Abstract
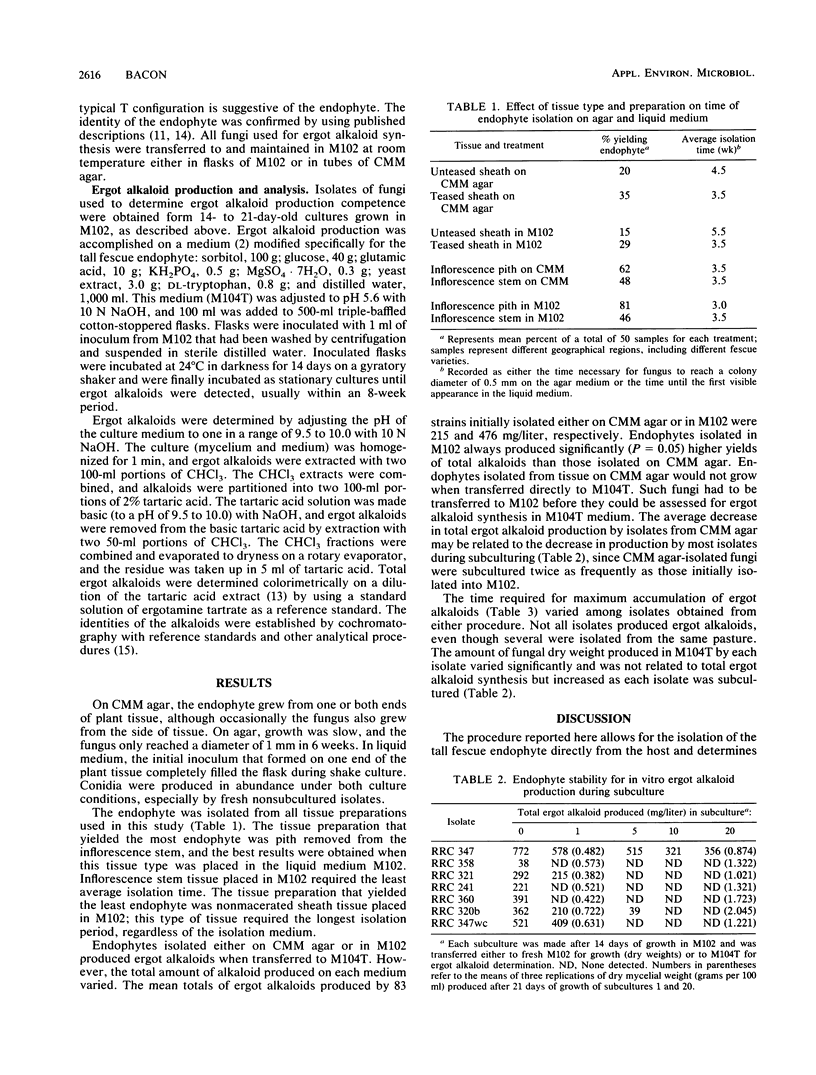
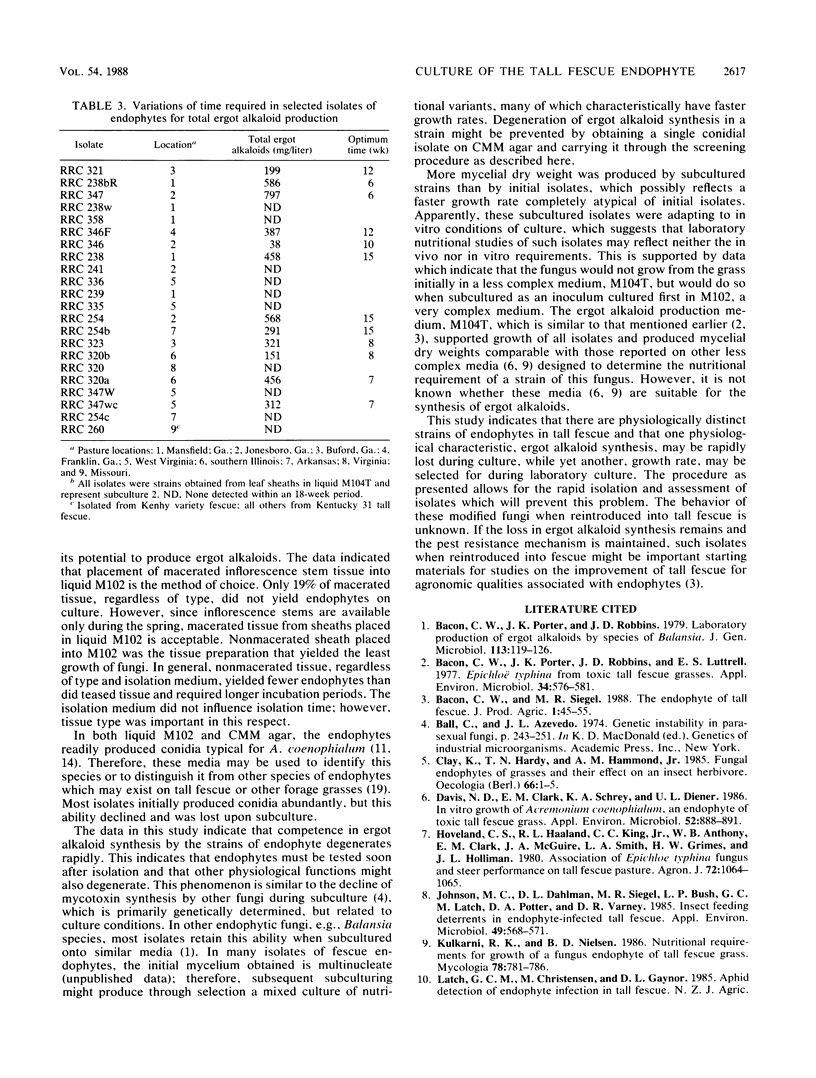
A procedure was developed to isolate and determine ergot alkaloid production by Acremonium coenophialum, the endophytic fungus of tall fescue. The procedure established that macerated leaf sheath or pith from inflorescence stem placed either in a liquid medium or on a corn meal-malt extract agar medium produced isolated mycelium and characteristic conidia within a 3- to 3.5-week period. Once isolated, each fungus was placed in another liquid medium, M104T, where competent strains produced total ergot alkaloids ranging from 38 to 797 mg/liter. Several isolates were negative for ergot alkaloid synthesis. The production of ergot alkaloids by individual isolates was unstable; isolates rapidly degenerated in their ability to produce ergot alkaloids during subculture. However, the procedure as presented allows the assessment of an isolate for ergot alkaloid synthesis during its initial isolation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon C. W., Porter J. K., Robbins J. D. Laboratory production of ergot alkaloids by species of balansia. J Gen Microbiol. 1979 Jul;113(1):119–126. doi: 10.1099/00221287-113-1-119. [DOI] [PubMed] [Google Scholar]
- Bacon C. W., Porter J. K., Robbins J. D., Luttrell E. S. Epichloë typhina from toxic tall fescue grasses. Appl Environ Microbiol. 1977 Nov;34(5):576–581. doi: 10.1128/aem.34.5.576-581.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. D., Clark E. M., Schrey K. A., Diener U. L. In Vitro Growth of Acremonium coenophialum, an Endophyte of Toxic Tall Fescue Grass. Appl Environ Microbiol. 1986 Oct;52(4):888–891. doi: 10.1128/aem.52.4.888-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Dahlman D. L., Siegel M. R., Bush L. P., Latch G. C., Potter D. A., Varney D. R. Insect feeding deterrents in endophyte-infected tall fescue. Appl Environ Microbiol. 1985 Mar;49(3):568–571. doi: 10.1128/aem.49.3.568-571.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons P. C., Plattner R. D., Bacon C. W. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science. 1986 Apr 25;232(4749):487–489. doi: 10.1126/science.3008328. [DOI] [PubMed] [Google Scholar]
- Porter J. K., Bacon C. W., Robbins J. D. Ergosine, ergosinine, and chanoclavine I from Epichloë typhina. J Agric Food Chem. 1979 May-Jun;27(3):595–598. doi: 10.1021/jf60223a045. [DOI] [PubMed] [Google Scholar]
- Schmidt S. P., Hoveland C. S., Clark E. M., Davis N. D., Smith L. A., Grimes H. W., Holliman J. L. Association of an endophytic fungus with fescue toxicity in steers fed Kentucky 31 tall fescue seed or hay. J Anim Sci. 1982 Dec;55(6):1259–1263. doi: 10.2527/jas1982.5561259x. [DOI] [PubMed] [Google Scholar]



