
In Arabidopsis thaliana, tryptophan pathway genes are induced in response to starvation, wounding, and pathogen attack, resulting in increased production of tryptophan and secondary metabolites important for development and defense. The Arabidopsis tryptophan pathway therefore provides an ideal system for elucidating how environmental stimuli are transduced into changes in plant gene expression. To characterize the factors that regulate the first gene in the pathway, ASA1, which is the key point of control, we have isolated altered tryptophan regulation (atr) mutants with deregulated expression of ASA1. One of these mutants, atr1D is dominant for increased transcription of ASA1 in specific seedling tissues. We have used atr1D to clone the ATR1 gene based on its map position. ATR1 encodes a Myb-like transcription factor that modulates ASA1 expression. The ATR1 transcript also includes a 5′ regulatory region with three short ORFs, one of which is prematurely terminated by the atr1D mutation. Thus, ATR1 defines the first characterized tryptophan gene regulator in plants, and the atr1D mutation defines a sequence important for ATR1 expression.
Keywords: Myb transcription factor, translational control
In plants the tryptophan pathway not only provides the amino acid tryptophan for protein synthesis, but it also gives rise to a wide array of secondary metabolites including the growth regulator indole-3-acetic acid and antipathogenic compounds (1). Therefore, control of tryptophan gene expression in plants is likely to be more complex than in microorganisms, where these secondary metabolites are not produced.
The first committed step of the tryptophan pathway in both microorganisms and plants is catalyzed by the enzyme anthranilate synthase (AS), which is composed of two catalytic α subunits and two glutamine amidotransferase β subunits (2–5). Because AS activity determines the flow of metabolites through the rest of the pathway it is the key point of regulatory control. For example, AS is regulated by tryptophan feedback inhibition in which free tryptophan, the end product of the primary pathway, binds to an allosteric site on the catalytic α subunits of AS and renders the enzyme inactive (4, 5). However, AS is also regulated at the transcriptional level. In the higher plant Arabidopsis there are two AS catalytic α subunit genes that display very different expression patterns (2). The major gene, ASA1, has 10-fold higher basal transcript levels than does the minor gene, ASA2. ASA1 transcription can be induced further by either wounding or pathogen attack whereas ASA2 expression is not responsive to these stimuli.
To understand tryptophan gene control in Arabidopsis we performed genetic screens to isolate altered tryptophan regulation (atr) mutants with deregulated transcription of the ASA1 gene. The factors identified by these deregulating mutations will define the signaling pathways that convert environmental stimuli such as pathogen attack into ASA1 transcriptional induction. In particular, we used two successive screening strategies to isolate mutants that are resistant to tryptophan feedback inhibition of AS α subunit activity with the expectation that some of these mutants will have increased expression of ASA1 so that the additional AS α subunits will dilute out the effects of feedback inhibitors.
We describe here the isolation, characterization, and positional cloning of one of our deregulating mutants, atr1D. The ATR1 locus encodes a Myb transcription factor with strongest homology to Myb factors from maize that regulate phenylalanine-derived pigment biosynthetic genes. In the atr1D mutant, increased expression of ATR1 leads to up-regulation of the ASA1 target gene. The atr1D mutation disrupts a short ORF upstream of the ATR1 ORF in the ATR1 transcript, suggesting that this short ORF plays a key role in controlling ATR1 levels.
MATERIALS AND METHODS
atr Mutant Isolation.
Columbia ecotype (Col) trp1–100 gl1–1 M2 seeds (90,000) generated as described (3) by ethyl methanesulfonate mutagenesis were screened for fluorescence in the presence of high tryptophan. Aliquots of 500 surface-sterilized seeds were plated on 150- × 25-mm Petri plates containing 100 ml of minimal plant nutrient plus 0.5% sucrose (PNS) medium (6) with 100 μM tryptophan. Plates were sealed with Parafilm and were incubated at 22°C for 7 days under continuous illumination (30–50 μmol m−2⋅s−1) with yellow long-pass filters (7). Seedlings then were screened for fluorescence by visual inspection under a hand-held short-wavelength UV light source in a darkroom. Mutant lines were tested for resistance to 5-methyl-tryptophan (5MT) by plating surface-sterilized seeds on PNS medium with 15 μM 5MT (solubilized in 100 mM NaOH). Plates were incubated as for the high-tryptophan screen, and seedlings were monitored between 7 and 10 days for root growth relative to a wild-type control on the same plate. In addition, 50,000 M2 wild-type Col seeds were screened directly for 5MT resistance. Several other atr mutants that are unlinked to ATR1, ASA1, or ASA2 were isolated from both screens and will be described elsewhere.
Positional Cloning of the atr1D Locus.
Mapping of the atr1D mutation using cleaved amplified polymorphic sequence analysis showed that atr1D was tightly linked to the LFY marker (8). Yeast artificial chromosome (YAC) end clones were plasmid-rescued from LFY-hybridizing YACs in the yUP (9) and CIC (10) Arabidopsis YAC libraries as described (11). Positions of end clones relative to other YACs in the LFY region were determined by cross-hybridization on Southern blots of total yeast genomic DNA prepared from YAC-containing strains. Positions of end clones relative to the atr1D genetic map were determined by identifying Col versus Landsberg erecta ecotype (Ler) restriction fragment length polymorphisms for each clone and scoring them on DNA prepared from Col atr1D × Ler yi cer3 recombinant progeny. Information about YAC end clone polymorphisms is available on request from J.B.
A library was constructed from atr1D mutant genomic DNA by ligation of Sau3AI-partially digested 15- to 20-kb fragments into BamHI-cut λDASH (Stratagene) arms and packaged with Gigapack II Gold (Stratagene) in vitro packaging extracts. A wild-type genomic ATR1 clone was obtained by hybridization screening of a wild-type Col genomic lambda library provided by J. Mulligan and R. Davis (Stanford University, Stanford, CA). Because the atr1D mutation creates an MseI restriction site (Fig. 4B), we were able to verify its presence in mutant versus wild-type genomic DNA by PCR amplification of the mutant region followed by cleavage with MseI. cDNAs in the ATR1 region of the genome were isolated by probing a Ler seedling cDNA library (12) with genomic DNA fragments. The AGL8 and RLK1 genes adjacent to ATR1 were cloned and sequenced previously (13, 14).
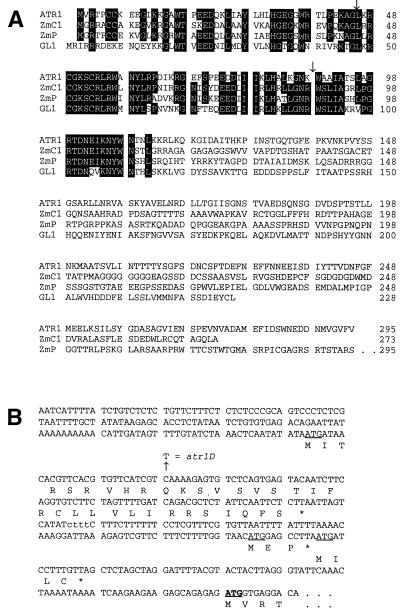
Figure 4.
ATR1 is a Myb-like transcription factor with three ORFs in its 5′ leader region. (A) Alignments of the predicted ATR1 amino acid sequence with Myb transcription factor homologues from Arabidopsis and maize. Sequences of ATR1, C1 (20), P (21), and GL1 (22) were aligned manually. Residues shared by at least three sequences are boxed. Positions of the two ATR1 introns are indicated by arrows. (B) The 5′ regulatory leader region of the ATR1 transcript. The nucleic acid sequence upstream of the ATR1 start codon in the longest ATR1 cDNA isolate is shown. All of 14 other cDNAs analyzed that include the entire ATR1 ORF ended within this sequence at least 15 bp 5′ of the first upstream ATG. Three out-of-frame upstream start codons are underlined, and the conserved Myb start codon is underlined and in bold. Predicted protein sequences encoded by each ORF are shown below the nucleic acid sequence. A 4-bp polymorphism present in the Col 5′ leader but absent in the Ler 5′ leader is shown in lowercase. A second 9-bp-length polymorphism internal to the coding sequence deletes from Ler the residues encoded at positions 134–136 (QTG) in Col.
Plant Transformation.
BamHI to SalI genomic DNA fragments (5.5 kb) of either wild-type ATR1 or the atr1D mutant were cloned into the plant transformation vector pBIN19 (15). Clones were transformed into wild-type Col plants by an Agrobacterium tumefaciens-mediated vacuum infiltration method (16). Independent transgenic lines that segregated 3:1 for the transgene kanamycin-resistance marker in the heterozygous T1 (first) generation were carried through T2 and T3 generations to isolate progeny that are homozygous for the transgene. T3 seeds of homozygous lines were tested for 5MT resistance as described above.
Northern Blot Analysis.
Total RNA was prepared from 2-week-old seedlings grown aseptically on PNS medium. Duplicate Northern blots of these RNA samples were probed with gel-purified DNA fragments of ASA1, ATR1, or RLK1. Blots were then stripped and reprobed with an α tubulin DNA probe to normalize for differences in loading. Band intensities were quantitated on a Fuji PhosphorImager by using macbas 2.2 software. All RNA preparations and Northern blots were performed in three independent experiments.
Histochemical Staining for β-Glucuronidase (GUS) Activity.
Seedlings were grown for 2 weeks on unsupplemented PNS medium followed by staining for GUS activity with 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc) for 24 hr at room temperature in X-Gluc buffer (17) containing 0.01% Triton X-100. Chlorophyll was removed from stained seedlings with ethanol. Seedlings were rehydrated in 50% glycerol before being mounted on microscope slides for photography. Staining experiments were performed on 20 seedlings of each strain grown on the same Petri plate in three independent trials and consistently gave the results shown.
RESULTS
Isolation of the atr1D Mutant.
The first part of our tryptophan deregulated mutant screening strategy took advantage of trp1–100, a leaky mutant with a defect in the structural gene encoding the second step of the tryptophan pathway such that the mutant accumulates blue fluorescent anthranilate compounds and displays a blue fluorescent leaf phenotype under UV light (18). When the trp1–100 mutant is grown on agar medium containing 100 μM tryptophan (high trp medium), its fluorescence is completely suppressed, presumably through feedback inhibition of AS activity and reduced production of anthranilate (Fig. 1A). We screened for plants in the trp1–100 strain background that are still fluorescent even on high trp medium to identify mutants resistant to this tryptophan repression.
Figure 1.
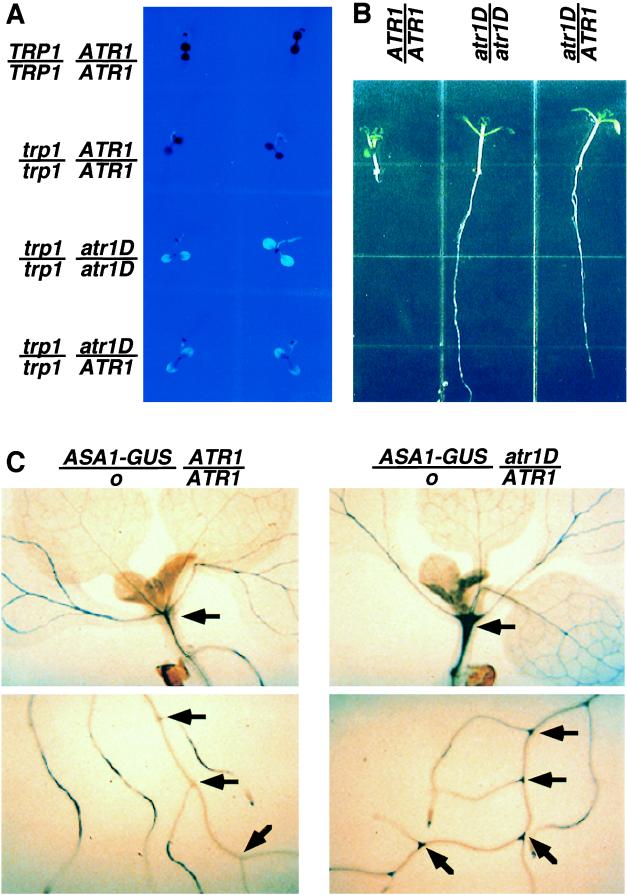
atr1D regulatory phenotypes. (A) The trp1–100 atr1D double mutant is fluorescent on high-tryptophan medium. One-week-old seedlings of wild-type Col (TRP1/TRP1 ATR1/ATR1), Col trp1–100 gl1–1 (trp1/trp1 ATR1/ATR1), Col trp1–100 gl1–1 atr1D homozygotes (trp1/trp1 atr1D/atr1D), and Col trp1–100 gl1–1 atr1D/ATR1 F1 heterozygotes made by crossing Col trp1–100 gl1–1 atr1D with Col trp1–100 gl1–1 ATR1D (trp1/trp1 atr1D/ATR1) are shown under UV light grown on PNS medium containing 100 μM tryptophan. (B) The atr1D mutation confers dominant 5MT resistance. Seedlings of wild-type Col (ATR1/ATR1), a Col atr1D homozygote (atr1D/atr1D) made by crossing the Col trp1–100 atr1D isolate to Col and identifying homozygous 5MT-resistant plants, and a Col atr1D/ATR1 F1 heterozygote made by crossing Col atr1D with wild-type Col are shown after being grown on PNS medium containing 15 μM 5MT under yellow long-pass-filtered light for 10 days. (C) The atr1D mutation activates ASA1 expression in hypocotyls and lateral root junctions. Representative 2-week-old F1 seedlings of Arabidopsis made by crossing a transgenic No-O strain carrying the ASA1-GUS reporter fusion with either wild-type Col (ASA1-GUS/o ATR1/ATR1) or Col atr1D (ASA1-GUS/o atr1D/ATR1) are shown after growth on unsupplemented PNS medium followed by staining for GUS activity. Arrows indicate tissues with increased GUS expression in the atr1D mutant relative to the ATR1 control.
Plants identified by the fluorescence screen subsequently were tested in a second screen for resistance to 5MT, a toxic compound that presumably acts by feedback inhibiting the AS α subunit without substituting for the nutritional role of true tryptophan. Wild-type or trp1–100 seedlings grown on agar medium containing 15 μM 5MT fail to develop primary roots, whereas resistant mutants display strong primary root growth (Fig. 1B).
We recovered one allele of atr1D from a fluorescence screen of 90,000 EMS-mutagenized Col trp1–100 M2 seeds (3). This mutant was selected for in-depth analysis because it displays a strong, dominant phenotype for resistance both to high tryptophan and 5MT (Fig. 1). In addition, atr1D is tightly linked to the LFY marker on the lower arm of chromosome 5 (8), a position unlinked to the AS α subunit structural genes ASA1 or ASA2 (2).
atr1D Activates ASA1 Expression.
To assess the effects of atr1D on ASA1 transcription, we crossed atr1D into a transgenic Arabidopsis strain carrying a reporter fusion construct of the ASA1 promoter to the bacterial β-glucuronidase gene uidA (ASA1-GUS) (19). This fusion allows expression controlled by the ASA1 promoter to be monitored by histochemical staining. We found that the atr1D mutation strongly increases the levels of ASA1-GUS expression in particular seedling tissues: the hypocotyl and the junctions between lateral roots and the primary root (Fig. 1C). However, in other tissues such as the leaf and root vasculature, atr1D has no significant effect on ASA1-GUS expression.
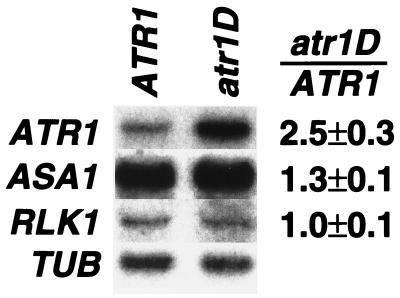
We further assessed the effects of atr1D on ASA1 expression by Northern blot analysis of steady-state transcript levels by using total RNA isolated from 2-week-old seedlings. By this assay we found that in atr1D mutant seedlings, expression of ASA1 is elevated approximately 1.3-fold relative to wild-type ATR1 plants (Fig. 3). Anthranilate synthase enzyme activity is also elevated approximately 1.5-fold in the atr1D mutant (data not shown).
Figure 3.
ATR1 transcript levels are elevated in the atr1D mutant. Total RNA was prepared from 2-week-old seedlings of wild-type Col (ATR1) or Col atr1D (atr1D) grown on unsupplemented PNS medium. Duplicate Northern blots of these samples were probed with an ATR1 cDNA probe (ATR1, 1-week exposure), an ASA1 cDNA probe (ASA1, 1-week exposure), or an RLK1 probe (RLK1, 1-week exposure). The blots were then stripped and reprobed with an α tubulin probe (TUB, 24-hr exposure) as a normalization control. The ATR1 blot reprobed with TUB is shown here. The quantitated ratio of transcripts detected in atr1D relative to ATR1 after normalization to TUB is given as the average of three independent experiments.
Molecular Isolation of the ATR1 Gene.
To characterize the atr1D mutation we cloned the ATR1 gene based on its map position. The Col atr1D mutant was crossed to a polymorphic double-visible marker mutant strain Ler cer3 yi. Segregation patterns of the cer3, atr1D 5MT-resistance, and yi phenotypes in the F2 progeny of this cross were used to determine that atr1D lies approximately midway between cer3 and yi. The visible markers then were used to identify 350 F2 progeny that crossed over between the markers and thus have recombination break points near atr1D. These recombinants define the minimum genetic distance of the atr1D region (Fig. 2). To obtain cloned genomic DNA covering this minimum genetic distance, a contig of YAC clones extending from the nearby LFY marker across the ATR1 region was built. The atr1D region was narrowed down to 25 kb by using new polymorphic markers internal to the contig. Southern blot analysis of this 25-kb region showed no evidence of structural differences between wild-type Col and atr1D.
Figure 2.
Cloning of the atr1D mutation. (A) Genetic analysis of F2 progeny from a three-factor cross between Col CER3 atr1D YI and Ler cer3 ATR1 yi localizes ATR1 between the visible markers. (B) A YAC clone contig spanning from the LFY gene northward over the ATR1 locus was constructed by hybridization and mapping analysis of end clones from LFY-containing YACs. Vertical lines represent end clones used for hybridization or detection of polymorphisms between Col and Ler. (C) λ clones spanning the ATR1 region between the left end of YAC CIC10A10 and the left end of YAC yUP17C2 were isolated from an atr1D genomic library. Genes within the ATR1 region were identified by sequence analysis and comparison with the GenBank database. Arrows indicate the direction of transcription. B, BamHI; S, SalI.
We used two methods to identify the ATR1 gene in the internal 25-kb region. First, we isolated expressed genes in this region by probing an Arabidopsis seedling cDNA library (12) with genomic subclones. These cDNAs were used to probe a Northern blot of total plant RNA prepared from seedlings of Col versus atr1D plants to determine whether any of the genes in the region had altered expression in the mutant. This analysis revealed that one cDNA in this region (ATR1) has approximately 2.5-fold elevated transcript levels in the atr1D mutant (Fig. 3). In contrast, the neighboring gene RLK1 had no difference in expression between ATR1 and atr1D mutant plants. Second, a 5.5-kb BamHI to SalI genomic clone of the region encoding ATR1 from the dominant atr1D mutant was transformed into wild-type Col plants. The resulting transgenic plants (ATR1/ATR1 atr1D/atr1D) are 5MT-resistant in three independent lines (data not shown). In addition, the analogous clone from wild-type Col transformed into wild-type Col plants (ATR1/ATR1 ATR1/ATR1) yielded 5MT-resistant progeny in three independent lines. In contrast, control Col lines transformed with the empty transformation vector or an ATR1-GUS promoter fusion are completely 5MT-sensitive. Thus, this analysis demonstrates that the ATR1 gene confers 5MT resistance if its levels are elevated either by increased dosage of the wild-type gene or by increased dosage plus deregulation of expression from the atr1D mutant gene.
ATR1 Encodes a Myb Transcription Factor.
Sequencing of genomic DNA and cDNAs reveals that the predicted protein encoded by ATR1 is homologous to Myb transcription factors in its amino-terminal DNA-binding region (Fig. 4). ATR1 is particularly homologous to Myb factors isolated from plants, including the C1 and P anthocyanin and phlobaphene pigment gene regulators in maize and the GL1 regulator of trichome (leaf hair) development in Arabidopsis (20–22). ATR1 and these other plant myb genes also have conserved intron junctions. Furthermore, the sequence of ATR1 cDNAs reveals that the ATR1 transcript includes an approximately 400-nt 5′ leader region with three short, out-of-frame ORFs upstream of the conserved Myb start position (Fig. 4). To identify the atr1D mutation we sequenced the entire 5.5-kb 5MT resistance-conferring fragment cloned from both wild-type and atr1D mutant plants. We found only a single-base difference between the two strains: a C-to-T transition mutation in the 5′ leader region of the atr1D transcript that creates a premature stop codon in the first upstream ORF (Fig. 4).
DISCUSSION
We have identified a Myb transcription factor, ATR1, that regulates tryptophan gene expression in Arabidopsis. The ATR1 locus was isolated by screening for tryptophan feedback inhibition-resistant mutants. Although tryptophan feedback inhibition resistance can be conferred by several different mechanisms—for example, by mutations in a structural gene for the anthranilate synthase catalytic subunit (4, 5)—we were particularly interested in recovering mutations that increase expression of the anthranilate synthase genes and thus titrate out the effects of feedback inhibitors. The atr1D feedback-resistant mutant was targeted for molecular analysis because it fulfills two genetic criteria of an up-regulated mutant: it is dominant, and it is unlinked to the positions of the AS catalytic subunit genes. Our finding that atr1D is an overexpression allele of the ATR1 Myb transcription factor suggests that ATR1 acts as a positive regulator of anthranilate synthase gene expression.
We found that the up-regulated atr1D allele increases expression of ASA1 in a tissue-specific manner. Experiments with an ASA1-GUS reporter fusion show that atr1D increases expression from the ASA1 promoter specifically in the hypocotyl and lateral root junctions, but not in other tissues such as leaves and the bulk of root tissue (Fig. 1C). We also directly tested the effects of the atr1D mutation on steady-state transcript levels of ASA1 by Northern blot analysis of total seedling RNA (Fig. 3). This analysis showed a small but reproducible up-regulation of ASA1. The modest up-regulation detected by whole-plant Northern blot analysis likely is to be a result of a dilution of hypocotyl-specific activation by the large excess of RNA extracted from other nonactivated tissues. Presumably other regulatory factors control ASA1 expression in other tissues of the Arabidopsis plant.
The nature of the atr1D deregulating mutation in a short ORF upstream of the main ATR1 ORF in the transcript suggests a molecular mechanism for how this mutation can elevate ATR1 expression. Given its position internal to the transcript (Fig. 4), the atr1D base change is likely to increase the steady-state levels of ATR1 transcripts by increasing message stability. The mutation could act directly by altering the secondary structure of the transcript such that it is less susceptible to degradation from the 5′ end. However, this model is unlikely because the predicted structure of the first 300 nt in the transcript is not significantly altered by the atr1D mutation (data not shown). Alternatively, the atr1D mutation could act indirectly by increasing translation of the ATR1 ORF. Based on studies from other plant systems, increased translation has the potential to protect a transcript from degradation (23, 24). Thus, the atr1D mutation might activate ATR1 expression at both the protein and the mRNA levels.
The finding that the ATR1 message has a long 5′ leader sequence with three short ORFs upstream of the Myb start position suggests that this gene is under translational control. The structure of the ATR1 5′ leader is similar to that of the Saccharomyces cerevisiae transcription factor Gcn4, where the efficiency of translation of four upstream ORFs regulates reinitiation at the downstream Gcn4 ORF (25, 26). By analogy to Gcn4 translational control, the atr1D nonsense mutation at the 10th position of the 33-aa first upstream ORF might act to prematurely free the ribosomes from this ORF and increase the efficiency of translation reinitiation at the internal ATR1 ORF. This model also has been proposed to explain translational control of the maize pigment gene transcription factor Lc, where mutations that destroy or truncate an upstream ORF can deregulate expression of the internally coded protein (27). Alternatively, the atr1D nonsense mutation might act by truncating a cis-acting peptide product of the first upstream ORF that serves as a repressor of translation from the internal ATR1 ORF. This model has been proposed in the case of translational regulation of the S. cerevisiae arginine biosynthesis gene CPA1 (28). A more detailed mutagenic analysis of the ATR1 5′ leader region will clarify which model for ATR1 translational regulation might apply.
By analogy with the translational induction of Gcn4 upon amino acid starvation (26) or the translational induction of Cpa1 upon arginine starvation (28), the 5′ leader region of the ATR1 transcript is likely to serve as an inducible switch for ATR1 protein expression. Depending on the mechanism of ATR1 translational control, this switch can be activated either by general changes in the efficiency of translation of the upstream ORFs relative to the ATR1 ORF, or by a specific inducer molecule that inactivates the ORF1 repressor peptide under appropriate conditions. Because the ATR1 target gene ASA1 is induced by wounding, pathogen attack (2), and tryptophan starvation (29), at least some of these stimuli might act by increasing ATR1 translation. Other plant transcription factors that carry upstream regulatory ORFs such as the maize pigment gene activator Lc (27) or the maize zein gene activator Opaque-2 (30) have not yet been characterized to determine whether the negative regulation from their upstream ORFs can be reversed by appropriate environmental signals.
It is interesting to note that the increase in steady-state transcript levels for ATR1 is approximately 2-fold greater than the activation of the target gene ASA1 (Fig. 3). In addition, ATR1 expression is likely to be enhanced at the translational level. The discrepancy between activator levels and degree of target gene activation could be because of aggregation, mislocalization, or degradation of excess protein when ATR1 is overproduced. Alternatively, ATR1 might activate ASA1 indirectly through intermediate factors, and these intermediate factors might dilute the degree of activation achieved. A third possibility is that ATR1 might work together with a second factor to directly activate ASA1, and the levels of this second factor could limit the degree of activation that can be achieved through increased ATR1 alone. Consistent with this third possibility, the maize Myb factors C1 and Pl require a second factor, a member of the B or R class of helix–loop–helix factors, for target gene activation (31). Future analysis of other Arabidopsis atr mutants might reveal an analogous cofactor for ATR1.
Our finding that deregulation of ATR1 expression can lead to deregulation of ASA1 transcription suggests that ATR1 or its orthologues from other plant species can be engineered to generate high-tryptophan crop plants. In particular, a combination of the overexpressed transcription factor ATR1 with a feedback-resistant mutant of ASA1 (4, 5, 19) might provide a strong enough deregulation of AS activity to increase significantly the levels of tryptophan and other pathway metabolites. In addition, the cloned ATR1 gene can be used to isolate interacting coregulators of tryptophan gene expression. Thus, the ATR1 gene provides the first molecular tool for analyzing and manipulating tryptophan pathway control in plants.
Acknowledgments
We thank K. Niyogi (University of California at Berkeley) and B. Keith (University of Chicago) for trp1–100 M2 seeds, P. Dunn and J. Ecker (University of Pennsylvania) for help building a YAC contig in the ATR1 region, and members of the Fink lab for help and advice. We also thank the Arabidopsis Biological Resource Center for plant strains and YAC clones. This work was supported by a Jane Coffin Childs postdoctoral fellowship to J.B. and a research grant from National Science Foundation (MCB9317175) to G.R.F.
ABBREVIATIONS
- 5MT
5-methyl-tryptophan
- Col
Columbia ecotype
- Ler
Landsberg erecta ecotype
- AS
anthranilate synthase
- PNS
plant nutrient plus 0.5% sucrose medium
- YAC
yeast artificial chromosome
- GUS
β-glucuronidase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U66462).
References
- 1.Radwanski E R, Last R L. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niyogi K K, Fink G R. Plant Cell. 1992;4:721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niyogi K K, Last R L, Fink G R, Keith B. Plant Cell. 1993;5:1011–1027. doi: 10.1105/tpc.5.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Last R L. Plant Physiol. 1996;110:51–59. doi: 10.1104/pp.110.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreps J A, Ponappa T, Dong W, Town C D. Plant Physiol. 1996;110:1159–1165. doi: 10.1104/pp.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haughn G W, Somerville C. Mol Gen Genet. 1986;204:430–434. [Google Scholar]
- 7.Stasinopoulos T C, Hangarter R P. Plant Physiol. 1990;93:1365–1369. doi: 10.1104/pp.93.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konieczny A, Ausubel F M. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- 9.Matallana E, Bell C J, Dunn P J, Lu M, Ecker J R. In: Methods in Arabidopsis Research. Koncz C, Chua N-H, Schell J, editors. Teaneck, NJ: World Scientific; 1992. pp. 144–169. [Google Scholar]
- 10.Creusot F, Fouilloux E, Dron M, Lafleuriel J, Picard G, Billault A, Le Paslier D, Cohen D, Chaboute M-E, Durr A, et al. Plant J. 1995;8:763–770. doi: 10.1046/j.1365-313x.1995.08050763.x. [DOI] [PubMed] [Google Scholar]
- 11.Hermanson G G, Hoekstra M F, McElligott D L, Evans G A. Nucleic Acids Res. 1991;19:4943–4948. doi: 10.1093/nar/19.18.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minet M, Dufour M-E, Lacroute F. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- 13.Mandel M A, Yanofsky M F. Plant Cell. 1995;7:1763–1771. doi: 10.1105/tpc.7.11.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker J C. Plant J. 1993;3:451–456. doi: 10.1111/j.1365-313x.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 15.Bevan M. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bent A F, Kunkel B N, Dahlbeck D, Brown K L, Schmidt R, Giraudat J, Leung J, Staskawicz B. Science. 1994;265:1856–1860. doi: 10.1126/science.8091210. [DOI] [PubMed] [Google Scholar]
- 17.Castle L A, Morris R O. Plant Mol Biol Rep. 1990;8:28–39. [Google Scholar]
- 18.Rose A B, Casselman A L, Last R L. Plant Physiol. 1992;100:582–592. doi: 10.1104/pp.100.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyogi K K. Ph.D. thesis. Boston: Massachusetts Institute of Technology; 1993. [Google Scholar]
- 20.Paz-Ares J, Ghosal D, Wienand U, Peterson P A, Saedler H. EMBO J. 1987;6:3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotewold E, Athma P, Peterson T. Proc Natl Acad Sci USA. 1991;88:4587–4591. doi: 10.1073/pnas.88.11.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppenhiemer D G, Herman P L, Sivakumaran S, Esch J, Marks M D. Cell. 1991;67:483–493. doi: 10.1016/0092-8674(91)90523-2. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan M L, Green P J. Plant Mol Biol. 1993;23:1091–1104. doi: 10.1007/BF00042344. [DOI] [PubMed] [Google Scholar]
- 24.Abler M L, Green P J. Plant Mol Biol. 1996;32:63–78. doi: 10.1007/BF00039377. [DOI] [PubMed] [Google Scholar]
- 25.Grant C M, Miller P F, Hinnebusch A G. Mol Cell Biol. 1994;14:2616–2628. doi: 10.1128/mcb.14.4.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinnebusch A G. Trends Biochem Sci. 1994;19:409–414. doi: 10.1016/0968-0004(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 27.Damiani R D, Wessler S R. Proc Natl Acad Sci USA. 1993;90:8244–8248. doi: 10.1073/pnas.90.17.8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delbecq P, Werner M, Feller A, Filipkowski R K, Messenguy F, Pierard A. Mol Cell Biol. 1994;14:2378–2390. doi: 10.1128/mcb.14.4.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barczak A J, Zhao J, Pruitt K D, Last R L. Genetics. 1995;140:303–313. doi: 10.1093/genetics/140.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohmer S, Maddaloni M, Motto M, Salamini F, Thompson R D. Plant Cell. 1993;5:65–73. doi: 10.1105/tpc.5.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooner H K, Robbins T P, Jorgensen R A. Annu Rev Genet. 1991;25:173–199. doi: 10.1146/annurev.ge.25.120191.001133. [DOI] [PubMed] [Google Scholar]